 Found 1583 hits with Last Name = 'jeong' and Initial = 'jw'
Found 1583 hits with Last Name = 'jeong' and Initial = 'jw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458125
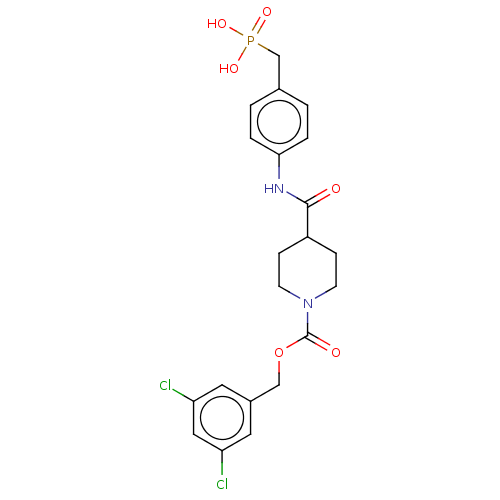
(CHEMBL4217352)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C21H23Cl2N2O6P/c22-17-9-15(10-18(23)11-17)12-31-21(27)25-7-5-16(6-8-25)20(26)24-19-3-1-14(2-4-19)13-32(28,29)30/h1-4,9-11,16H,5-8,12-13H2,(H,24,26)(H2,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458125

(CHEMBL4217352)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C21H23Cl2N2O6P/c22-17-9-15(10-18(23)11-17)12-31-21(27)25-7-5-16(6-8-25)20(26)24-19-3-1-14(2-4-19)13-32(28,29)30/h1-4,9-11,16H,5-8,12-13H2,(H,24,26)(H2,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of ENPP2 in human A2058 cells using LPC as substrate preincubated for 15 mins followed by substrate addition measured after 3 hrs by LC-MS... |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458132

(CHEMBL4205127)Show SMILES OP(O)(=O)Cc1ccc(cc1)C(=O)NC1CCN(CC1)C(=O)OCc1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C21H23Cl2N2O6P/c22-17-9-15(10-18(23)11-17)12-31-21(27)25-7-5-19(6-8-25)24-20(26)16-3-1-14(2-4-16)13-32(28,29)30/h1-4,9-11,19H,5-8,12-13H2,(H,24,26)(H2,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458135
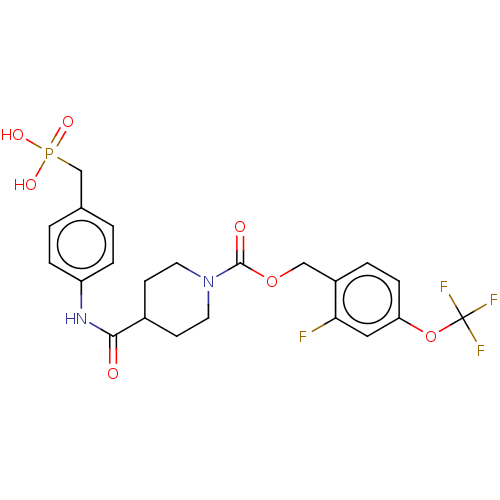
(CHEMBL4214731)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2ccc(OC(F)(F)F)cc2F)cc1 Show InChI InChI=1S/C22H23F4N2O7P/c23-19-11-18(35-22(24,25)26)6-3-16(19)12-34-21(30)28-9-7-15(8-10-28)20(29)27-17-4-1-14(2-5-17)13-36(31,32)33/h1-6,11,15H,7-10,12-13H2,(H,27,29)(H2,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458131

(CHEMBL4212398)Show SMILES OP(O)(=O)Cc1csc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)n1 Show InChI InChI=1S/C18H20Cl2N3O6PS/c19-13-5-11(6-14(20)7-13)8-29-18(25)23-3-1-12(2-4-23)16(24)22-17-21-15(10-31-17)9-30(26,27)28/h5-7,10,12H,1-4,8-9H2,(H,21,22,24)(H2,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458127
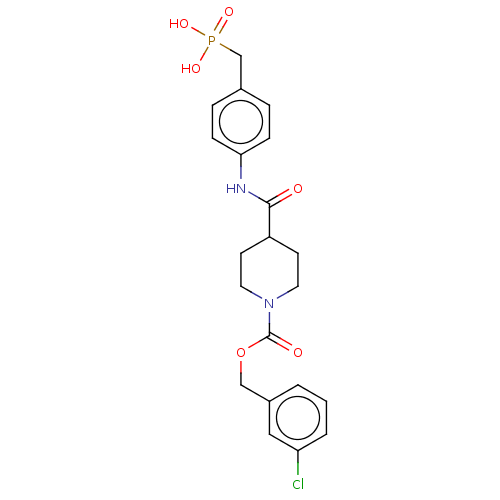
(CHEMBL4211269)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cccc(Cl)c2)cc1 Show InChI InChI=1S/C21H24ClN2O6P/c22-18-3-1-2-16(12-18)13-30-21(26)24-10-8-17(9-11-24)20(25)23-19-6-4-15(5-7-19)14-31(27,28)29/h1-7,12,17H,8-11,13-14H2,(H,23,25)(H2,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458126

(CHEMBL4203076)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2ccc(OC(F)(F)F)cc2)cc1 Show InChI InChI=1S/C22H24F3N2O7P/c23-22(24,25)34-19-7-3-15(4-8-19)13-33-21(29)27-11-9-17(10-12-27)20(28)26-18-5-1-16(2-6-18)14-35(30,31)32/h1-8,17H,9-14H2,(H,26,28)(H2,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458125

