

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB PubMed | 0.00120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity against Wild-type human DHFR | J Med Chem 38: 745-52 (1995) BindingDB Entry DOI: 10.7270/Q2JQ11PK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50026273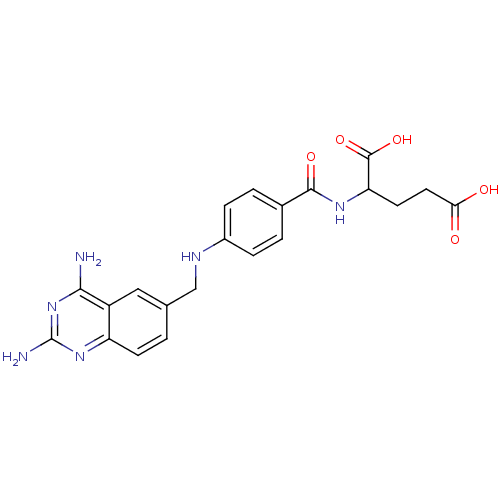 (2-{4-[(2,4-Diamino-quinazolin-6-ylmethyl)-amino]-b...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase | J Med Chem 38: 745-52 (1995) BindingDB Entry DOI: 10.7270/Q2JQ11PK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50026273 (2-{4-[(2,4-Diamino-quinazolin-6-ylmethyl)-amino]-b...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase | J Med Chem 38: 745-52 (1995) BindingDB Entry DOI: 10.7270/Q2JQ11PK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase | J Med Chem 38: 745-52 (1995) BindingDB Entry DOI: 10.7270/Q2JQ11PK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50367055 (4-Aminofolic acid | 4-Aminopteroic acid | AMINOPTE...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase | J Med Chem 38: 745-52 (1995) BindingDB Entry DOI: 10.7270/Q2JQ11PK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50367055 (4-Aminofolic acid | 4-Aminopteroic acid | AMINOPTE...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase | J Med Chem 38: 745-52 (1995) BindingDB Entry DOI: 10.7270/Q2JQ11PK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50229783 (6-[(2-(4-(2,3-dichlorophenyl)piperazin-1-yl)ethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50229776 (6-[(2-(4-(2-methoxyphenyl)piperazin-1-yl)ethyl)(pr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50229776 (6-[(2-(4-(2-methoxyphenyl)piperazin-1-yl)ethyl)(pr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50036495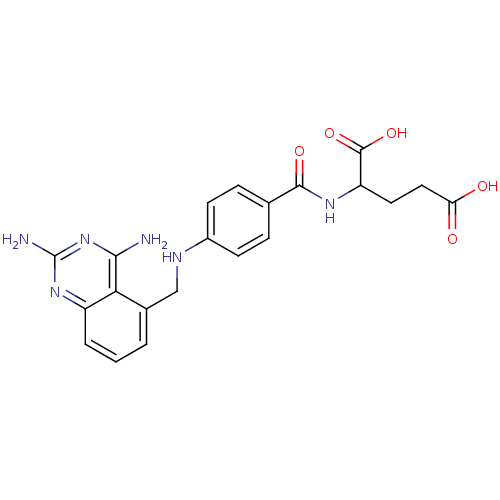 (2-{4-[(2,4-Diamino-quinazolin-5-ylmethyl)-amino]-b...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity against Wild-type human DHFR | J Med Chem 38: 745-52 (1995) BindingDB Entry DOI: 10.7270/Q2JQ11PK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM29589 (Faridak | LBH-589 | LBH-589B | Panobinostat | US10...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd. Curated by ChEMBL | Assay Description Competitive inhibition of HDAC4 using KI-104 as substrate by fluorescence assay | J Med Chem 54: 4694-720 (2011) Article DOI: 10.1021/jm2003552 BindingDB Entry DOI: 10.7270/Q29S1RD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 5 (Homo sapiens (Human)) | BDBM29589 (Faridak | LBH-589 | LBH-589B | Panobinostat | US10...