

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50024714 (2-(4-Carboxymethyl-5-oxo-3-thiophen-2-yl-[1,4]thia...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate | J Med Chem 30: 1984-91 (1987) BindingDB Entry DOI: 10.7270/Q2Q52NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50023299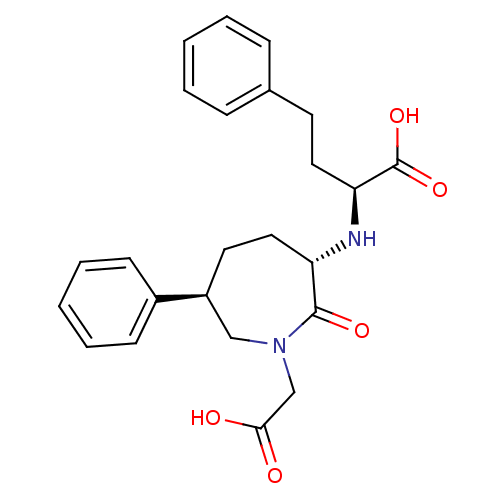 (2-(1-Carboxymethyl-2-oxo-6-phenyl-azepan-3-ylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of rabbit lung Angiotensin I converting enzyme with 5 mM hippuryl-histidyl-leucine as substrate | J Med Chem 31: 422-8 (1988) BindingDB Entry DOI: 10.7270/Q2S75GWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50023298 (2-(4-Carboxymethyl-5-oxo-3-phenyl-[1,4]thiazepan-6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of rabbit lung Angiotensin I converting enzyme with 5 mM hippuryl-histidyl-leucine as substrate | J Med Chem 31: 422-8 (1988) BindingDB Entry DOI: 10.7270/Q2S75GWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50023298 (2-(4-Carboxymethyl-5-oxo-3-phenyl-[1,4]thiazepan-6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate | J Med Chem 30: 1984-91 (1987) BindingDB Entry DOI: 10.7270/Q2Q52NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50023296 (2-(1-Carboxymethyl-2-oxo-7-phenyl-azepan-3-ylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of rabbit lung Angiotensin I converting enzyme with 5 mM hippuryl-histidyl-leucine as substrate | J Med Chem 31: 422-8 (1988) BindingDB Entry DOI: 10.7270/Q2S75GWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50024710 (2-(4-Carboxymethyl-5-oxo-2-thiophen-2-yl-[1,4]thia...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate | J Med Chem 30: 1984-91 (1987) BindingDB Entry DOI: 10.7270/Q2Q52NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50023295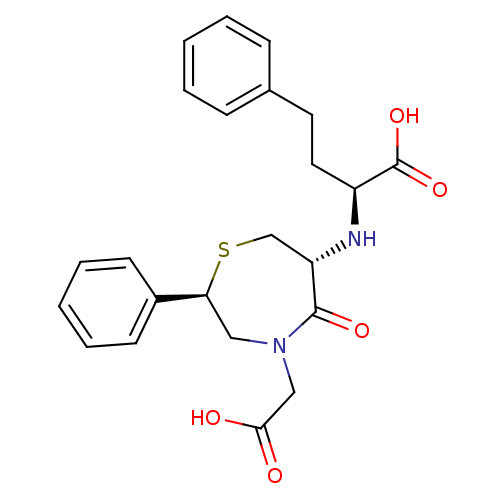 (2-(4-Carboxymethyl-5-oxo-2-phenyl-[1,4]thiazepan-6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of rabbit lung Angiotensin I converting enzyme with 5 mM hippuryl-histidyl-leucine as substrate | J Med Chem 31: 422-8 (1988) BindingDB Entry DOI: 10.7270/Q2S75GWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50023295 (2-(4-Carboxymethyl-5-oxo-2-phenyl-[1,4]thiazepan-6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate | J Med Chem 30: 1984-91 (1987) BindingDB Entry DOI: 10.7270/Q2Q52NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50024713 (2-(4-Carboxymethyl-5-oxo-2-thiophen-3-yl-[1,4]thia...