 Found 266 hits with Last Name = 'jorgensen' and Initial = 'wt'
Found 266 hits with Last Name = 'jorgensen' and Initial = 'wt' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50205990

(CHEMBL395429 | OXYTOCIN)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C43H66N12O12S2/c1-5-22(4)35-42(66)49-26(12-13-32(45)57)38(62)51-29(17-33(46)58)39(63)53-30(20-69-68-19-25(44)36(60)50-28(40(64)54-35)16-23-8-10-24(56)11-9-23)43(67)55-14-6-7-31(55)41(65)52-27(15-21(2)3)37(61)48-18-34(47)59/h8-11,21-22,25-31,35,56H,5-7,12-20,44H2,1-4H3,(H2,45,57)(H2,46,58)(H2,47,59)(H,48,61)(H,49,66)(H,50,60)(H,51,62)(H,52,65)(H,53,63)(H,54,64)/t22-,25-,26-,27-,28-,29-,30-,31-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Agonist activity at human oxytocin receptor expressed in HEK293 cells assessed as increase in IP1 accumulation preincubated for 1 hr followed by addi... |
Eur J Med Chem 108: 730-40 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.050
BindingDB Entry DOI: 10.7270/Q2XW4MNF |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H](+)-pentazocine from sigma 1 receptor in rat brain homogenate |
Bioorg Med Chem Lett 21: 3622-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.098
BindingDB Entry DOI: 10.7270/Q2ZS2WTH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylspiperone from human D2 receptor expressed in HEK cells |
Bioorg Med Chem Lett 21: 3622-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.098
BindingDB Entry DOI: 10.7270/Q2ZS2WTH |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50170660
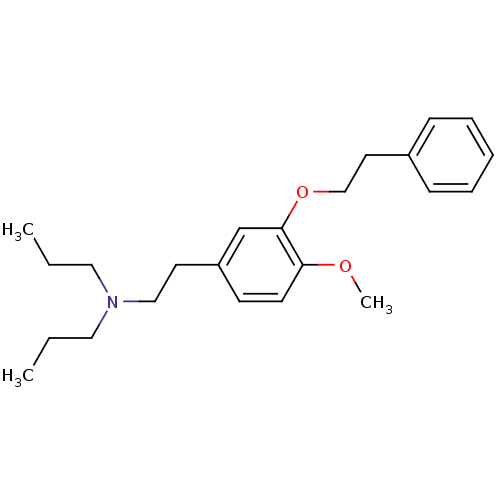
(CHEMBL190883 | CHEMBL521582 | N,N-dipropyl-2-[4-me...)Show InChI InChI=1S/C23H33NO2/c1-4-15-24(16-5-2)17-13-21-11-12-22(25-3)23(19-21)26-18-14-20-9-7-6-8-10-20/h6-12,19H,4-5,13-18H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.03 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H](+)-pentazocine from sigma 1 receptor in rat brain homogenate |
Bioorg Med Chem Lett 21: 3622-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.098
BindingDB Entry DOI: 10.7270/Q2ZS2WTH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50334458
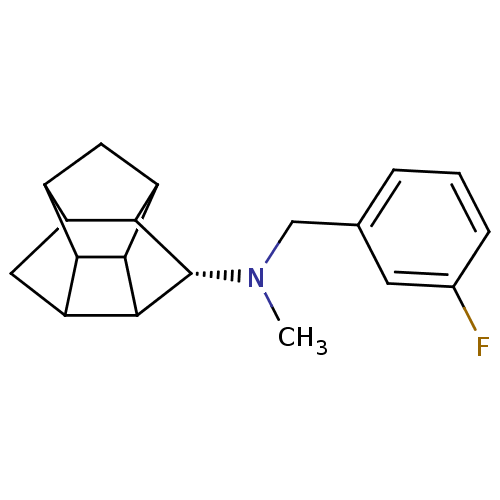
(CHEMBL1643901 | N-(3-Fluorobenzyl)-N-methyl-8-amin...)Show SMILES CN(Cc1cccc(F)c1)[C@@H]1C2C3CC4C5CC(C2C35)C14 |r,THB:11:18:16:14.20,10:20:18.19:16,12:19:16:14.20,13:14:18.19:16| Show InChI InChI=1S/C19H22FN/c1-21(8-9-3-2-4-10(20)5-9)19-16-12-7-13-15-11(12)6-14(16)17(15)18(13)19/h2-5,11-19H,6-8H2,1H3/t11?,12?,13?,14?,15?,16?,17?,18?,19-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN-35428 form human DAT expressed in HEK cell membranes |
Bioorg Med Chem Lett 21: 38-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.075
BindingDB Entry DOI: 10.7270/Q20K28VR |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylspiperone from human D4 receptor expressed in HEK cells |
Bioorg Med Chem Lett 21: 3622-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.098
BindingDB Entry DOI: 10.7270/Q2ZS2WTH |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50026948
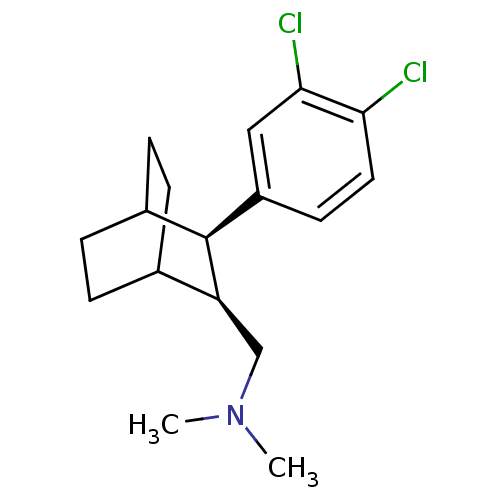
((trans) [3-(3,4-Dichloro-phenyl)-bicyclo[2.2.2]oct...)Show SMILES CN(C)C[C@H]1C2CCC(CC2)[C@H]1c1ccc(Cl)c(Cl)c1 |wD:11.13,4.3,(7.55,1.59,;6.22,.82,;4.89,1.59,;6.22,-.68,;4.89,-1.49,;3.54,-.72,;2.22,-1.49,;2.22,-3.03,;3.54,-3.8,;4.09,-2.55,;2.77,-2.03,;4.89,-3.03,;6.2,-3.8,;6.2,-5.34,;7.55,-6.11,;8.89,-5.34,;10.21,-6.11,;8.87,-3.8,;10.2,-2.99,;7.54,-3.03,)| Show InChI InChI=1S/C17H23Cl2N/c1-20(2)10-14-11-3-5-12(6-4-11)17(14)13-7-8-15(18)16(19)9-13/h7-9,11-12,14,17H,3-6,10H2,1-2H3/t11?,12?,14-,17-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of DAT |
Bioorg Med Chem Lett 21: 38-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.075
BindingDB Entry DOI: 10.7270/Q20K28VR |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50344628
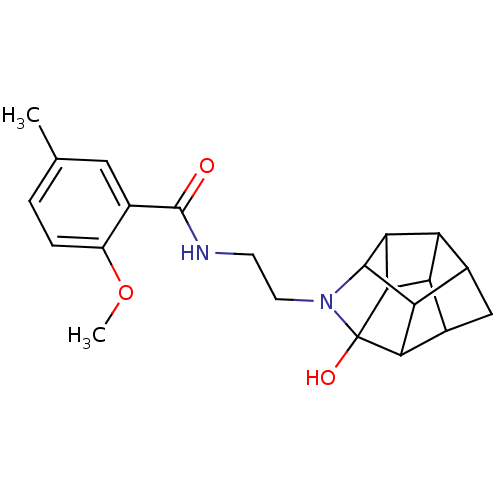
(CHEMBL1779060 | N-(2-(2-methoxy-5-methylbenzamido)...)Show SMILES COc1ccc(C)cc1C(=O)NCCN1C2C3C4C5C3C1(O)C1C5CC4C21 |TLB:15:26:17.18:24,20:22:17.18:24,THB:16:17:26.22:24,25:26:16.19:14,17:16:26.22:14,19:18:26.22:24,23:22:16.19:14,18:19:26.22:14,13:14:16.19:26.22| Show InChI InChI=1S/C22H26N2O3/c1-9-3-4-13(27-2)10(7-9)21(25)23-5-6-24-20-16-11-8-12-15-14(11)17(20)19(15)22(24,26)18(12)16/h3-4,7,11-12,14-20,26H,5-6,8H2,1-2H3,(H,23,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H](+)-pentazocine from sigma 1 receptor in rat brain homogenate |
Bioorg Med Chem Lett 21: 3622-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.098
BindingDB Entry DOI: 10.7270/Q2ZS2WTH |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50175145
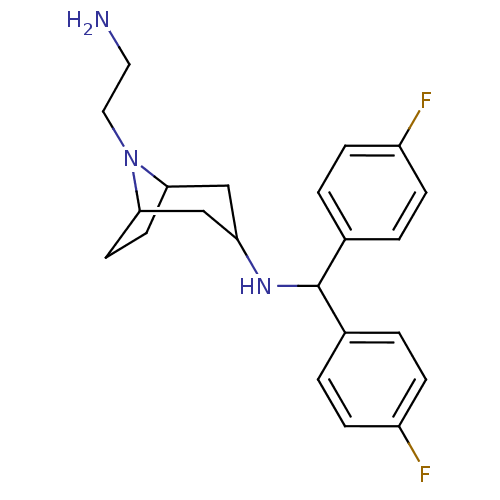
(8-(2-aminoethyl)-N-(bis(4-fluorophenyl)methyl)-8-a...)Show SMILES NCCN1C2CCC1CC(C2)NC(c1ccc(F)cc1)c1ccc(F)cc1 |TLB:2:3:5.6:8.10.9,THB:11:9:5.6:3| Show InChI InChI=1S/C22H27F2N3/c23-17-5-1-15(2-6-17)22(16-3-7-18(24)8-4-16)26-19-13-20-9-10-21(14-19)27(20)12-11-25/h1-8,19-22,26H,9-14,25H2 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of DAT |
Bioorg Med Chem Lett 21: 38-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.075
BindingDB Entry DOI: 10.7270/Q20K28VR |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50334461
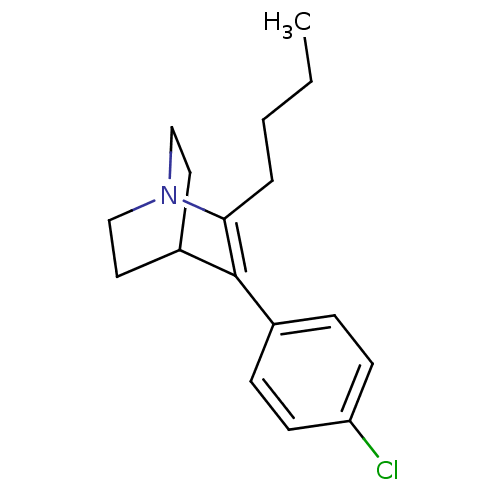
(2-butyl-3-(4-chlorophenyl)-1-azabicyclo[2.2.2]oct-...)Show SMILES CCCCC1=C(C2CCN1CC2)c1ccc(Cl)cc1 |t:4,(.06,-16.55,;-1.41,-17.03,;-2.55,-16,;-4.01,-16.47,;-5.2,-15.49,;-5.2,-13.3,;-7.34,-12.86,;-6.01,-13.62,;-6.01,-15.16,;-7.35,-15.93,;-8.69,-15.16,;-8.66,-13.62,;-3.99,-12.34,;-2.55,-12.89,;-1.36,-11.93,;-1.59,-10.41,;-.39,-9.44,;-3.03,-9.85,;-4.23,-10.82,)| Show InChI InChI=1S/C17H22ClN/c1-2-3-4-16-17(13-5-7-15(18)8-6-13)14-9-11-19(16)12-10-14/h5-8,14H,2-4,9-12H2,1H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of DAT |
Bioorg Med Chem Lett 21: 38-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.075
BindingDB Entry DOI: 10.7270/Q20K28VR |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50334459
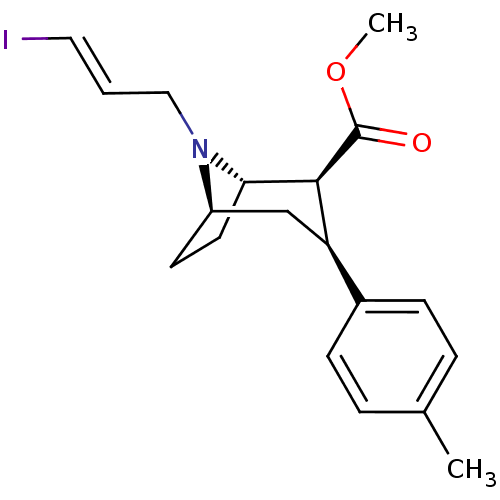
((1S,2S,3S,5R)-methyl 8-(3-iodoallyl)-3-p-tolyl-8-a...)Show SMILES COC(=O)[C@@H]1[C@@H]2CC[C@H](C[C@@H]1c1ccc(C)cc1)N2C\C=C\I |r,TLB:19:18:10.9.4:7.6| Show InChI InChI=1S/C19H24INO2/c1-13-4-6-14(7-5-13)16-12-15-8-9-17(18(16)19(22)23-2)21(15)11-3-10-20/h3-7,10,15-18H,8-9,11-12H2,1-2H3/b10-3+/t15-,16-,17+,18+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of DAT |
Bioorg Med Chem Lett 21: 38-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.075
BindingDB Entry DOI: 10.7270/Q20K28VR |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50334455