(CHEMBL4217352)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C21H23Cl2N2O6P/c22-17-9-15(10-18(23)11-17)12-31-21(27)25-7-5-16(6-8-25)20(26)24-19-3-1-14(2-4-19)13-32(28,29)30/h1-4,9-11,16H,5-8,12-13H2,(H,24,26)(H2,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using bis-pNPP as substrate measured every 10 secs for 10 mins by spectrophotometric assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458135

(CHEMBL4214731)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2ccc(OC(F)(F)F)cc2F)cc1 Show InChI InChI=1S/C22H23F4N2O7P/c23-19-11-18(35-22(24,25)26)6-3-16(19)12-34-21(30)28-9-7-15(8-10-28)20(29)27-17-4-1-14(2-5-17)13-36(31,32)33/h1-6,11,15H,7-10,12-13H2,(H,27,29)(H2,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using bis-pNPP as substrate measured every 10 secs for 10 mins by spectrophotometric assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458126

(CHEMBL4203076)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2ccc(OC(F)(F)F)cc2)cc1 Show InChI InChI=1S/C22H24F3N2O7P/c23-22(24,25)34-19-7-3-15(4-8-19)13-33-21(29)27-11-9-17(10-12-27)20(28)26-18-5-1-16(2-6-18)14-35(30,31)32/h1-8,17H,9-14H2,(H,26,28)(H2,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using bis-pNPP as substrate measured every 10 secs for 10 mins by spectrophotometric assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458136
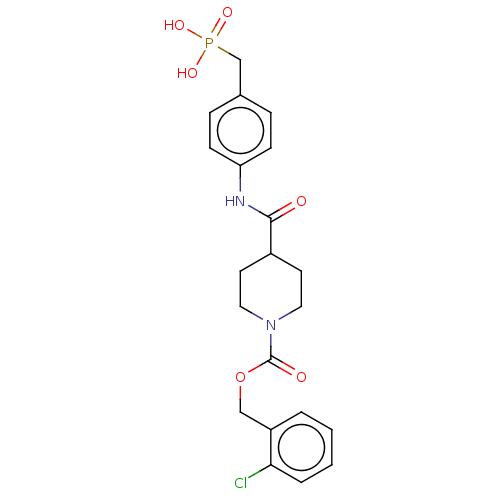
(CHEMBL4216236)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2ccccc2Cl)cc1 Show InChI InChI=1S/C21H24ClN2O6P/c22-19-4-2-1-3-17(19)13-30-21(26)24-11-9-16(10-12-24)20(25)23-18-7-5-15(6-8-18)14-31(27,28)29/h1-8,16H,9-14H2,(H,23,25)(H2,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458133

(CHEMBL4216825)Show SMILES OP(O)(=O)Cc1csc(n1)C(=O)NC1CCN(C1)C(=O)OCc1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C17H18Cl2N3O6PS/c18-11-3-10(4-12(19)5-11)7-28-17(24)22-2-1-13(6-22)20-15(23)16-21-14(9-30-16)8-29(25,26)27/h3-5,9,13H,1-2,6-8H2,(H,20,23)(H2,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458124
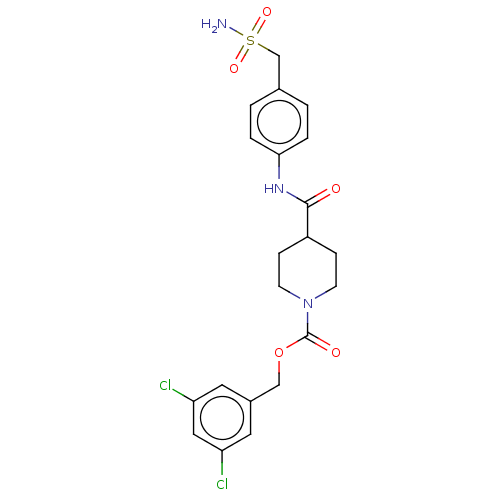
(CHEMBL4208368)Show SMILES NS(=O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C21H23Cl2N3O5S/c22-17-9-15(10-18(23)11-17)12-31-21(28)26-7-5-16(6-8-26)20(27)25-19-3-1-14(2-4-19)13-32(24,29)30/h1-4,9-11,16H,5-8,12-13H2,(H,25,27)(H2,24,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50380413

(CHEMBL2018573)Show SMILES Fc1ccc(C[C@@H]2C[C@H](N(C2)C(=O)Cn2nccn2)C(=O)Nc2ccc(Oc3ccc(F)cc3)cc2)cc1 |r| Show InChI InChI=1S/C28H25F2N5O3/c29-21-3-1-19(2-4-21)15-20-16-26(34(17-20)27(36)18-35-31-13-14-32-35)28(37)33-23-7-11-25(12-8-23)38-24-9-5-22(30)6-10-24/h1-14,20,26H,15-18H2,(H,33,37)/t20-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometry |
J Med Chem 55: 1368-81 (2012)
Article DOI: 10.1021/jm201533b
BindingDB Entry DOI: 10.7270/Q2416Z1H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393357

(CHEMBL2152148)Show SMILES CNC(=O)c1nc(cnc1N)-c1ccc(Cl)c(c1)S(=O)(=O)Nc1ccc(F)cc1Cl Show InChI InChI=1S/C18H14Cl2FN5O3S/c1-23-18(27)16-17(22)24-8-14(25-16)9-2-4-11(19)15(6-9)30(28,29)26-13-5-3-10(21)7-12(13)20/h2-8,26H,1H3,(H2,22,24)(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458132