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd. Curated by ChEMBL | Assay Description Competitive inhibition of HDAC5 using KI-104 as substrate by fluorescence assay | J Med Chem 54: 4694-720 (2011) Article DOI: 10.1021/jm2003552 BindingDB Entry DOI: 10.7270/Q29S1RD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM29589 (Faridak | LBH-589 | LBH-589B | Panobinostat | US10...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd. Curated by ChEMBL | Assay Description Competitive inhibition of HDAC6 using KI-104 as substrate by fluorescence assay | J Med Chem 54: 4694-720 (2011) Article DOI: 10.1021/jm2003552 BindingDB Entry DOI: 10.7270/Q29S1RD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50109927 ((+)-7-[(4-(4-phenylpiperazin-1-yl)butyl)(propyl)am...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50109927 ((+)-7-[(4-(4-phenylpiperazin-1-yl)butyl)(propyl)am...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50109931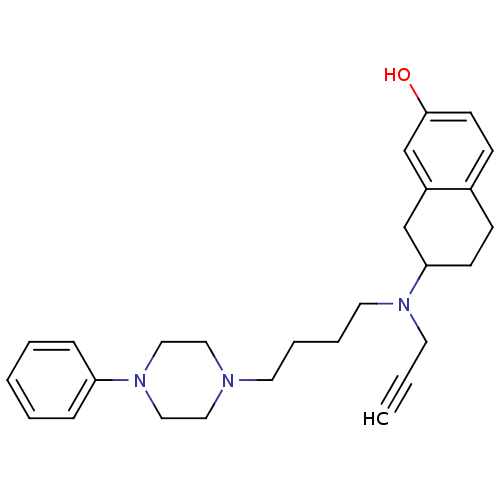 ((+)-7-{[4-(4-phenylpiperazin-1-yl)butyl]prop-2-yny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50109931 ((+)-7-{[4-(4-phenylpiperazin-1-yl)butyl]prop-2-yny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50229775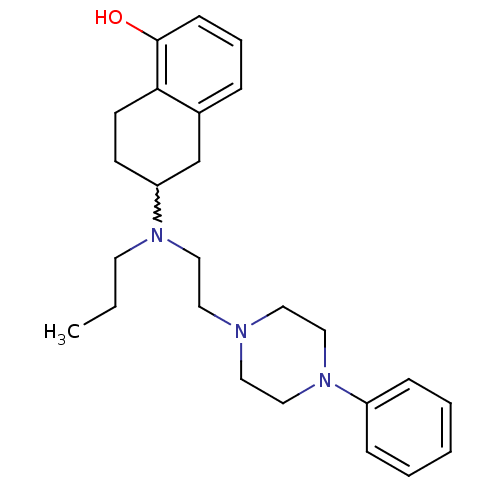 ((+)-6-[(2-(4-phenylpiperazin-1-yl)ethyl)(propyl)am...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50229775 ((+)-6-[(2-(4-phenylpiperazin-1-yl)ethyl)(propyl)am...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50229775 ((+)-6-[(2-(4-phenylpiperazin-1-yl)ethyl)(propyl)am...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50116766 ((-)-Pramipexole | (6S)-N(6)-propyl-4,5,6,7-tetrahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Neurological Disorders and Stroke Curated by ChEMBL | Assay Description Displacement of [3H]-N-methylspiperone from D3 receptor (unknown origin) measured after 90 mins by microbeta scintillation counting method | J Med Chem 63: 5526-5567 (2020) Article DOI: 10.1021/acs.jmedchem.0c00424 BindingDB Entry DOI: 10.7270/Q28D00QN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50261383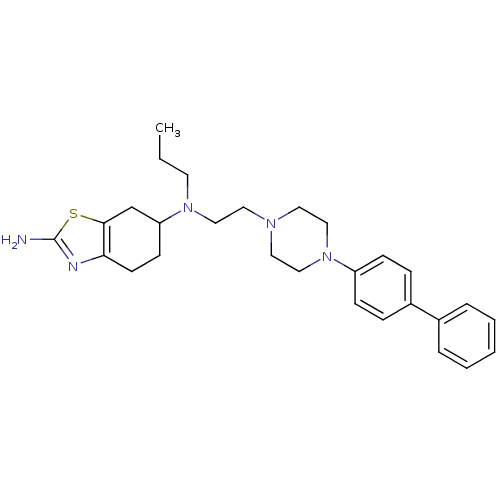 ((-)-N6-(2-(4-(Biphenyl-4-yl)piperazin-1-yl)-ethyl)...