(CHEMBL1643900 | N-(2-(3'-Fluorophenyl)ethyl)-8-ami...)Show SMILES Fc1cccc(CCN[C@@H]2C3C4CC5C6CC(C3C46)C25)c1 |r,THB:10:17:15:13.19,9:19:17.18:15,11:18:15:13.19,12:13:17.18:15| Show InChI InChI=1S/C19H22FN/c20-10-3-1-2-9(6-10)4-5-21-19-16-12-8-13-15-11(12)7-14(16)17(15)18(13)19/h1-3,6,11-19,21H,4-5,7-8H2/t11?,12?,13?,14?,15?,16?,17?,18?,19-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of (+)-[3H]pentazocine from sigma 1 receptor in rat PC12 cells |
Bioorg Med Chem Lett 21: 38-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.075
BindingDB Entry DOI: 10.7270/Q20K28VR |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50344629
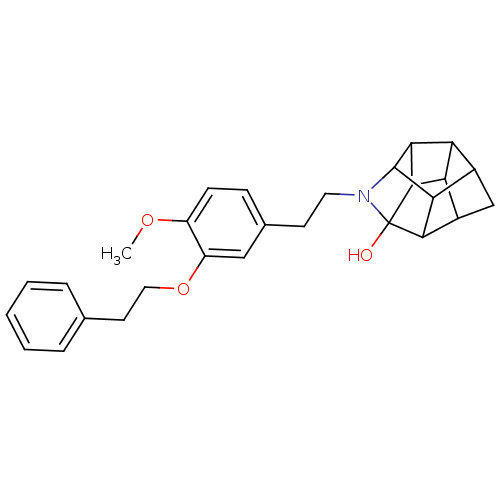
(CHEMBL1779059 | N-(2-(4-methoxy-3-phenethoxyphenyl...)Show SMILES COc1ccc(CCN2C3C4C5C6C4C2(O)C2C6CC5C32)cc1OCCc1ccccc1 |TLB:9:20:11.12:18,14:16:11.12:18,THB:10:11:20.16:18,19:20:10.13:8,11:10:20.16:8,13:12:20.16:18,17:16:10.13:8,12:13:20.16:8,7:8:10.13:20.16| Show InChI InChI=1S/C28H31NO3/c1-31-19-8-7-16(13-20(19)32-12-10-15-5-3-2-4-6-15)9-11-29-27-23-17-14-18-22-21(17)24(27)26(22)28(29,30)25(18)23/h2-8,13,17-18,21-27,30H,9-12,14H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H](+)-pentazocine from sigma 1 receptor in rat brain homogenate |
Bioorg Med Chem Lett 21: 3622-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.098
BindingDB Entry DOI: 10.7270/Q2ZS2WTH |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH233930 from human D1 receptor expressed in HEK cells |
Bioorg Med Chem Lett 21: 3622-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.098
BindingDB Entry DOI: 10.7270/Q2ZS2WTH |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50139178
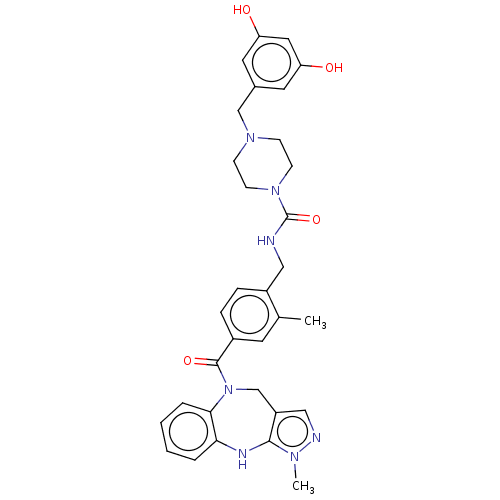
(CHEMBL3763342)Show SMILES Cc1cc(ccc1CNC(=O)N1CCN(Cc2cc(O)cc(O)c2)CC1)C(=O)N1Cc2cnn(C)c2Nc2ccccc12 Show InChI InChI=1S/C32H35N7O4/c1-21-13-23(31(42)39-20-25-18-34-36(2)30(25)35-28-5-3-4-6-29(28)39)7-8-24(21)17-33-32(43)38-11-9-37(10-12-38)19-22-14-26(40)16-27(41)15-22/h3-8,13-16,18,35,40-41H,9-12,17,19-20H2,1-2H3,(H,33,43) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Agonist activity at human oxytocin receptor expressed in HEK293 cells assessed as increase in IP1 accumulation preincubated for 1 hr followed by addi... |
Eur J Med Chem 108: 730-40 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.050
BindingDB Entry DOI: 10.7270/Q2XW4MNF |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50139178