(CHEMBL4205127)Show SMILES OP(O)(=O)Cc1ccc(cc1)C(=O)NC1CCN(CC1)C(=O)OCc1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C21H23Cl2N2O6P/c22-17-9-15(10-18(23)11-17)12-31-21(27)25-7-5-19(6-8-25)24-20(26)16-3-1-14(2-4-16)13-32(28,29)30/h1-4,9-11,19H,5-8,12-13H2,(H,24,26)(H2,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using bis-pNPP as substrate measured every 10 secs for 10 mins by spectrophotometric assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50380417
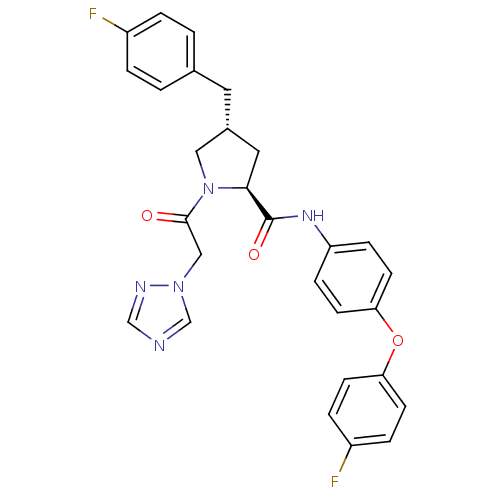
(CHEMBL2018485)Show SMILES Fc1ccc(C[C@@H]2C[C@H](N(C2)C(=O)Cn2cncn2)C(=O)Nc2ccc(Oc3ccc(F)cc3)cc2)cc1 |r| Show InChI InChI=1S/C28H25F2N5O3/c29-21-3-1-19(2-4-21)13-20-14-26(35(15-20)27(36)16-34-18-31-17-32-34)28(37)33-23-7-11-25(12-8-23)38-24-9-5-22(30)6-10-24/h1-12,17-18,20,26H,13-16H2,(H,33,37)/t20-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometry |
J Med Chem 55: 1368-81 (2012)
Article DOI: 10.1021/jm201533b
BindingDB Entry DOI: 10.7270/Q2416Z1H |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50380418

(CHEMBL2018571)Show SMILES Fc1ccc(C[C@@H]2C[C@H](N(C2)C(=O)Cn2ccnn2)C(=O)Nc2ccc(Oc3ccc(F)cc3)cc2)cc1 |r| Show InChI InChI=1S/C28H25F2N5O3/c29-21-3-1-19(2-4-21)15-20-16-26(35(17-20)27(36)18-34-14-13-31-33-34)28(37)32-23-7-11-25(12-8-23)38-24-9-5-22(30)6-10-24/h1-14,20,26H,15-18H2,(H,32,37)/t20-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometry |
J Med Chem 55: 1368-81 (2012)
Article DOI: 10.1021/jm201533b
BindingDB Entry DOI: 10.7270/Q2416Z1H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393365
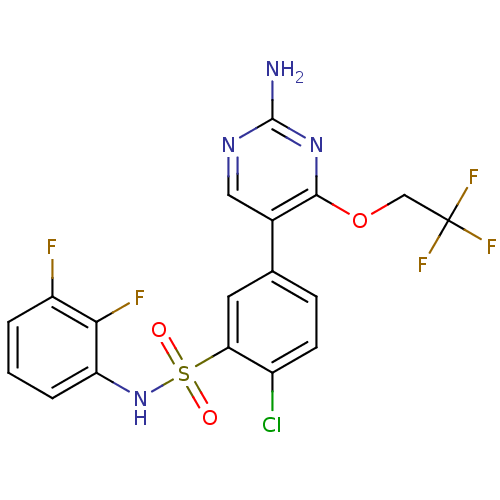
(CHEMBL2152257)Show SMILES Nc1ncc(-c2ccc(Cl)c(c2)S(=O)(=O)Nc2cccc(F)c2F)c(OCC(F)(F)F)n1 Show InChI InChI=1S/C18H12ClF5N4O3S/c19-11-5-4-9(10-7-26-17(25)27-16(10)31-8-18(22,23)24)6-14(11)32(29,30)28-13-3-1-2-12(20)15(13)21/h1-7,28H,8H2,(H2,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50310205

(7-(3'-Cyclopropylmethoxy-4'-difluoromethoxyphenyl)...)Show SMILES FC(F)Oc1ccc(cc1OCC1CC1)-c1ccnc2cc(nn12)-c1cccc(I)c1 Show InChI InChI=1S/C23H18F2IN3O2/c24-23(25)31-20-7-6-16(11-21(20)30-13-14-4-5-14)19-8-9-27-22-12-18(28-29(19)22)15-2-1-3-17(26)10-15/h1-3,6-12,14,23H,4-5,13H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D |
Bioorg Med Chem Lett 20: 922-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.070
BindingDB Entry DOI: 10.7270/Q2SF2W9S |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50380410

(CHEMBL2018484)Show SMILES Fc1ccc(Oc2ccc(NC(=O)[C@@H]3C[C@@H](Cc4ccccc4)CN3C(=O)Cn3cncn3)cc2)cc1 |r| Show InChI InChI=1S/C28H26FN5O3/c29-22-6-10-24(11-7-22)37-25-12-8-23(9-13-25)32-28(36)26-15-21(14-20-4-2-1-3-5-20)16-34(26)27(35)17-33-19-30-18-31-33/h1-13,18-19,21,26H,14-17H2,(H,32,36)/t21-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometry |
J Med Chem 55: 1368-81 (2012)
Article DOI: 10.1021/jm201533b
BindingDB Entry DOI: 10.7270/Q2416Z1H |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458128

