

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50561628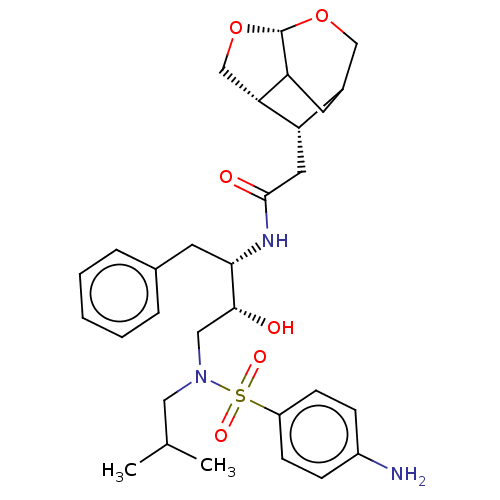 (CHEMBL4800615) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease by fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00670 BindingDB Entry DOI: 10.7270/Q2GF0Z75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50561630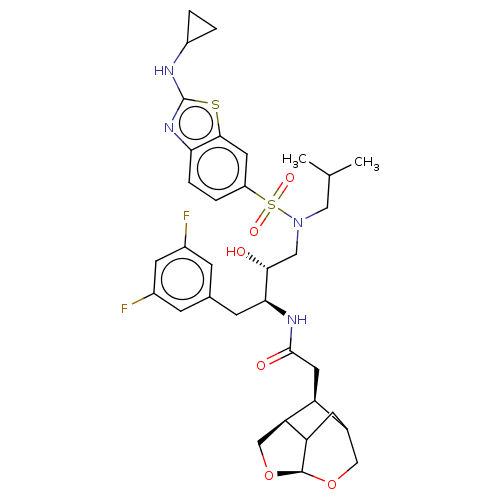 (CHEMBL4743665) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease by fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00670 BindingDB Entry DOI: 10.7270/Q2GF0Z75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50561627 (CHEMBL4744116) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease by fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00670 BindingDB Entry DOI: 10.7270/Q2GF0Z75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50561629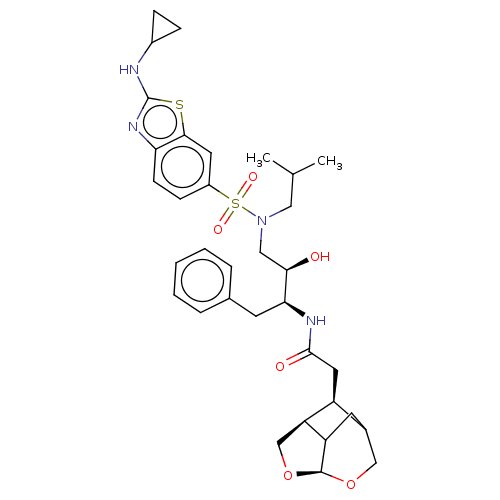 (CHEMBL4740468) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease by fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00670 BindingDB Entry DOI: 10.7270/Q2GF0Z75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50561623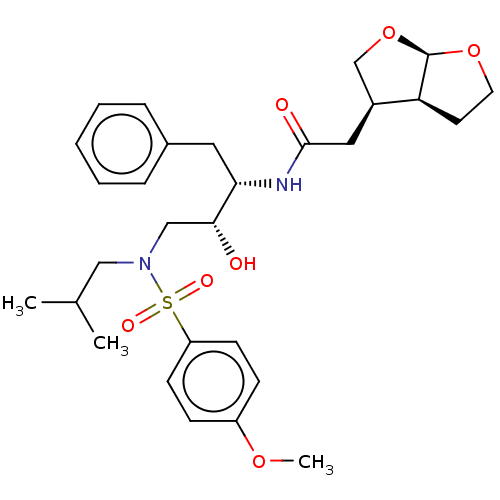 (CHEMBL256107 | GRL-0026A) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease by fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00670 BindingDB Entry DOI: 10.7270/Q2GF0Z75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50561626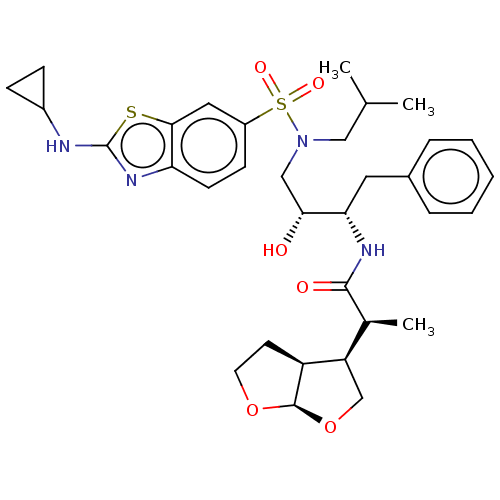 (CHEMBL4763723) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease by fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00670 BindingDB Entry DOI: 10.7270/Q2GF0Z75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50561624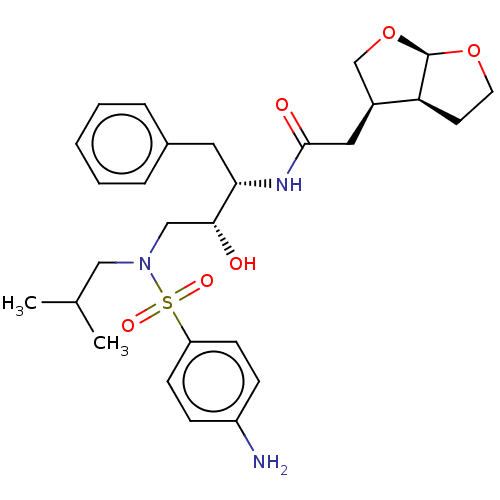 (CHEMBL4764958) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease by fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00670 BindingDB Entry DOI: 10.7270/Q2GF0Z75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452511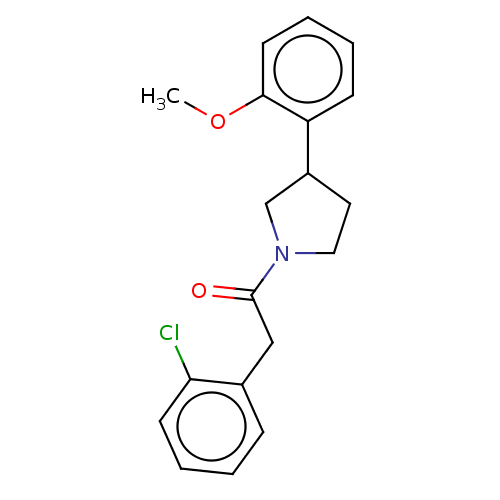 (CHEMBL4204517) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50561625 (CHEMBL4784475) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 284 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 protease by fluorescence based assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00670 BindingDB Entry DOI: 10.7270/Q2GF0Z75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Francisella tularensis subsp. tularensis (strain S...) | BDBM50392117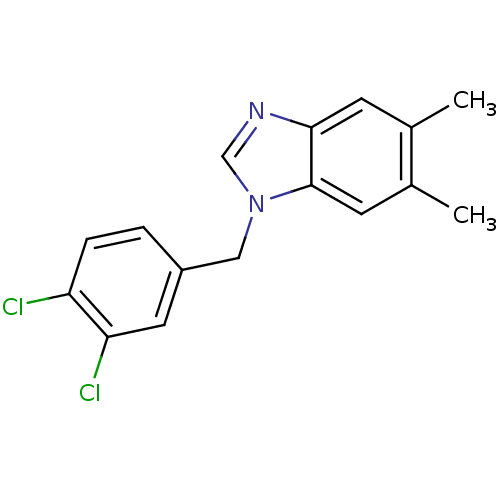 (CHEMBL1945507) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Competitive inhibition of Francisella tularensis subsp. tularensis SCHU4 N-terminal His-tagged FabI expressed in Escherichia coli BL21(DE3) using cro... | J Med Chem 55: 5933-41 (2012) Article DOI: 10.1021/jm300489v BindingDB Entry DOI: 10.7270/Q2S46T27 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452501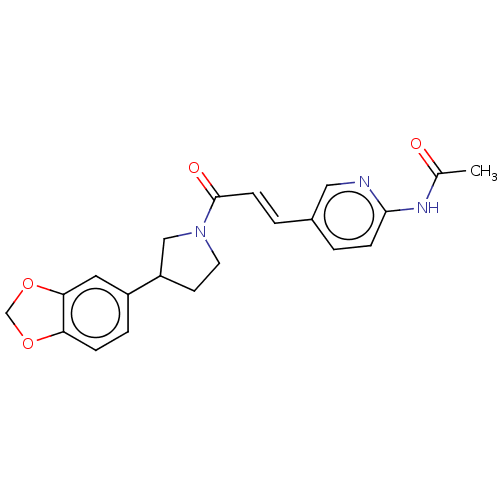 (CHEMBL4204415) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452502 (CHEMBL4209594) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452479 (CHEMBL4210008) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452494 (CHEMBL4204858) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452516 (CHEMBL4211939) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452512 (CHEMBL4205638) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452506 (CHEMBL4216771) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452510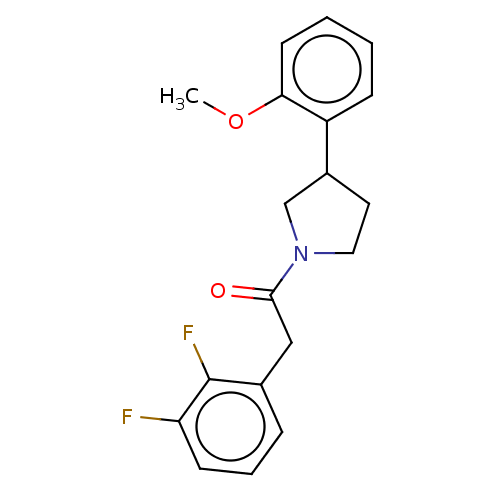 (CHEMBL4210599) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452496 (CHEMBL4217852) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452515 (CHEMBL4214096) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452482 (CHEMBL4208104) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452477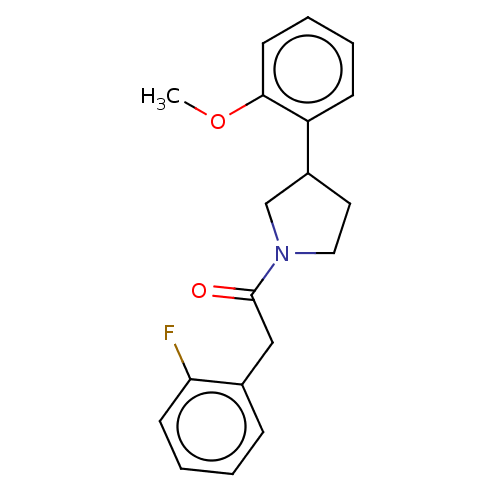 (CHEMBL4209685) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452483 (CHEMBL4210639) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452495 (CHEMBL4218049) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452499 (CHEMBL4213777) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452507 (CHEMBL4205014) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452509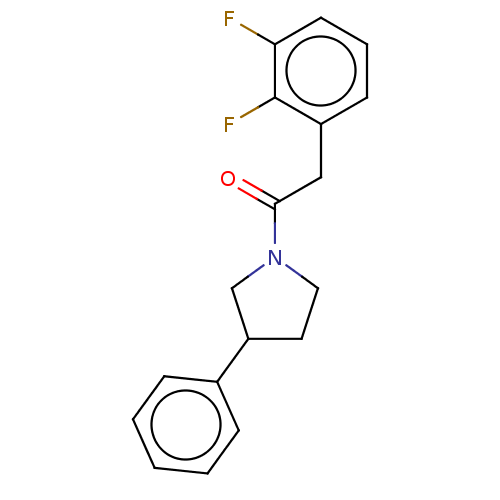 (CHEMBL4217672) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452497 (CHEMBL4205565) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452508 (CHEMBL4209393) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452481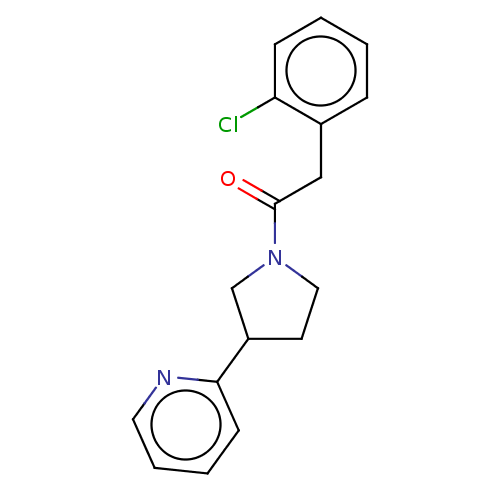 (CHEMBL4216697) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ORF1a (Middle East respiratory syndrome coronavirus (Viru...) | BDBM154573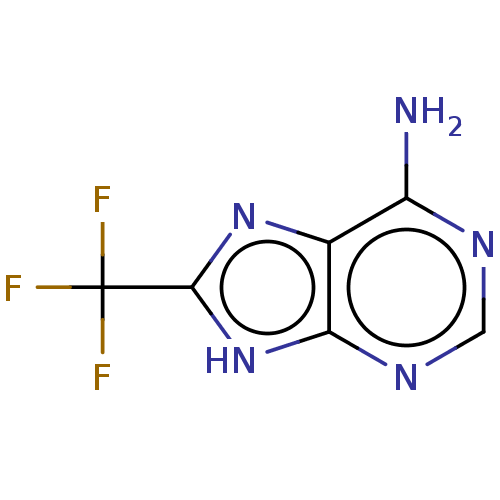 (8-(trifluoromethyl)-9H-purin-6-amine (4) | acs.jme...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 7.60E+3 | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Illinois at Chicago | Assay Description done in a single pass in black 384-well plates (Matrix Technologies). The SARS-PLpro enzyme (20 nM final concentration) was prepared in an assay buff... | ACS Chem Biol 10: 1456-65 (2015) Article DOI: 10.1021/cb500917m BindingDB Entry DOI: 10.7270/Q2086426 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452498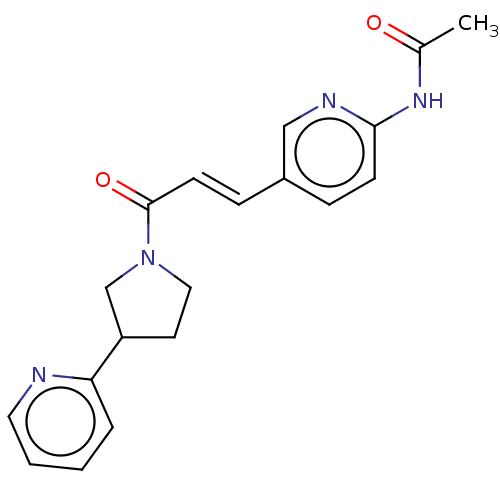 (CHEMBL4217928) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM154573 (8-(trifluoromethyl)-9H-purin-6-amine (4) | acs.jme...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Mixed-type inhibition of SARS coronavirus 3C-like protease using 5-FAM-TSATLQSGFRK(QXL520)-NH2 as substrate assessed as enzyme-inhibitor complex by D... | Bioorg Med Chem 22: 167-77 (2014) Article DOI: 10.1016/j.bmc.2013.11.041 BindingDB Entry DOI: 10.7270/Q2G73HPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM154573 (8-(trifluoromethyl)-9H-purin-6-amine (4) | acs.jme...