(CHEMBL3763342)Show SMILES Cc1cc(ccc1CNC(=O)N1CCN(Cc2cc(O)cc(O)c2)CC1)C(=O)N1Cc2cnn(C)c2Nc2ccccc12 Show InChI InChI=1S/C32H35N7O4/c1-21-13-23(31(42)39-20-25-18-34-36(2)30(25)35-28-5-3-4-6-29(28)39)7-8-24(21)17-33-32(43)38-11-9-37(10-12-38)19-22-14-26(40)16-27(41)15-22/h3-8,13-16,18,35,40-41H,9-12,17,19-20H2,1-2H3,(H,33,43) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H]-vasopressin from human vasopressin V1a receptor expressed in HEK293 cell membranes after 90 mins by radioligand binding assay |
Eur J Med Chem 143: 1644-1656 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.059
BindingDB Entry DOI: 10.7270/Q2MS3W92 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50344630
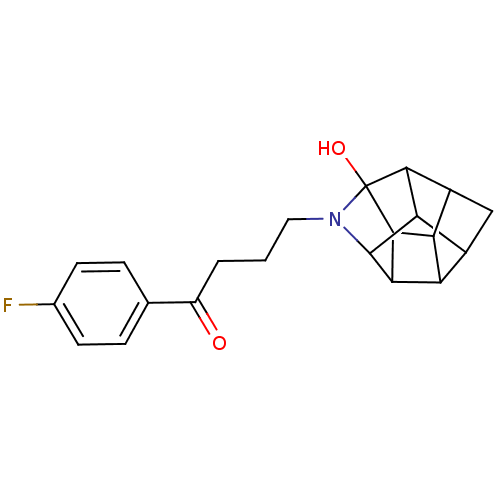
(CHEMBL1779058 | N-(4-(4-Fluoro)butyrophenone)-4-az...)Show SMILES OC12C3C4C5C3C(C3C5CC4C13)N2CCCC(=O)c1ccc(F)cc1 |TLB:6:7:4.3:9,8:7:5.2:12,4:5:7.11:12,1:11:4.3:9,10:11:5.2:12,3:2:7.11:12,THB:5:4:7.11:9,2:3:7.11:9,13:12:5.2:7.11| Show InChI InChI=1S/C21H22FNO2/c22-10-5-3-9(4-6-10)13(24)2-1-7-23-20-16-11-8-12-15-14(11)17(20)19(15)21(23,25)18(12)16/h3-6,11-12,14-20,25H,1-2,7-8H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H](+)-pentazocine from sigma 1 receptor in rat brain homogenate |
Bioorg Med Chem Lett 21: 3622-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.098
BindingDB Entry DOI: 10.7270/Q2ZS2WTH |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylspiperone from human D3 receptor expressed in HEK cells |
Bioorg Med Chem Lett 21: 3622-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.098
BindingDB Entry DOI: 10.7270/Q2ZS2WTH |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50026948

((trans) [3-(3,4-Dichloro-phenyl)-bicyclo[2.2.2]oct...)Show SMILES CN(C)C[C@H]1C2CCC(CC2)[C@H]1c1ccc(Cl)c(Cl)c1 |wD:11.13,4.3,(7.55,1.59,;6.22,.82,;4.89,1.59,;6.22,-.68,;4.89,-1.49,;3.54,-.72,;2.22,-1.49,;2.22,-3.03,;3.54,-3.8,;4.09,-2.55,;2.77,-2.03,;4.89,-3.03,;6.2,-3.8,;6.2,-5.34,;7.55,-6.11,;8.89,-5.34,;10.21,-6.11,;8.87,-3.8,;10.2,-2.99,;7.54,-3.03,)| Show InChI InChI=1S/C17H23Cl2N/c1-20(2)10-14-11-3-5-12(6-4-11)17(14)13-7-8-15(18)16(19)9-13/h7-9,11-12,14,17H,3-6,10H2,1-2H3/t11?,12?,14-,17-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of NET |
Bioorg Med Chem Lett 21: 38-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.075
BindingDB Entry DOI: 10.7270/Q20K28VR |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH233930 from human D5 receptor expressed in HEK cells |
Bioorg Med Chem Lett 21: 3622-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.098
BindingDB Entry DOI: 10.7270/Q2ZS2WTH |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50026948