(CHEMBL4206899)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2ccccc2F)cc1 Show InChI InChI=1S/C21H24FN2O6P/c22-19-4-2-1-3-17(19)13-30-21(26)24-11-9-16(10-12-24)20(25)23-18-7-5-15(6-8-18)14-31(27,28)29/h1-8,16H,9-14H2,(H,23,25)(H2,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458129
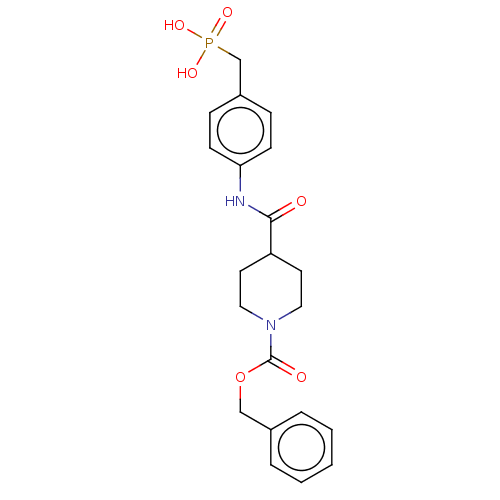
(CHEMBL4209155)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2ccccc2)cc1 Show InChI InChI=1S/C21H25N2O6P/c24-20(22-19-8-6-17(7-9-19)15-30(26,27)28)18-10-12-23(13-11-18)21(25)29-14-16-4-2-1-3-5-16/h1-9,18H,10-15H2,(H,22,24)(H2,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458125

(CHEMBL4217352)Show SMILES OP(O)(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C21H23Cl2N2O6P/c22-17-9-15(10-18(23)11-17)12-31-21(27)25-7-5-16(6-8-25)20(26)24-19-3-1-14(2-4-19)13-32(28,29)30/h1-4,9-11,16H,5-8,12-13H2,(H,24,26)(H2,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of ENPP2 in human SKHEP1 cells using LPC as substrate preincubated for 15 mins followed by substrate addition measured after 3 hrs by LC-M... |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393359
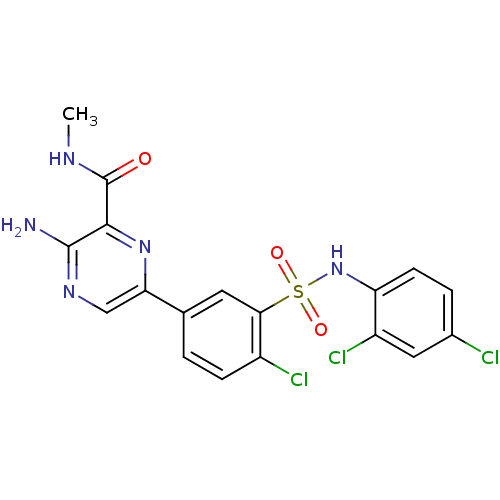
(CHEMBL2152150)Show SMILES CNC(=O)c1nc(cnc1N)-c1ccc(Cl)c(c1)S(=O)(=O)Nc1ccc(Cl)cc1Cl Show InChI InChI=1S/C18H14Cl3N5O3S/c1-23-18(27)16-17(22)24-8-14(25-16)9-2-4-11(20)15(6-9)30(28,29)26-13-5-3-10(19)7-12(13)21/h2-8,26H,1H3,(H2,22,24)(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458130
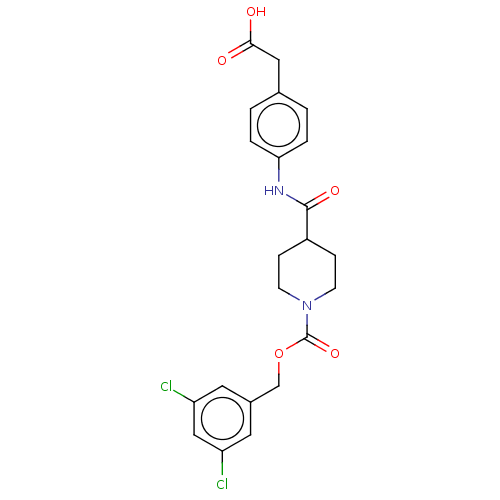
(CHEMBL4204027)Show SMILES OC(=O)Cc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C22H22Cl2N2O5/c23-17-9-15(10-18(24)12-17)13-31-22(30)26-7-5-16(6-8-26)21(29)25-19-3-1-14(2-4-19)11-20(27)28/h1-4,9-10,12,16H,5-8,11,13H2,(H,25,29)(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458134

(CHEMBL4203499)Show SMILES NS(=O)(=O)Nc1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C20H22Cl2N4O5S/c21-15-9-13(10-16(22)11-15)12-31-20(28)26-7-5-14(6-8-26)19(27)24-17-1-3-18(4-2-17)25-32(23,29)30/h1-4,9-11,14,25H,5-8,12H2,(H,24,27)(H2,23,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50310235

(3-[7-(3'-Cyclopropylmethoxy-4'-difluoromethoxyphen...)Show SMILES OC(=O)c1cccc(c1)-c1cc2nccc(-c3ccc(OC(F)F)c(OCC4CC4)c3)n2n1 Show InChI InChI=1S/C24H19F2N3O4/c25-24(26)33-20-7-6-16(11-21(20)32-13-14-4-5-14)19-8-9-27-22-12-18(28-29(19)22)15-2-1-3-17(10-15)23(30)31/h1-3,6-12,14,24H,4-5,13H2,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D |
Bioorg Med Chem Lett 20: 922-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.070
BindingDB Entry DOI: 10.7270/Q2SF2W9S |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 30 [57-517]
(Homo sapiens (Human)) | BDBM592760