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Mixed-type inhibition of SARS coronavirus 3C-like protease using 5-FAM-TSATLQSGFRK(QXL520)-NH2 as substrate assessed as enzyme-substrate-inhibitor co... | Bioorg Med Chem 22: 167-77 (2014) Article DOI: 10.1016/j.bmc.2013.11.041 BindingDB Entry DOI: 10.7270/Q2G73HPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab [1541-1855] (Human SARS coronavirus (SARS-CoV)) | BDBM154573 (8-(trifluoromethyl)-9H-purin-6-amine (4) | acs.jme...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.15E+4 | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Illinois at Chicago | Assay Description done in a single pass in black 384-well plates (Matrix Technologies). The SARS-PLpro enzyme (20 nM final concentration) was prepared in an assay buff... | ACS Chem Biol 10: 1456-65 (2015) Article DOI: 10.1021/cb500917m BindingDB Entry DOI: 10.7270/Q2086426 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM47844 (4-chloranyl-N-(1-methylbenzimidazol-5-yl)benzamide...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 2.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Competitive inhibition of SARS coronavirus 3C-like protease using 5-FAM-TSATLQSGFRK(QXL520)-NH2 as substrate by Dixon plot analysis | Bioorg Med Chem 22: 167-77 (2014) Article DOI: 10.1016/j.bmc.2013.11.041 BindingDB Entry DOI: 10.7270/Q2G73HPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Middle East respiratory syndrome-related coronavir...) | BDBM50523945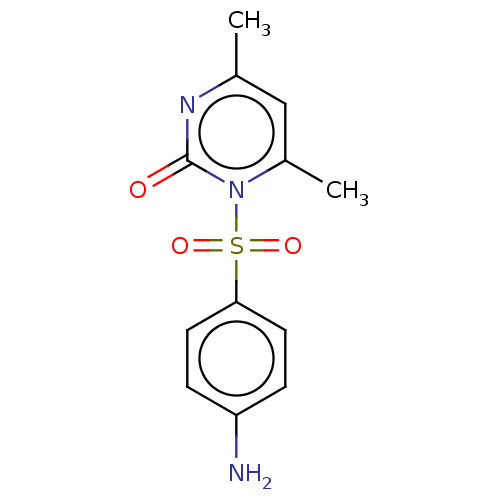 (CHEMBL4475926) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 3.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Competitive-inhibition of MERS-CoV papain-like protease using varying levels of Z-Arg-Leu-Arg-Gly-Gly-AMC as substrate by Dixon-plot analysis | Bioorg Med Chem 27: 1981-1989 (2019) Article DOI: 10.1016/j.bmc.2019.03.050 BindingDB Entry DOI: 10.7270/Q20868RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452480 (CHEMBL4206150) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | >3.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452503 (CHEMBL4204726) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | >3.