((trans) [3-(3,4-Dichloro-phenyl)-bicyclo[2.2.2]oct...)Show SMILES CN(C)C[C@H]1C2CCC(CC2)[C@H]1c1ccc(Cl)c(Cl)c1 |wD:11.13,4.3,(7.55,1.59,;6.22,.82,;4.89,1.59,;6.22,-.68,;4.89,-1.49,;3.54,-.72,;2.22,-1.49,;2.22,-3.03,;3.54,-3.8,;4.09,-2.55,;2.77,-2.03,;4.89,-3.03,;6.2,-3.8,;6.2,-5.34,;7.55,-6.11,;8.89,-5.34,;10.21,-6.11,;8.87,-3.8,;10.2,-2.99,;7.54,-3.03,)| Show InChI InChI=1S/C17H23Cl2N/c1-20(2)10-14-11-3-5-12(6-4-11)17(14)13-7-8-15(18)16(19)9-13/h7-9,11-12,14,17H,3-6,10H2,1-2H3/t11?,12?,14-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of SERT |
Bioorg Med Chem Lett 21: 38-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.075
BindingDB Entry DOI: 10.7270/Q20K28VR |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50139178

(CHEMBL3763342)Show SMILES Cc1cc(ccc1CNC(=O)N1CCN(Cc2cc(O)cc(O)c2)CC1)C(=O)N1Cc2cnn(C)c2Nc2ccccc12 Show InChI InChI=1S/C32H35N7O4/c1-21-13-23(31(42)39-20-25-18-34-36(2)30(25)35-28-5-3-4-6-29(28)39)7-8-24(21)17-33-32(43)38-11-9-37(10-12-38)19-22-14-26(40)16-27(41)15-22/h3-8,13-16,18,35,40-41H,9-12,17,19-20H2,1-2H3,(H,33,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H]-vasopressin from human vasopressin 1a receptor expressed in HEK293 cells after 90 mins by microbeta 2 microplate-reader method |
Eur J Med Chem 108: 730-40 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.050
BindingDB Entry DOI: 10.7270/Q2XW4MNF |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50139179
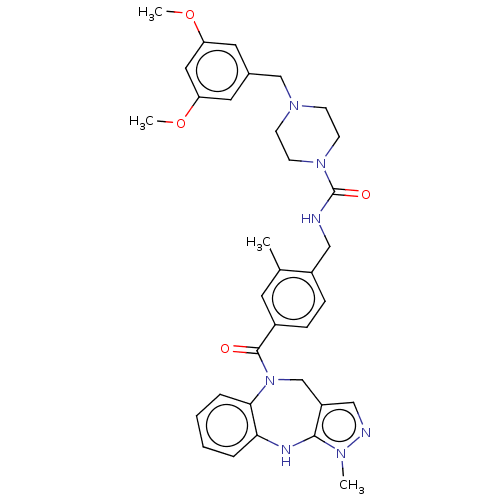
(CHEMBL3763951)Show SMILES COc1cc(CN2CCN(CC2)C(=O)NCc2ccc(cc2C)C(=O)N2Cc3cnn(C)c3Nc3ccccc23)cc(OC)c1 Show InChI InChI=1S/C34H39N7O4/c1-23-15-25(33(42)41-22-27-20-36-38(2)32(27)37-30-7-5-6-8-31(30)41)9-10-26(23)19-35-34(43)40-13-11-39(12-14-40)21-24-16-28(44-3)18-29(17-24)45-4/h5-10,15-18,20,37H,11-14,19,21-22H2,1-4H3,(H,35,43) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Agonist activity at human oxytocin receptor expressed in HEK293 cells assessed as increase in IP1 accumulation preincubated for 1 hr followed by addi... |
Eur J Med Chem 108: 730-40 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.050
BindingDB Entry DOI: 10.7270/Q2XW4MNF |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50139179

(CHEMBL3763951)Show SMILES COc1cc(CN2CCN(CC2)C(=O)NCc2ccc(cc2C)C(=O)N2Cc3cnn(C)c3Nc3ccccc23)cc(OC)c1 Show InChI InChI=1S/C34H39N7O4/c1-23-15-25(33(42)41-22-27-20-36-38(2)32(27)37-30-7-5-6-8-31(30)41)9-10-26(23)19-35-34(43)40-13-11-39(12-14-40)21-24-16-28(44-3)18-29(17-24)45-4/h5-10,15-18,20,37H,11-14,19,21-22H2,1-4H3,(H,35,43) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H]-vasopressin from human vasopressin V1a receptor expressed in HEK293 cell membranes after 90 mins by radioligand binding assay |
Eur J Med Chem 143: 1644-1656 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.059
BindingDB Entry DOI: 10.7270/Q2MS3W92 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50139373
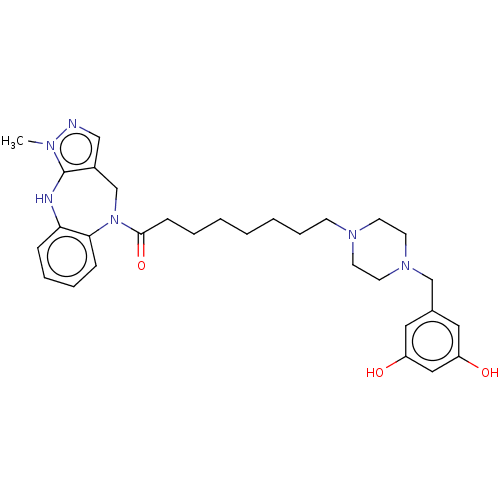
(CHEMBL3763823)Show SMILES Cn1ncc2CN(C(=O)CCCCCCCN3CCN(Cc4cc(O)cc(O)c4)CC3)c3ccccc3Nc12 Show InChI InChI=1S/C30H40N6O3/c1-33-30-24(20-31-33)22-36(28-10-7-6-9-27(28)32-30)29(39)11-5-3-2-4-8-12-34-13-15-35(16-14-34)21-23-17-25(37)19-26(38)18-23/h6-7,9-10,17-20,32,37-38H,2-5,8,11-16,21-22H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Antagonist activity at human vasopressin 1a receptor expressed in HEK293 cells assessed as inhibition of vasopressin induced IP1 accumulation pretrea... |
Eur J Med Chem 108: 730-40 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.050
BindingDB Entry DOI: 10.7270/Q2XW4MNF |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50139373

(CHEMBL3763823)Show SMILES Cn1ncc2CN(C(=O)CCCCCCCN3CCN(Cc4cc(O)cc(O)c4)CC3)c3ccccc3Nc12 Show InChI InChI=1S/C30H40N6O3/c1-33-30-24(20-31-33)22-36(28-10-7-6-9-27(28)32-30)29(39)11-5-3-2-4-8-12-34-13-15-35(16-14-34)21-23-17-25(37)19-26(38)18-23/h6-7,9-10,17-20,32,37-38H,2-5,8,11-16,21-22H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H]-vasopressin from human vasopressin V1a receptor expressed in HEK293 cell membranes after 90 mins by radioligand binding assay |
Eur J Med Chem 143: 1644-1656 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.059
BindingDB Entry DOI: 10.7270/Q2MS3W92 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50344630