(2-chloro-N-(8-cyano-8- azabicyclo[3.2.1]octan-2-yl...)Show SMILES Cc1cnn(c1)-c1ccc(C(=O)N[C@@H]2CCC3CCC2N3C#N)c(Cl)c1 |r,THB:21:20:18.17:13.15.14| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
USP30 inhibition was tested with recombinant human USP30 using the fluorescent substrate Ubiquitin-Rhodamine 110 (UBPBio) in 1536-well format. Compou... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NZ8CKC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393333
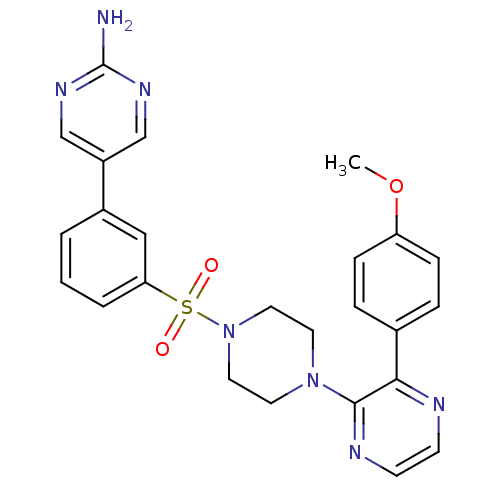
(CHEMBL2151050)Show SMILES COc1ccc(cc1)-c1nccnc1N1CCN(CC1)S(=O)(=O)c1cccc(c1)-c1cnc(N)nc1 Show InChI InChI=1S/C25H25N7O3S/c1-35-21-7-5-18(6-8-21)23-24(28-10-9-27-23)31-11-13-32(14-12-31)36(33,34)22-4-2-3-19(15-22)20-16-29-25(26)30-17-20/h2-10,15-17H,11-14H2,1H3,(H2,26,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | |
GTPase KRas [1-169,G12C,C118A]
(Homo sapiens (Human)) | BDBM515895

(2-(5-Chloro-2- cyclopropyl-3- ((5-methoxy-3,4- dih...)Show SMILES COc1nccc2CN(CCc12)C(=O)c1c(C2CC2)n(CC(=O)NC2CN(C2)C(=O)C=C)c2c(C)cc(Cl)cc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Purified GDP-bound KRAS protein (aa 1-169), containing both G12C and C118A amino acid substitutions and an N-terminal His-tag, was pre-incubated in a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2DZ0CGT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393348
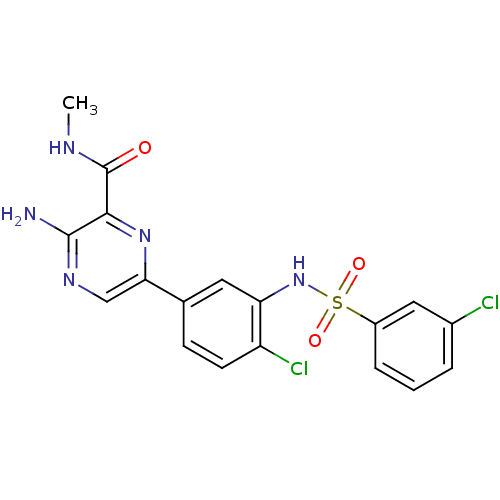
(CHEMBL2152139)Show SMILES CNC(=O)c1nc(cnc1N)-c1ccc(Cl)c(NS(=O)(=O)c2cccc(Cl)c2)c1 Show InChI InChI=1S/C18H15Cl2N5O3S/c1-22-18(26)16-17(21)23-9-15(24-16)10-5-6-13(20)14(7-10)25-29(27,28)12-4-2-3-11(19)8-12/h2-9,25H,1H3,(H2,21,23)(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50310214

(2-(3-Bromophenyl)-7-(3'-cyclopropylmethoxy-4'-difl...)Show SMILES FC(F)Oc1ccc(cc1OCC1CC1)-c1ccnc2cc(nn12)-c1cccc(Br)c1 Show InChI InChI=1S/C23H18BrF2N3O2/c24-17-3-1-2-15(10-17)18-12-22-27-9-8-19(29(22)28-18)16-6-7-20(31-23(25)26)21(11-16)30-13-14-4-5-14/h1-3,6-12,14,23H,4-5,13H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D |
Bioorg Med Chem Lett 20: 922-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.070
BindingDB Entry DOI: 10.7270/Q2SF2W9S |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50380414

(CHEMBL2018467)Show SMILES Fc1ccc(Oc2ccc(NC(=O)[C@H](COCc3ccccc3)NC(=O)Cc3cnc[nH]3)cc2)cc1 |r| Show InChI InChI=1S/C27H25FN4O4/c28-20-6-10-23(11-7-20)36-24-12-8-21(9-13-24)31-27(34)25(17-35-16-19-4-2-1-3-5-19)32-26(33)14-22-15-29-18-30-22/h1-13,15,18,25H,14,16-17H2,(H,29,30)(H,31,34)(H,32,33)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometry |
J Med Chem 55: 1368-81 (2012)
Article DOI: 10.1021/jm201533b
BindingDB Entry DOI: 10.7270/Q2416Z1H |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50380417