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from rat dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 3005-19 (2008) Article DOI: 10.1021/jm701524h BindingDB Entry DOI: 10.7270/Q2BV7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50116766 ((-)-Pramipexole | (6S)-N(6)-propyl-4,5,6,7-tetrahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Neurological Disorders and Stroke Curated by ChEMBL | Assay Description Displacement of [3H]-7-OH-DPAT from human D3R expressed in HEK293 cell membranes measured after 90 mins in Tris buffer by topcount assay | J Med Chem 63: 5526-5567 (2020) Article DOI: 10.1021/acs.jmedchem.0c00424 BindingDB Entry DOI: 10.7270/Q28D00QN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50116766 ((-)-Pramipexole | (6S)-N(6)-propyl-4,5,6,7-tetrahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Neurological Disorders and Stroke Curated by ChEMBL | Assay Description Displacement of [3H]-7-OH-DPAT from human D3R expressed in HEK293 cell membranes measured after 90 mins in EBSS buffer by topcount assay | J Med Chem 63: 5526-5567 (2020) Article DOI: 10.1021/acs.jmedchem.0c00424 BindingDB Entry DOI: 10.7270/Q28D00QN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM119438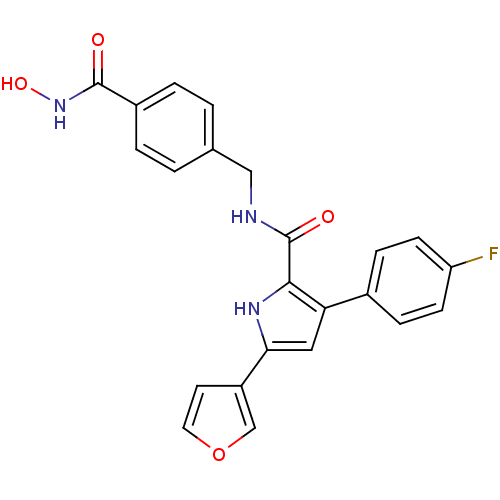 (BDBM119693 | US8685986, 12) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | -52.0 | n/a | n/a | n/a | n/a | n/a | 6.0 | 30 |
Ube Industries, Ltd. US Patent | Assay Description Measurement of EP2 receptor binding affinity was carried out according to the method of Abramovitz et al. (Biochimica et Biophysica Acta, 1483, 285 (... | US Patent US8685986 (2014) BindingDB Entry DOI: 10.7270/Q28S4NK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM29589 (Faridak | LBH-589 | LBH-589B | Panobinostat | US10...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd. Curated by ChEMBL | Assay Description Competitive inhibition of HDAC9 using KI-104 as substrate by fluorescence assay | J Med Chem 54: 4694-720 (2011) Article DOI: 10.1021/jm2003552 BindingDB Entry DOI: 10.7270/Q29S1RD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50229778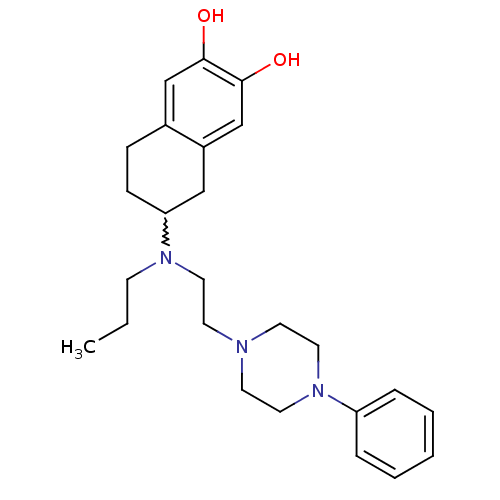 (6-[(2-(4-phenylpiperazin-1-yl)ethyl)(propyl)amino]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM119442 (US8685986, 17) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.5 | -51.2 | n/a | n/a | n/a | n/a | n/a | 6.0 | 30 |