(CHEMBL1779058 | N-(4-(4-Fluoro)butyrophenone)-4-az...)Show SMILES OC12C3C4C5C3C(C3C5CC4C13)N2CCCC(=O)c1ccc(F)cc1 |TLB:6:7:4.3:9,8:7:5.2:12,4:5:7.11:12,1:11:4.3:9,10:11:5.2:12,3:2:7.11:12,THB:5:4:7.11:9,2:3:7.11:9,13:12:5.2:7.11| Show InChI InChI=1S/C21H22FNO2/c22-10-5-3-9(4-6-10)13(24)2-1-7-23-20-16-11-8-12-15-14(11)17(20)19(15)21(23,25)18(12)16/h3-6,11-12,14-20,25H,1-2,7-8H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylspiperone from human D4 receptor expressed in HEK cells |
Bioorg Med Chem Lett 21: 3622-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.098
BindingDB Entry DOI: 10.7270/Q2ZS2WTH |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50334456
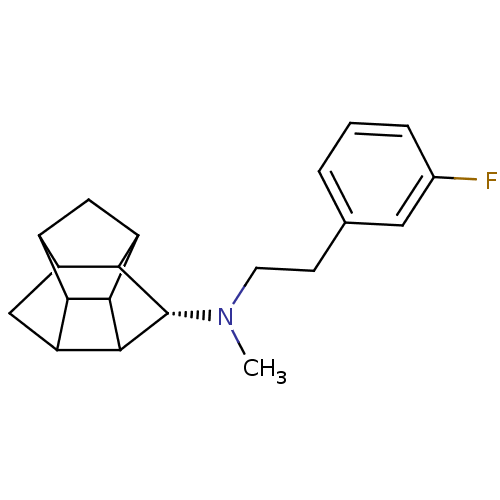
(CHEMBL1643902 | N-(2-(3'-Fluorophenyl)ethyl)-N-met...)Show SMILES CN(CCc1cccc(F)c1)[C@@H]1C2C3CC4C5CC(C2C35)C14 |r,THB:12:19:17:15.21,11:21:19.20:17,13:20:17:15.21,14:15:19.20:17| Show InChI InChI=1S/C20H24FN/c1-22(6-5-10-3-2-4-11(21)7-10)20-17-13-9-14-16-12(13)8-15(17)18(16)19(14)20/h2-4,7,12-20H,5-6,8-9H2,1H3/t12?,13?,14?,15?,16?,17?,18?,19?,20-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN-35428 form human DAT expressed in HEK cell membranes |
Bioorg Med Chem Lett 21: 38-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.075
BindingDB Entry DOI: 10.7270/Q20K28VR |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50139178

(CHEMBL3763342)Show SMILES Cc1cc(ccc1CNC(=O)N1CCN(Cc2cc(O)cc(O)c2)CC1)C(=O)N1Cc2cnn(C)c2Nc2ccccc12 Show InChI InChI=1S/C32H35N7O4/c1-21-13-23(31(42)39-20-25-18-34-36(2)30(25)35-28-5-3-4-6-29(28)39)7-8-24(21)17-33-32(43)38-11-9-37(10-12-38)19-22-14-26(40)16-27(41)15-22/h3-8,13-16,18,35,40-41H,9-12,17,19-20H2,1-2H3,(H,33,43) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H]-vasopressin from human vasopressin 1a receptor expressed in HEK293 cells after 90 mins by microbeta 2 microplate-reader method |
Eur J Med Chem 108: 730-40 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.050
BindingDB Entry DOI: 10.7270/Q2XW4MNF |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50139183
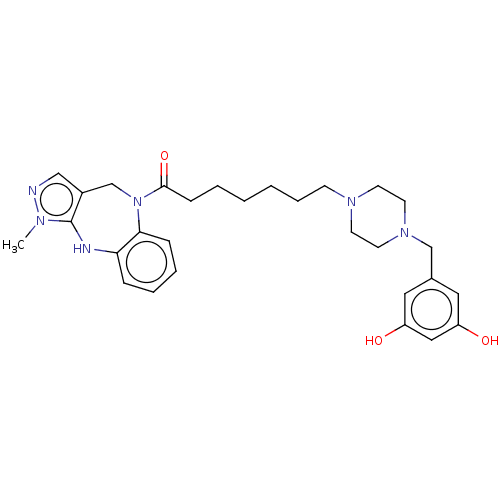
(CHEMBL3763217)Show SMILES Cn1ncc2CN(C(=O)CCCCCCN3CCN(Cc4cc(O)cc(O)c4)CC3)c3ccccc3Nc12 Show InChI InChI=1S/C29H38N6O3/c1-32-29-23(19-30-32)21-35(27-9-6-5-8-26(27)31-29)28(38)10-4-2-3-7-11-33-12-14-34(15-13-33)20-22-16-24(36)18-25(37)17-22/h5-6,8-9,16-19,31,36-37H,2-4,7,10-15,20-21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Antagonist activity at human vasopressin 1a receptor expressed in HEK293 cells assessed as inhibition of vasopressin induced IP1 accumulation pretrea... |
Eur J Med Chem 108: 730-40 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.050
BindingDB Entry DOI: 10.7270/Q2XW4MNF |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50139176
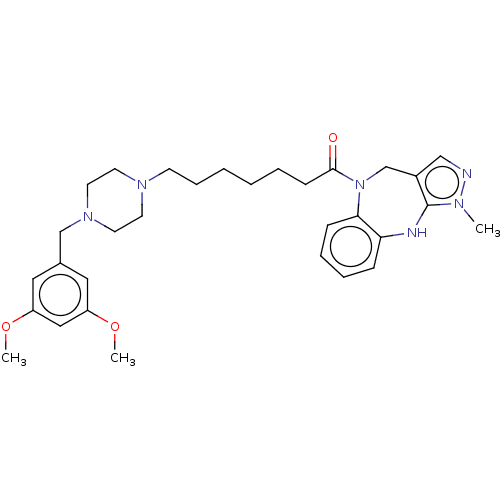
(CHEMBL3763174)Show SMILES COc1cc(CN2CCN(CCCCCCC(=O)N3Cc4cnn(C)c4Nc4ccccc34)CC2)cc(OC)c1 Show InChI InChI=1S/C31H42N6O3/c1-34-31-25(21-32-34)23-37(29-11-8-7-10-28(29)33-31)30(38)12-6-4-5-9-13-35-14-16-36(17-15-35)22-24-18-26(39-2)20-27(19-24)40-3/h7-8,10-11,18-21,33H,4-6,9,12-17,22-23H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H]-vasopressin from human vasopressin V1a receptor expressed in HEK293 cell membranes after 90 mins by radioligand binding assay |
Eur J Med Chem 143: 1644-1656 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.059
BindingDB Entry DOI: 10.7270/Q2MS3W92 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50139176