(CHEMBL2018485)Show SMILES Fc1ccc(C[C@@H]2C[C@H](N(C2)C(=O)Cn2cncn2)C(=O)Nc2ccc(Oc3ccc(F)cc3)cc2)cc1 |r| Show InChI InChI=1S/C28H25F2N5O3/c29-21-3-1-19(2-4-21)13-20-14-26(35(15-20)27(36)16-34-18-31-17-32-34)28(37)33-23-7-11-25(12-8-23)38-24-9-5-22(30)6-10-24/h1-12,17-18,20,26H,13-16H2,(H,33,37)/t20-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced tango beta-arrestin recruitment preincubated for... |
J Med Chem 55: 1368-81 (2012)
Article DOI: 10.1021/jm201533b
BindingDB Entry DOI: 10.7270/Q2416Z1H |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50380437
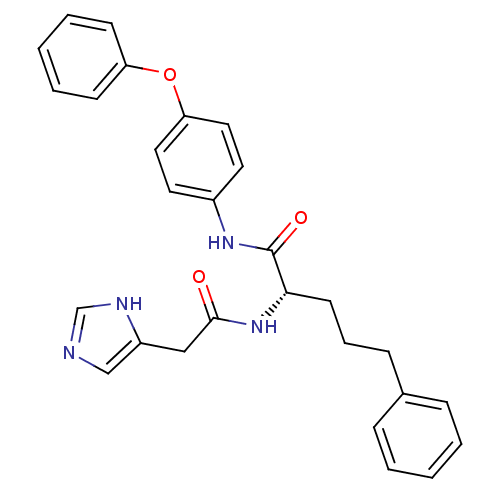
(CHEMBL2018474)Show SMILES O=C(Cc1cnc[nH]1)N[C@@H](CCCc1ccccc1)C(=O)Nc1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C28H28N4O3/c33-27(18-23-19-29-20-30-23)32-26(13-7-10-21-8-3-1-4-9-21)28(34)31-22-14-16-25(17-15-22)35-24-11-5-2-6-12-24/h1-6,8-9,11-12,14-17,19-20,26H,7,10,13,18H2,(H,29,30)(H,31,34)(H,32,33)/t26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Antagonist activity at S1P1 receptor expressed in HEK293 cells assessed as inhibition of S1P-induced cAMP response after 90 mins by spectrophotometry |
J Med Chem 55: 1368-81 (2012)
Article DOI: 10.1021/jm201533b
BindingDB Entry DOI: 10.7270/Q2416Z1H |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50269714

(CHEMBL4092264)Show SMILES OP(O)(=O)Cc1ccc(cc1)-c1csc(n1)C(=O)NC1CCN(CC1)C(=O)OCc1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C24H24Cl2N3O6PS/c25-18-9-16(10-19(26)11-18)12-35-24(31)29-7-5-20(6-8-29)27-22(30)23-28-21(14-37-23)17-3-1-15(2-4-17)13-36(32,33)34/h1-4,9-11,14,20H,5-8,12-13H2,(H,27,30)(H2,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of ENPP2 in human plasma using LPC as substrate preincubated for 15 mins followed by substrate addition measured after 3 hrs by LC-MS/MS a... |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393339
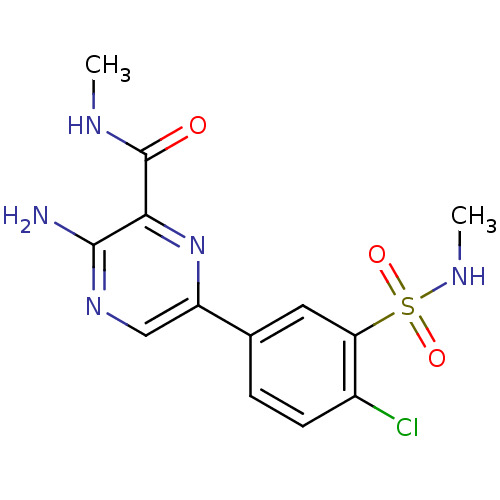
(CHEMBL2152131)Show SMILES CNC(=O)c1nc(cnc1N)-c1ccc(Cl)c(c1)S(=O)(=O)NC Show InChI InChI=1S/C13H14ClN5O3S/c1-16-13(20)11-12(15)18-6-9(19-11)7-3-4-8(14)10(5-7)23(21,22)17-2/h3-6,17H,1-2H3,(H2,15,18)(H,16,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 30 [57-517]
(Homo sapiens (Human)) | BDBM592741
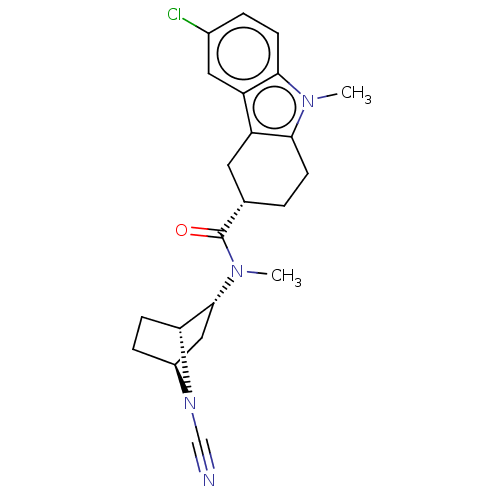
((3S)-6-chloro-N-((1R,2R,4S)-7-cyano-7- azabicyclo[...)Show SMILES CN([C@@H]1C[C@@H]2CC[C@H]1N2C#N)C(=O)[C@H]1CCc2c(C1)c1cc(Cl)ccc1n2C |r,THB:9:8:6.5:2.3| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
USP30 inhibition was tested with recombinant human USP30 using the fluorescent substrate Ubiquitin-Rhodamine 110 (UBPBio) in 1536-well format. Compou... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NZ8CKC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393360
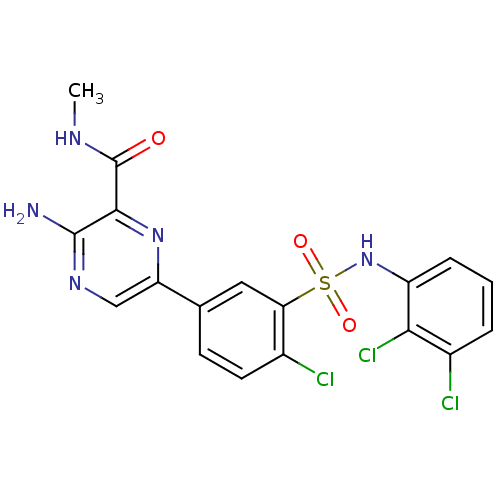
(CHEMBL2152250)Show SMILES CNC(=O)c1nc(cnc1N)-c1ccc(Cl)c(c1)S(=O)(=O)Nc1cccc(Cl)c1Cl Show InChI InChI=1S/C18H14Cl3N5O3S/c1-23-18(27)16-17(22)24-8-13(25-16)9-5-6-10(19)14(7-9)30(28,29)26-12-4-2-3-11(20)15(12)21/h2-8,26H,1H3,(H2,22,24)(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393342