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452505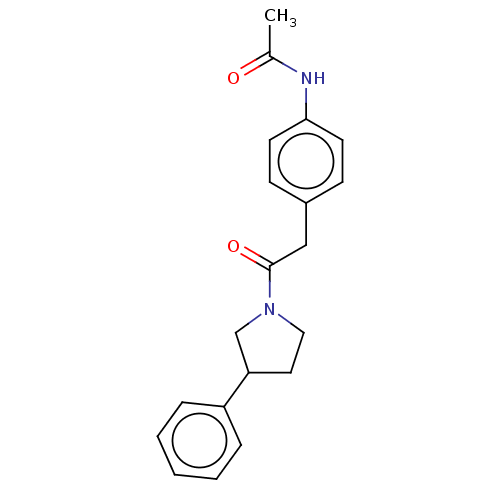 (CHEMBL4214922) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | >3.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452513 (CHEMBL4213468) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | >3.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452514 (CHEMBL4217867) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | >3.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452504 (CHEMBL4218400) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | >3.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM50452500 (CHEMBL4213757) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | >3.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novalex Therapeutics Curated by ChEMBL | Assay Description Competitive inhibition of Staphylococcus aureus subsp. aureus Rosenbach ATCC 43300 FabI using crotonyl-CoA as substrate in presence of NADPH/NADH aft... | Bioorg Med Chem 26: 65-76 (2018) Article DOI: 10.1016/j.bmc.2017.11.018 BindingDB Entry DOI: 10.7270/Q2QF8WF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Middle East respiratory syndrome-related coronavir...) | BDBM50523946 (ONO-1078) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.27E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Competitive-inhibition of MERS-CoV papain-like protease using varying levels of Z-Arg-Leu-Arg-Gly-Gly-AMC as substrate by Dixon-plot analysis | Bioorg Med Chem 27: 1981-1989 (2019) Article DOI: 10.1016/j.bmc.2019.03.050 BindingDB Entry DOI: 10.7270/Q20868RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM387821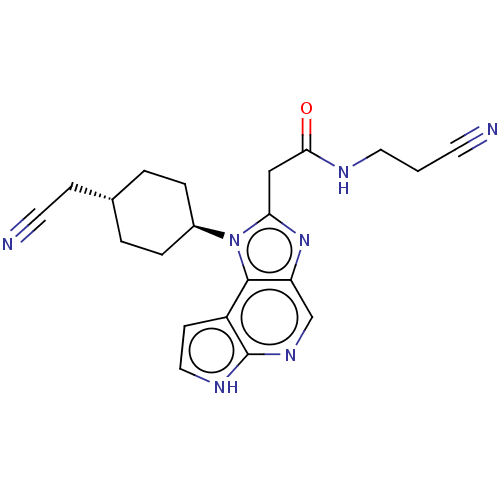 (N-(2-Cyanoethyl)-2-(1-((1r,4r)-4-(cyanomethyl)cycl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inhibition of JAK1-JH1/JH2 domain (574 to 1154 residues) (unknown origin) pre-incubated before NH2-KGGEEEEYFELVKK-CO2 substrate addition and measured... | J Med