(CHEMBL3763174)Show SMILES COc1cc(CN2CCN(CCCCCCC(=O)N3Cc4cnn(C)c4Nc4ccccc34)CC2)cc(OC)c1 Show InChI InChI=1S/C31H42N6O3/c1-34-31-25(21-32-34)23-37(29-11-8-7-10-28(29)33-31)30(38)12-6-4-5-9-13-35-14-16-36(17-15-35)22-24-18-26(39-2)20-27(19-24)40-3/h7-8,10-11,18-21,33H,4-6,9,12-17,22-23H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Antagonist activity at human vasopressin 1a receptor expressed in HEK293 cells assessed as inhibition of vasopressin induced IP1 accumulation pretrea... |
Eur J Med Chem 108: 730-40 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.050
BindingDB Entry DOI: 10.7270/Q2XW4MNF |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50334457
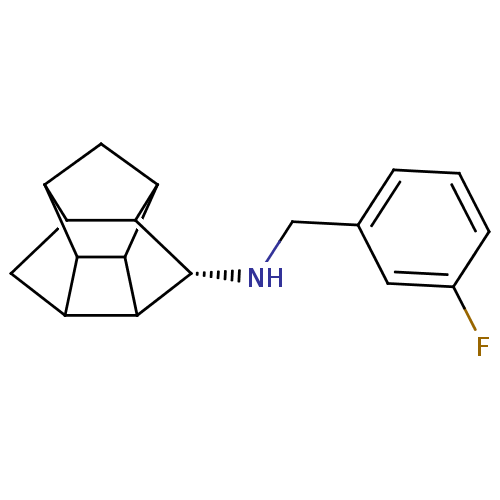
(CHEMBL1643899 | N-(3-Fluorobenzyl)-8-aminopentacyc...)Show SMILES Fc1cccc(CN[C@@H]2C3C4CC5C6CC(C3C46)C25)c1 |r,THB:9:16:14:12.18,8:18:16.17:14,10:17:14:12.18,11:12:16.17:14| Show InChI InChI=1S/C18H20FN/c19-9-3-1-2-8(4-9)7-20-18-15-11-6-12-14-10(11)5-13(15)16(14)17(12)18/h1-4,10-18,20H,5-7H2/t10?,11?,12?,13?,14?,15?,16?,17?,18-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 198 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of (+)-[3H]pentazocine from sigma 1 receptor in rat PC12 cells |
Bioorg Med Chem Lett 21: 38-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.075
BindingDB Entry DOI: 10.7270/Q20K28VR |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50344630

(CHEMBL1779058 | N-(4-(4-Fluoro)butyrophenone)-4-az...)Show SMILES OC12C3C4C5C3C(C3C5CC4C13)N2CCCC(=O)c1ccc(F)cc1 |TLB:6:7:4.3:9,8:7:5.2:12,4:5:7.11:12,1:11:4.3:9,10:11:5.2:12,3:2:7.11:12,THB:5:4:7.11:9,2:3:7.11:9,13:12:5.2:7.11| Show InChI InChI=1S/C21H22FNO2/c22-10-5-3-9(4-6-10)13(24)2-1-7-23-20-16-11-8-12-15-14(11)17(20)19(15)21(23,25)18(12)16/h3-6,11-12,14-20,25H,1-2,7-8H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 209 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH233930 from human D1 receptor expressed in HEK cells |
Bioorg Med Chem Lett 21: 3622-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.098
BindingDB Entry DOI: 10.7270/Q2ZS2WTH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50139178

(CHEMBL3763342)Show SMILES Cc1cc(ccc1CNC(=O)N1CCN(Cc2cc(O)cc(O)c2)CC1)C(=O)N1Cc2cnn(C)c2Nc2ccccc12 Show InChI InChI=1S/C32H35N7O4/c1-21-13-23(31(42)39-20-25-18-34-36(2)30(25)35-28-5-3-4-6-29(28)39)7-8-24(21)17-33-32(43)38-11-9-37(10-12-38)19-22-14-26(40)16-27(41)15-22/h3-8,13-16,18,35,40-41H,9-12,17,19-20H2,1-2H3,(H,33,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H]-oxytocin from human OTR expressed in HEK293 cell membranes after 90 mins by radioligand binding assay |
Eur J Med Chem 143: 1644-1656 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.059
BindingDB Entry DOI: 10.7270/Q2MS3W92 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50139178