(CHEMBL2152252)Show SMILES CNC(=O)c1nc(cnc1N)-c1ccc(Cl)c(c1)S(=O)(=O)Nc1cccc(F)c1F Show InChI InChI=1S/C18H14ClF2N5O3S/c1-23-18(27)16-17(22)24-8-13(25-16)9-5-6-10(19)14(7-9)30(28,29)26-12-4-2-3-11(20)15(12)21/h2-8,26H,1H3,(H2,22,24)(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393335
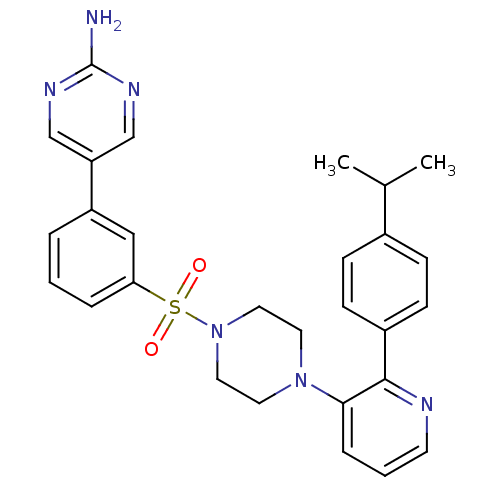
(CHEMBL2152127)Show SMILES CC(C)c1ccc(cc1)-c1ncccc1N1CCN(CC1)S(=O)(=O)c1cccc(c1)-c1cnc(N)nc1 Show InChI InChI=1S/C28H30N6O2S/c1-20(2)21-8-10-22(11-9-21)27-26(7-4-12-30-27)33-13-15-34(16-14-33)37(35,36)25-6-3-5-23(17-25)24-18-31-28(29)32-19-24/h3-12,17-20H,13-16H2,1-2H3,(H2,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50310226

(Acetic acid 3-[7-(3'-cyclopropylmethoxy-4'-difluor...)Show SMILES CC(=O)Oc1cccc(c1)-c1cc2nccc(-c3ccc(OC(F)F)c(OCC4CC4)c3)n2n1 Show InChI InChI=1S/C25H21F2N3O4/c1-15(31)33-19-4-2-3-17(11-19)20-13-24-28-10-9-21(30(24)29-20)18-7-8-22(34-25(26)27)23(12-18)32-14-16-5-6-16/h2-4,7-13,16,25H,5-6,14H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D |
Bioorg Med Chem Lett 20: 922-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.070
BindingDB Entry DOI: 10.7270/Q2SF2W9S |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393334
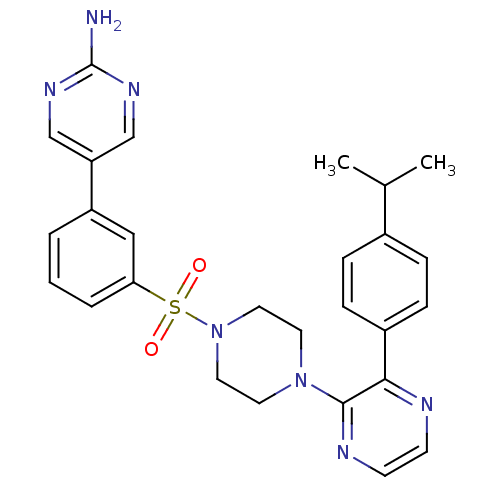
(CHEMBL2152126)Show SMILES CC(C)c1ccc(cc1)-c1nccnc1N1CCN(CC1)S(=O)(=O)c1cccc(c1)-c1cnc(N)nc1 Show InChI InChI=1S/C27H29N7O2S/c1-19(2)20-6-8-21(9-7-20)25-26(30-11-10-29-25)33-12-14-34(15-13-33)37(35,36)24-5-3-4-22(16-24)23-17-31-27(28)32-18-23/h3-11,16-19H,12-15H2,1-2H3,(H2,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
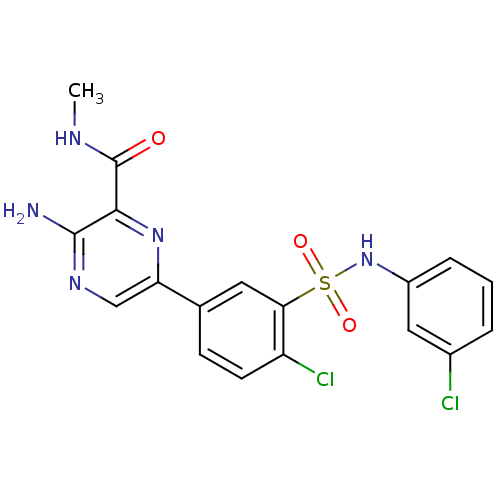
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393353
(CHEMBL2152144)Show SMILES CNC(=O)c1nc(cnc1N)-c1ccc(Cl)c(c1)S(=O)(=O)Nc1cccc(Cl)c1 Show InChI InChI=1S/C18H15Cl2N5O3S/c1-22-18(26)16-17(21)23-9-14(24-16)10-5-6-13(20)15(7-10)29(27,28)25-12-4-2-3-11(19)8-12/h2-9,25H,1H3,(H2,21,23)(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50458123