Ube Industries, Ltd. US Patent | Assay Description Measurement of EP2 receptor binding affinity was carried out according to the method of Abramovitz et al. (Biochimica et Biophysica Acta, 1483, 285 (... | US Patent US8685986 (2014) BindingDB Entry DOI: 10.7270/Q28S4NK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50109926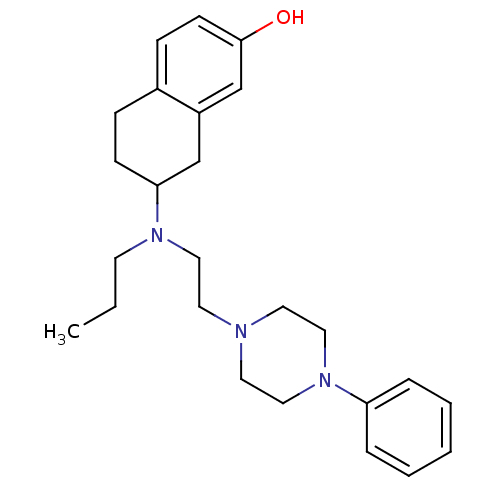 (7-((2-(4-phenylpiperazin-1-yl)ethyl)(propyl)amino)...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from rat dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 3005-19 (2008) Article DOI: 10.1021/jm701524h BindingDB Entry DOI: 10.7270/Q2BV7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50109926 (7-((2-(4-phenylpiperazin-1-yl)ethyl)(propyl)amino)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50065258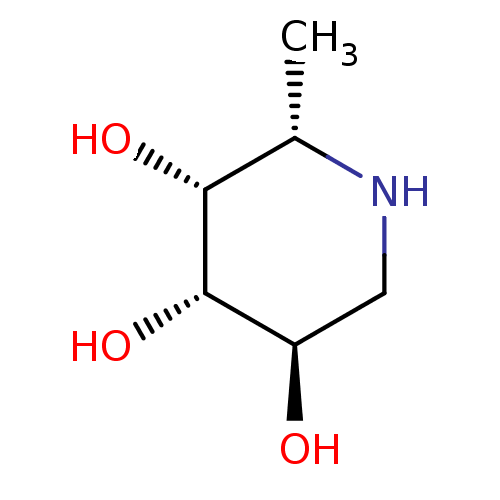 ((2S,3R,4S,5R)-2-Methyl-piperidine-3,4,5-triol | (2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Ki value against bovine alpha-L-fucosidase | J Med Chem 48: 2036-44 (2005) Article DOI: 10.1021/jm0495881 BindingDB Entry DOI: 10.7270/Q2DF6S0M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50261279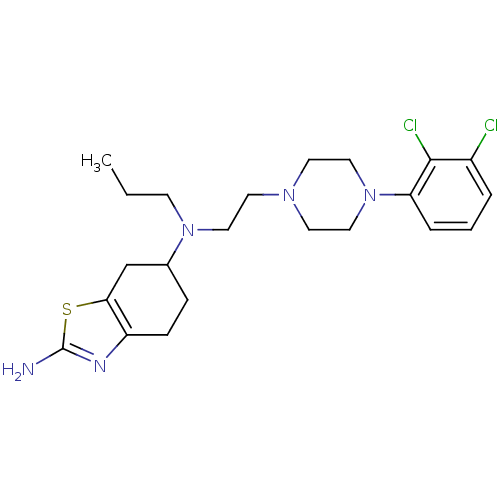 ((+)-N6-(2-(4-(2,3-Dichlorophenyl)piperazin-1-yl)et...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from rat dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 3005-19 (2008) Article DOI: 10.1021/jm701524h BindingDB Entry DOI: 10.7270/Q2BV7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50261279 ((+)-N6-(2-(4-(2,3-Dichlorophenyl)piperazin-1-yl)et...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from rat dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 3005-19 (2008) Article DOI: 10.1021/jm701524h BindingDB Entry DOI: 10.7270/Q2BV7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50036484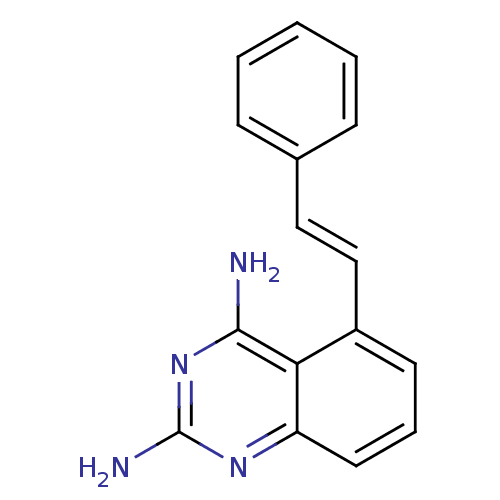 (5-((E)-Styryl)-quinazoline-2,4-diamine | CHEMBL164...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase | J Med Chem 38: 745-52 (1995) BindingDB Entry DOI: 10.7270/Q2JQ11PK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50261279 ((+)-N6-(2-(4-(2,3-Dichlorophenyl)piperazin-1-yl)et...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from rat dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 3005-19 (2008) Article DOI: 10.1021/jm701524h BindingDB Entry DOI: 10.7270/Q2BV7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM29589 (Faridak | LBH-589 | LBH-589B | Panobinostat | US10...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd. Curated by ChEMBL | Assay Description Competitive inhibition of HDAC2 using KI-104 as substrate by fluorescence assay | J Med Chem 54: 4694-720 (2011) Article DOI: 10.1021/jm2003552 BindingDB Entry DOI: 10.7270/Q29S1RD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM119432 (US8685986, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.90 | -50.6 | n/a | n/a | n/a | n/a | n/a | 6.0 | 30 |