(CHEMBL3763342)Show SMILES Cc1cc(ccc1CNC(=O)N1CCN(Cc2cc(O)cc(O)c2)CC1)C(=O)N1Cc2cnn(C)c2Nc2ccccc12 Show InChI InChI=1S/C32H35N7O4/c1-21-13-23(31(42)39-20-25-18-34-36(2)30(25)35-28-5-3-4-6-29(28)39)7-8-24(21)17-33-32(43)38-11-9-37(10-12-38)19-22-14-26(40)16-27(41)15-22/h3-8,13-16,18,35,40-41H,9-12,17,19-20H2,1-2H3,(H,33,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Agonist activity at human oxytocin receptor expressed in HEK293 cells assessed as increase in IP1 accumulation preincubated for 1 hr followed by addi... |
Eur J Med Chem 108: 730-40 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.050
BindingDB Entry DOI: 10.7270/Q2XW4MNF |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50139177
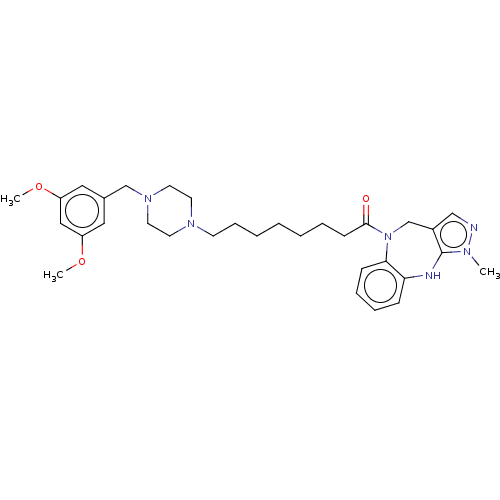
(CHEMBL3765689)Show SMILES COc1cc(CN2CCN(CCCCCCCC(=O)N3Cc4cnn(C)c4Nc4ccccc34)CC2)cc(OC)c1 Show InChI InChI=1S/C32H44N6O3/c1-35-32-26(22-33-35)24-38(30-12-9-8-11-29(30)34-32)31(39)13-7-5-4-6-10-14-36-15-17-37(18-16-36)23-25-19-27(40-2)21-28(20-25)41-3/h8-9,11-12,19-22,34H,4-7,10,13-18,23-24H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 248 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Agonist activity at human oxytocin receptor expressed in HEK293 cells assessed as increase in IP1 accumulation preincubated for 1 hr followed by addi... |
Eur J Med Chem 108: 730-40 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.050
BindingDB Entry DOI: 10.7270/Q2XW4MNF |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50283980
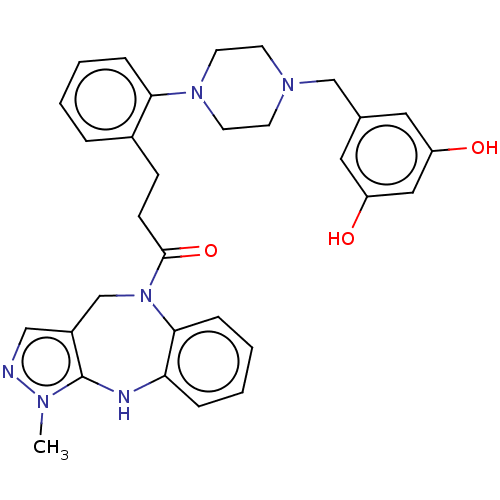
(CHEMBL4166008)Show SMILES Cn1ncc2CN(C(=O)CCc3ccccc3N3CCN(Cc4cc(O)cc(O)c4)CC3)c3ccccc3Nc12 Show InChI InChI=1S/C31H34N6O3/c1-34-31-24(19-32-34)21-37(29-9-5-3-7-27(29)33-31)30(40)11-10-23-6-2-4-8-28(23)36-14-12-35(13-15-36)20-22-16-25(38)18-26(39)17-22/h2-9,16-19,33,38-39H,10-15,20-21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H]-vasopressin from human vasopressin V1a receptor expressed in HEK293 cell membranes after 90 mins by radioligand binding assay |
Eur J Med Chem 143: 1644-1656 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.059
BindingDB Entry DOI: 10.7270/Q2MS3W92 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50139146
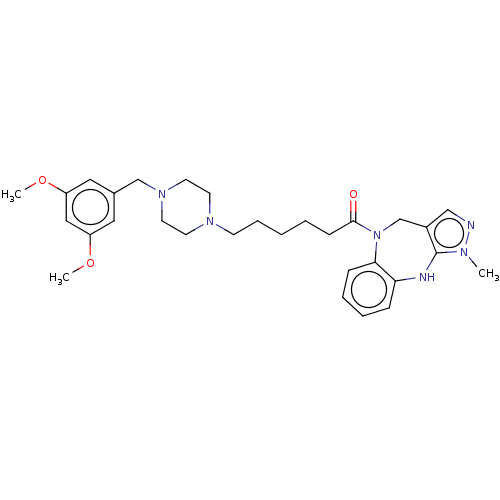
(CHEMBL3765356)Show SMILES COc1cc(CN2CCN(CCCCCC(=O)N3Cc4cnn(C)c4Nc4ccccc34)CC2)cc(OC)c1 Show InChI InChI=1S/C30H40N6O3/c1-33-30-24(20-31-33)22-36(28-10-7-6-9-27(28)32-30)29(37)11-5-4-8-12-34-13-15-35(16-14-34)21-23-17-25(38-2)19-26(18-23)39-3/h6-7,9-10,17-20,32H,4-5,8,11-16,21-22H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 266 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Antagonist activity at human vasopressin 1a receptor expressed in HEK293 cells assessed as inhibition of vasopressin induced IP1 accumulation pretrea... |
Eur J Med Chem 108: 730-40 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.050
BindingDB Entry DOI: 10.7270/Q2XW4MNF |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50139138
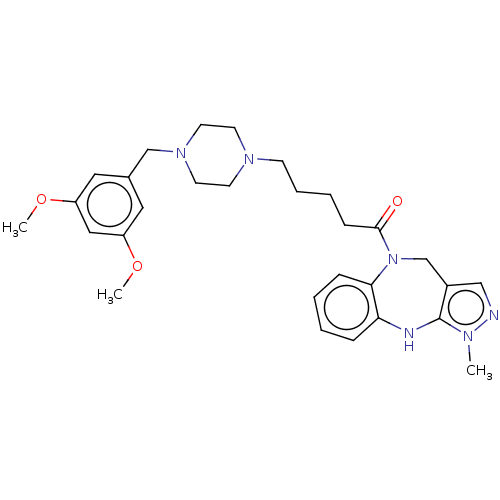
(CHEMBL3763620)Show SMILES COc1cc(CN2CCN(CCCCC(=O)N3Cc4cnn(C)c4Nc4ccccc34)CC2)cc(OC)c1 Show InChI InChI=1S/C29H38N6O3/c1-32-29-23(19-30-32)21-35(27-9-5-4-8-26(27)31-29)28(36)10-6-7-11-33-12-14-34(15-13-33)20-22-16-24(37-2)18-25(17-22)38-3/h4-5,8-9,16-19,31H,6-7,10-15,20-21H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 409 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Antagonist activity at human vasopressin 1a receptor expressed in HEK293 cells assessed as inhibition of vasopressin induced IP1 accumulation pretrea... |
Eur J Med Chem 108: 730-40 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.050
BindingDB Entry DOI: 10.7270/Q2XW4MNF |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50139182
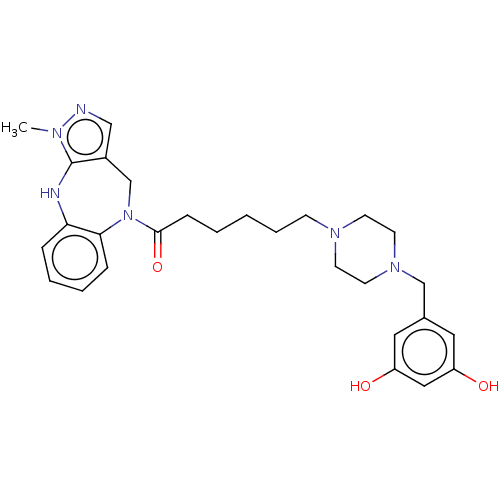
(CHEMBL3765525)Show SMILES Cn1ncc2CN(C(=O)CCCCCN3CCN(Cc4cc(O)cc(O)c4)CC3)c3ccccc3Nc12 Show InChI InChI=1S/C28H36N6O3/c1-31-28-22(18-29-31)20-34(26-8-5-4-7-25(26)30-28)27(37)9-3-2-6-10-32-11-13-33(14-12-32)19-21-15-23(35)17-24(36)16-21/h4-5,7-8,15-18,30,35-36H,2-3,6,9-14,19-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 449 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H]-vasopressin from human vasopressin 1a receptor expressed in HEK293 cells after 90 mins by microbeta 2 microplate-reader method |
Eur J Med Chem 108: 730-40 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.050
BindingDB Entry DOI: 10.7270/Q2XW4MNF |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50139180
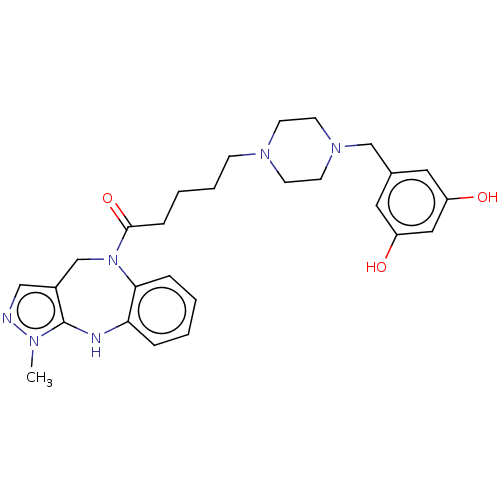
(CHEMBL3764572)Show SMILES Cn1ncc2CN(C(=O)CCCCN3CCN(Cc4cc(O)cc(O)c4)CC3)c3ccccc3Nc12 Show InChI InChI=1S/C27H34N6O3/c1-30-27-21(17-28-30)19-33(25-7-3-2-6-24(25)29-27)26(36)8-4-5-9-31-10-12-32(13-11-31)18-20-14-22(34)16-23(35)15-20/h2-3,6-7,14-17,29,34-35H,4-5,8-13,18-19H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 466 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H]-oxytocin from human oxytocin receptor expressed in HEK293 cells after 90 mins by microbeta 2 microplate reader analysis |
Eur J Med Chem 108: 730-40 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.050
BindingDB Entry DOI: 10.7270/Q2XW4MNF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50334459