Chem 63: 2915-2929 (2020) Article DOI: 10.1021/acs.jmedchem.9b01439 BindingDB Entry DOI: 10.7270/Q2H135FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50527405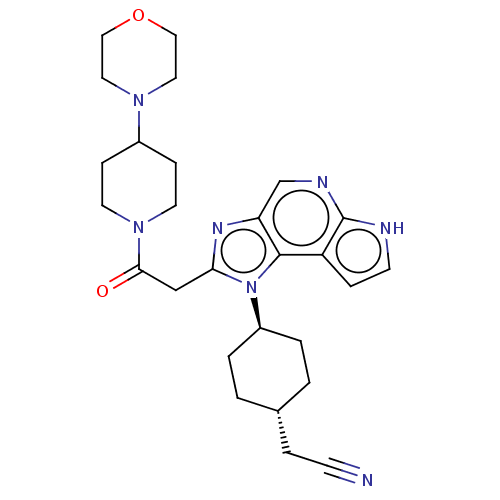 (CHEMBL4456630 | US10981911, Example 26) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inhibition of JAK1-JH1/JH2 domain (574 to 1154 residues) (unknown origin) pre-incubated before NH2-KGGEEEEYFELVKK-CO2 substrate addition and measured... | J Med Chem 63: 2915-2929 (2020) Article DOI: 10.1021/acs.jmedchem.9b01439 BindingDB Entry DOI: 10.7270/Q2H135FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM387815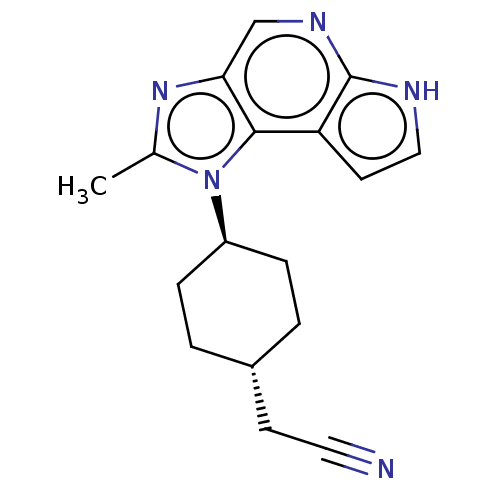 (US10294226, Compound A | US10487083, Example A | U...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inhibition of JAK1-JH1/JH2 domain (574 to 1154 residues) (unknown origin) pre-incubated before NH2-KGGEEEEYFELVKK-CO2 substrate addition and measured... | J Med Chem 63: 2915-2929 (2020) Article DOI: 10.1021/acs.jmedchem.9b01439 BindingDB Entry DOI: 10.7270/Q2H135FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM387815 (US10294226, Compound A | US10487083, Example A | U...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inhibition of JAK2-JH1/JH2 domain (532 to 1132 residues) (unknown origin) pre-incubated before NH2-KGGEEEEYFELVKK-CO2 substrate addition and measured... | J Med Chem 63: 2915-2929 (2020) Article DOI: 10.1021/acs.jmedchem.9b01439 BindingDB Entry DOI: 10.7270/Q2H135FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM387824 (N-(2-Cyano-2-methylpropyl)-2-(1-((1r,4r)-4-(cyanom...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inhibition of JAK1-JH1/JH2 domain (574 to 1154 residues) (unknown origin) pre-incubated before NH2-KGGEEEEYFELVKK-CO2 substrate addition and measured... | J Med Chem 63: 2915-2929 (2020) Article DOI: 10.1021/acs.jmedchem.9b01439 BindingDB Entry DOI: 10.7270/Q2H135FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 295 total ) | Next | Last >> |