Ube Industries, Ltd. US Patent | Assay Description Measurement of EP2 receptor binding affinity was carried out according to the method of Abramovitz et al. (Biochimica et Biophysica Acta, 1483, 285 (... | US Patent US8685986 (2014) BindingDB Entry DOI: 10.7270/Q28S4NK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM29589 (Faridak | LBH-589 | LBH-589B | Panobinostat | US10...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd. Curated by ChEMBL | Assay Description Competitive inhibition of HDAC3 using KI-104 as substrate by fluorescence assay | J Med Chem 54: 4694-720 (2011) Article DOI: 10.1021/jm2003552 BindingDB Entry DOI: 10.7270/Q29S1RD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 7 (Homo sapiens (Human)) | BDBM29589 (Faridak | LBH-589 | LBH-589B | Panobinostat | US10...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd. Curated by ChEMBL | Assay Description Competitive inhibition of HDAC7 using KI-104 as substrate by fluorescence assay | J Med Chem 54: 4694-720 (2011) Article DOI: 10.1021/jm2003552 BindingDB Entry DOI: 10.7270/Q29S1RD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50020222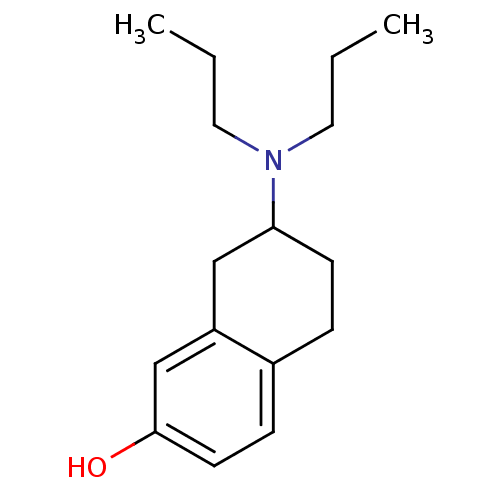 ((+/-)-7-(dipropylamino)-5,6,7,8-tetrahydronaphthal...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from rat dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 3005-19 (2008) Article DOI: 10.1021/jm701524h BindingDB Entry DOI: 10.7270/Q2BV7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50054067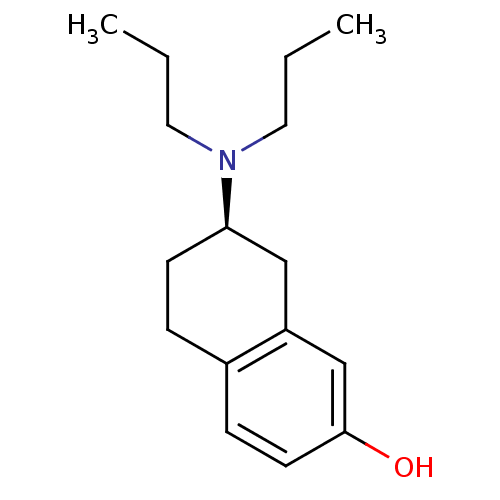 ((2R)-7-Dipropylamino-5,6,7,8-tetrahydro-naphthalen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50353233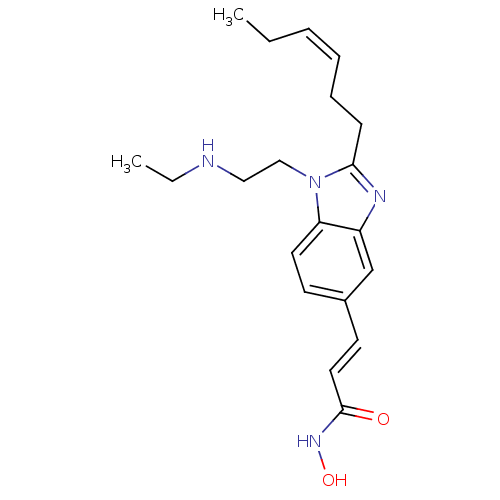 (CHEMBL1830536) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd. Curated by ChEMBL | Assay Description Competitive inhibition of HDAC4 using KI-104 as substrate by fluorescence assay | J Med Chem 54: 4694-720 (2011) Article DOI: 10.1021/jm2003552 BindingDB Entry DOI: 10.7270/Q29S1RD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM29589 (Faridak | LBH-589 | LBH-589B | Panobinostat | US10...