((1S,2S,3S,5R)-methyl 8-(3-iodoallyl)-3-p-tolyl-8-a...)Show SMILES COC(=O)[C@@H]1[C@@H]2CC[C@H](C[C@@H]1c1ccc(C)cc1)N2C\C=C\I |r,TLB:19:18:10.9.4:7.6| Show InChI InChI=1S/C19H24INO2/c1-13-4-6-14(7-5-13)16-12-15-8-9-17(18(16)19(22)23-2)21(15)11-3-10-20/h3-7,10,15-18H,8-9,11-12H2,1-2H3/b10-3+/t15-,16-,17+,18+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of SERT |
Bioorg Med Chem Lett 21: 38-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.075
BindingDB Entry DOI: 10.7270/Q20K28VR |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50283972
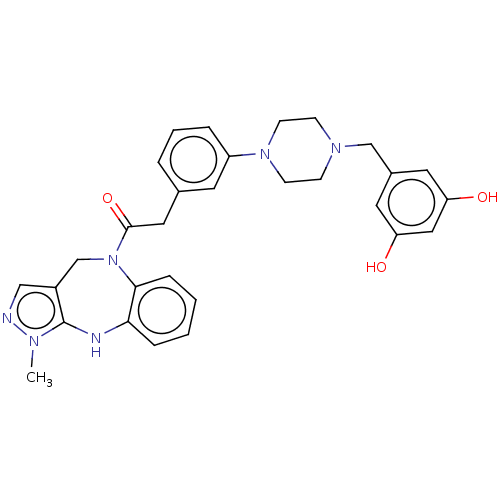
(CHEMBL4162565)Show SMILES Cn1ncc2CN(C(=O)Cc3cccc(c3)N3CCN(Cc4cc(O)cc(O)c4)CC3)c3ccccc3Nc12 Show InChI InChI=1S/C30H32N6O3/c1-33-30-23(18-31-33)20-36(28-8-3-2-7-27(28)32-30)29(39)16-21-5-4-6-24(13-21)35-11-9-34(10-12-35)19-22-14-25(37)17-26(38)15-22/h2-8,13-15,17-18,32,37-38H,9-12,16,19-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 643 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H]-vasopressin from human vasopressin V1a receptor expressed in HEK293 cell membranes after 90 mins by radioligand binding assay |
Eur J Med Chem 143: 1644-1656 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.059
BindingDB Entry DOI: 10.7270/Q2MS3W92 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50334455

(CHEMBL1643900 | N-(2-(3'-Fluorophenyl)ethyl)-8-ami...)Show SMILES Fc1cccc(CCN[C@@H]2C3C4CC5C6CC(C3C46)C25)c1 |r,THB:10:17:15:13.19,9:19:17.18:15,11:18:15:13.19,12:13:17.18:15| Show InChI InChI=1S/C19H22FN/c20-10-3-1-2-9(6-10)4-5-21-19-16-12-8-13-15-11(12)7-14(16)17(15)18(13)19/h1-3,6,11-19,21H,4-5,7-8H2/t11?,12?,13?,14?,15?,16?,17?,18?,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 705 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of muscarinic M4 receptor |
Bioorg Med Chem Lett 21: 38-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.075
BindingDB Entry DOI: 10.7270/Q20K28VR |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50283952
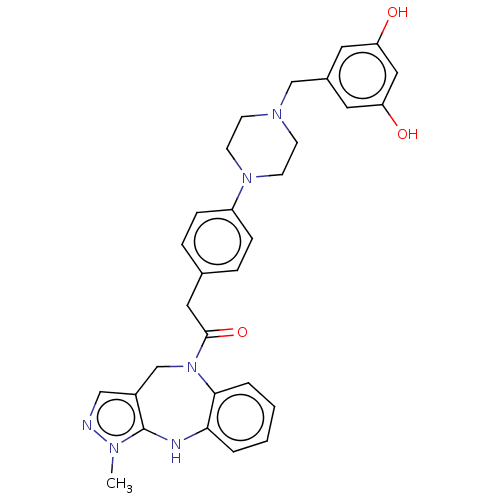
(CHEMBL4173250)Show SMILES Cn1ncc2CN(C(=O)Cc3ccc(cc3)N3CCN(Cc4cc(O)cc(O)c4)CC3)c3ccccc3Nc12 Show InChI InChI=1S/C30H32N6O3/c1-33-30-23(18-31-33)20-36(28-5-3-2-4-27(28)32-30)29(39)16-21-6-8-24(9-7-21)35-12-10-34(11-13-35)19-22-14-25(37)17-26(38)15-22/h2-9,14-15,17-18,32,37-38H,10-13,16,19-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 795 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H]-vasopressin from human vasopressin V1a receptor expressed in HEK293 cell membranes after 90 mins by radioligand binding assay |
Eur J Med Chem 143: 1644-1656 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.059
BindingDB Entry DOI: 10.7270/Q2MS3W92 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50139179

(CHEMBL3763951)Show SMILES COc1cc(CN2CCN(CC2)C(=O)NCc2ccc(cc2C)C(=O)N2Cc3cnn(C)c3Nc3ccccc23)cc(OC)c1 Show InChI InChI=1S/C34H39N7O4/c1-23-15-25(33(42)41-22-27-20-36-38(2)32(27)37-30-7-5-6-8-31(30)41)9-10-26(23)19-35-34(43)40-13-11-39(12-14-40)21-24-16-28(44-3)18-29(17-24)45-4/h5-10,15-18,20,37H,11-14,19,21-22H2,1-4H3,(H,35,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 801 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H]-oxytocin from human OTR expressed in HEK293 cell membranes after 90 mins by radioligand binding assay |
Eur J Med Chem 143: 1644-1656 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.059
BindingDB Entry DOI: 10.7270/Q2MS3W92 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50139179

(CHEMBL3763951)Show SMILES COc1cc(CN2CCN(CC2)C(=O)NCc2ccc(cc2C)C(=O)N2Cc3cnn(C)c3Nc3ccccc23)cc(OC)c1 Show InChI InChI=1S/C34H39N7O4/c1-23-15-25(33(42)41-22-27-20-36-38(2)32(27)37-30-7-5-6-8-31(30)41)9-10-26(23)19-35-34(43)40-13-11-39(12-14-40)21-24-16-28(44-3)18-29(17-24)45-4/h5-10,15-18,20,37H,11-14,19,21-22H2,1-4H3,(H,35,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 801 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Antagonist activity at human vasopressin 1a receptor expressed in HEK293 cells assessed as inhibition of vasopressin induced IP1 accumulation pretrea... |
Eur J Med Chem 108: 730-40 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.050
BindingDB Entry DOI: 10.7270/Q2XW4MNF |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50283978

(CHEMBL4176616)Show SMILES Cn1ncc2CN(C(=O)CCc3cccc(c3)N3CCN(Cc4cc(O)cc(O)c4)CC3)c3ccccc3Nc12 Show InChI InChI=1S/C31H34N6O3/c1-34-31-24(19-32-34)21-37(29-8-3-2-7-28(29)33-31)30(40)10-9-22-5-4-6-25(15-22)36-13-11-35(12-14-36)20-23-16-26(38)18-27(39)17-23/h2-8,15-19,33,38-39H,9-14,20-21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 874 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H]-vasopressin from human vasopressin V1a receptor expressed in HEK293 cell membranes after 90 mins by radioligand binding assay |
Eur J Med Chem 143: 1644-1656 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.059
BindingDB Entry DOI: 10.7270/Q2MS3W92 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50334459

((1S,2S,3S,5R)-methyl 8-(3-iodoallyl)-3-p-tolyl-8-a...)Show SMILES COC(=O)[C@@H]1[C@@H]2CC[C@H](C[C@@H]1c1ccc(C)cc1)N2C\C=C\I |r,TLB:19:18:10.9.4:7.6| Show InChI InChI=1S/C19H24INO2/c1-13-4-6-14(7-5-13)16-12-15-8-9-17(18(16)19(22)23-2)21(15)11-3-10-20/h3-7,10,15-18H,8-9,11-12H2,1-2H3/b10-3+/t15-,16-,17+,18+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of NET |
Bioorg Med Chem Lett 21: 38-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.075
BindingDB Entry DOI: 10.7270/Q20K28VR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data