(CHEMBL4215710)Show SMILES ONC(=O)c1ccc(NC(=O)C2CCN(CC2)C(=O)OCc2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C21H21Cl2N3O5/c22-16-9-13(10-17(23)11-16)12-31-21(29)26-7-5-15(6-8-26)19(27)24-18-3-1-14(2-4-18)20(28)25-30/h1-4,9-11,15,30H,5-8,12H2,(H,24,27)(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay |
Eur J Med Chem 148: 397-409 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.049
BindingDB Entry DOI: 10.7270/Q2VH5RGT |
More data for this
Ligand-Target Pair | |
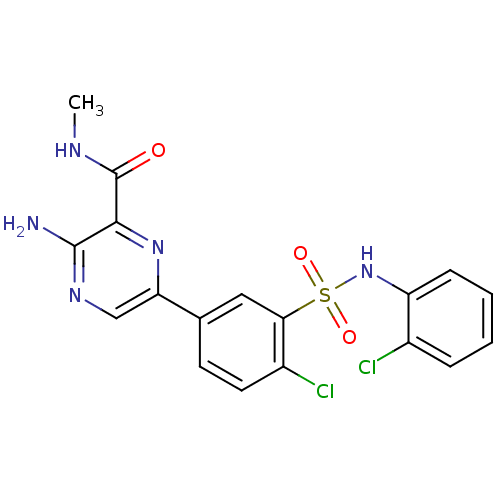
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393354
(CHEMBL2152145)Show SMILES CNC(=O)c1nc(cnc1N)-c1ccc(Cl)c(c1)S(=O)(=O)Nc1ccccc1Cl Show InChI InChI=1S/C18H15Cl2N5O3S/c1-22-18(26)16-17(21)23-9-14(24-16)10-6-7-12(20)15(8-10)29(27,28)25-13-5-3-2-4-11(13)19/h2-9,25H,1H3,(H2,21,23)(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50310210

(7-(3'-Cyclopentyloxy-4'-methoxyphenyl)-2-(3-chloro...)Show SMILES COc1ccc(cc1OC1CCCC1)-c1ccnc2cc(nn12)-c1cccc(Cl)c1 Show InChI InChI=1S/C24H22ClN3O2/c1-29-22-10-9-17(14-23(22)30-19-7-2-3-8-19)21-11-12-26-24-15-20(27-28(21)24)16-5-4-6-18(25)13-16/h4-6,9-15,19H,2-3,7-8H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D |
Bioorg Med Chem Lett 20: 922-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.070
BindingDB Entry DOI: 10.7270/Q2SF2W9S |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 30 [57-517]
(Homo sapiens (Human)) | BDBM592645

((3S)-6-chloro-N-((1R,2R,4S)-7-cyano-7- azabicyclo[...)Show SMILES Clc1ccc2[nH]c3CC[C@@H](Cc3c2c1)C(=O)N[C@@H]1C[C@@H]2CC[C@H]1N2C#N |r,THB:24:23:21.20:17.18| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
USP30 inhibition was tested with recombinant human USP30 using the fluorescent substrate Ubiquitin-Rhodamine 110 (UBPBio) in 1536-well format. Compou... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NZ8CKC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50393364
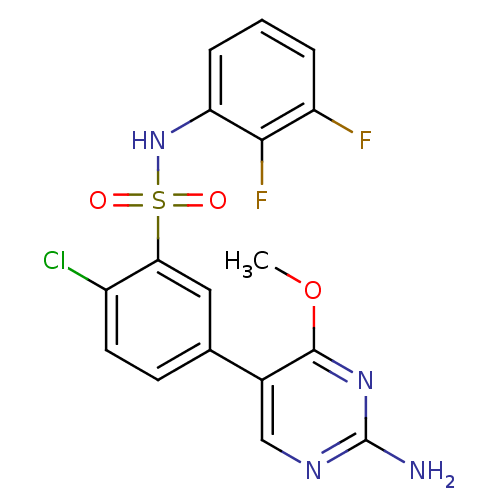
(CHEMBL2152256)Show SMILES COc1nc(N)ncc1-c1ccc(Cl)c(c1)S(=O)(=O)Nc1cccc(F)c1F Show InChI InChI=1S/C17H13ClF2N4O3S/c1-27-16-10(8-22-17(21)23-16)9-5-6-11(18)14(7-9)28(25,26)24-13-4-2-3-12(19)15(13)20/h2-8,24H,1H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma expressed in sf9 cells assessed as amount of ATP consumed by luciferase-luciferin chemiluminescence assay |
J Med Chem 55: 5467-82 (2012)
Article DOI: 10.1021/jm300403a
BindingDB Entry DOI: 10.7270/Q2DF6S9N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data