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd. Curated by ChEMBL | Assay Description Competitive inhibition of HDAC1 using KI-104 as substrate by fluorescence assay | J Med Chem 54: 4694-720 (2011) Article DOI: 10.1021/jm2003552 BindingDB Entry DOI: 10.7270/Q29S1RD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50036495 (2-{4-[(2,4-Diamino-quinazolin-5-ylmethyl)-amino]-b...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase | J Med Chem 38: 745-52 (1995) BindingDB Entry DOI: 10.7270/Q2JQ11PK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM119433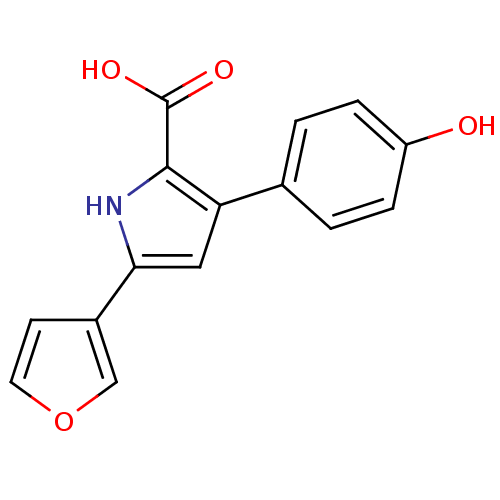 (US8685986, 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.80 | -49.6 | n/a | n/a | n/a | n/a | n/a | 6.0 | 30 |
Ube Industries, Ltd. US Patent | Assay Description Measurement of EP2 receptor binding affinity was carried out according to the method of Abramovitz et al. (Biochimica et Biophysica Acta, 1483, 285 (... | US Patent US8685986 (2014) BindingDB Entry DOI: 10.7270/Q28S4NK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50229776 (6-[(2-(4-(2-methoxyphenyl)piperazin-1-yl)ethyl)(pr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50229782 (6-[(4-(4-phenylpiperazin-1-yl)butyl)(propyl)amino]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50036483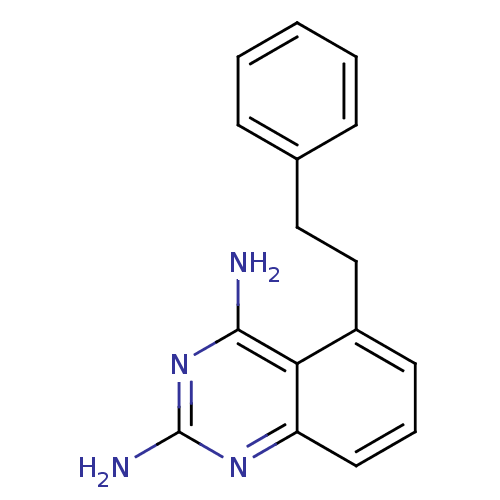 (5-Phenethyl-quinazoline-2,4-diamine | CHEMBL341703) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase | J Med Chem 38: 745-52 (1995) BindingDB Entry DOI: 10.7270/Q2JQ11PK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 5 (Homo sapiens (Human)) | BDBM50353233 (CHEMBL1830536) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd. Curated by ChEMBL | Assay Description Competitive inhibition of HDAC5 using KI-104 as substrate by fluorescence assay | J Med Chem 54: 4694-720 (2011) Article DOI: 10.1021/jm2003552 BindingDB Entry DOI: 10.7270/Q29S1RD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM119441 (US8685986, 16) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.10 | -49.4 | n/a | n/a | n/a | n/a | n/a | 6.0 | 30 |
Ube Industries, Ltd. US Patent | Assay Description Measurement of EP2 receptor binding affinity was carried out according to the method of Abramovitz et al. (Biochimica et Biophysica Acta, 1483, 285 (... | US Patent US8685986 (2014) BindingDB Entry DOI: 10.7270/Q28S4NK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1800 total ) | Next | Last >> |