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate | J Med Chem 30: 1984-91 (1987) BindingDB Entry DOI: 10.7270/Q2Q52NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50023300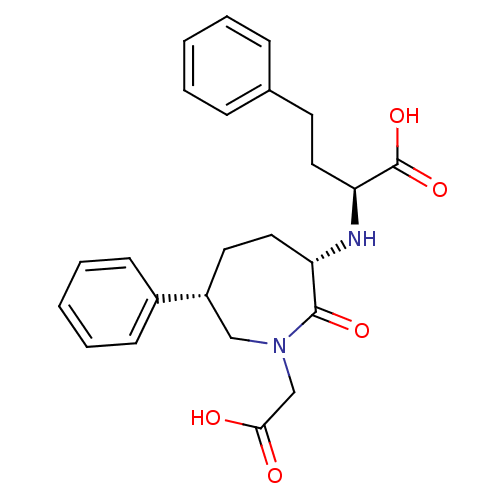 (2-(1-Carboxymethyl-2-oxo-6-phenyl-azepan-3-ylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of rabbit lung Angiotensin I converting enzyme with 5 mM hippuryl-histidyl-leucine as substrate | J Med Chem 31: 422-8 (1988) BindingDB Entry DOI: 10.7270/Q2S75GWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049128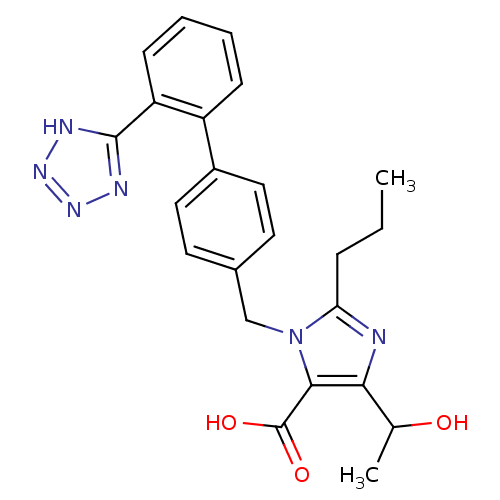 (5-(1-Hydroxy-ethyl)-2-propyl-3-[2'-(2H-tetrazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049118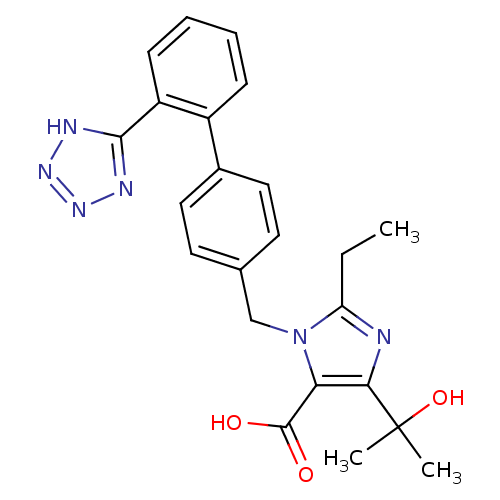 (2-Ethyl-5-(1-hydroxy-1-methyl-ethyl)-3-[2'-(2H-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50011192 ((R)-1-[(S)-2-((S)-1-Carboxy-3-phenyl-propylamino)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate | J Med Chem 30: 1984-91 (1987) BindingDB Entry DOI: 10.7270/Q2Q52NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50367254 (ENALAPRILAT) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of rabbit lung Angiotensin I converting enzyme with 5 mM hippuryl-histidyl-leucine as substrate | J Med Chem 31: 422-8 (1988) BindingDB Entry DOI: 10.7270/Q2S75GWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049123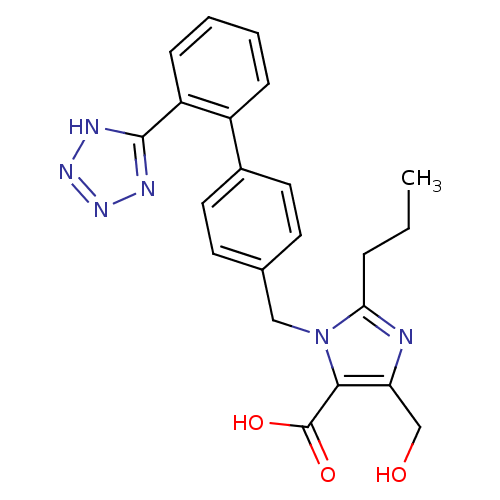 (5-Hydroxymethyl-2-propyl-3-[2'-(2H-tetrazol-5-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50241364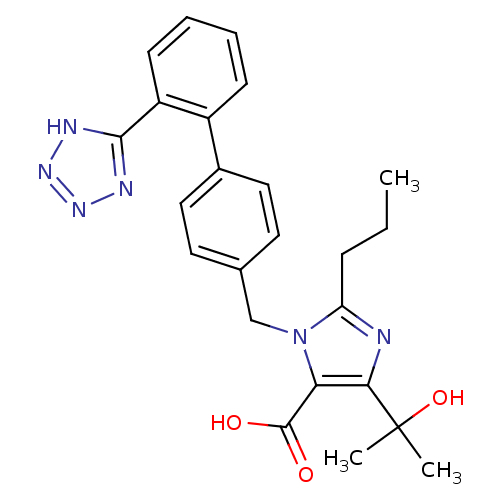 (4-(1-hydroxy-1-methylethyl)-2-propyl-1-{[2'-(1H-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049107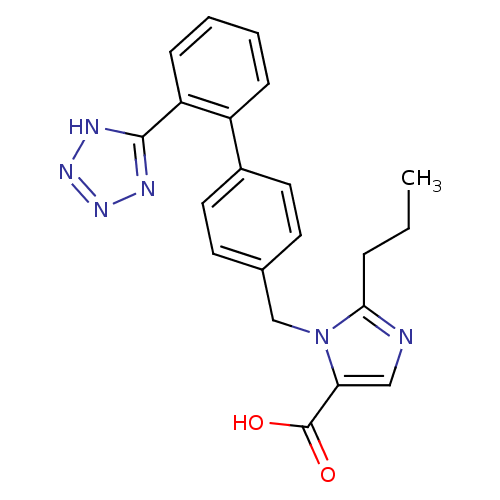 (2-Propyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-ylmet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049109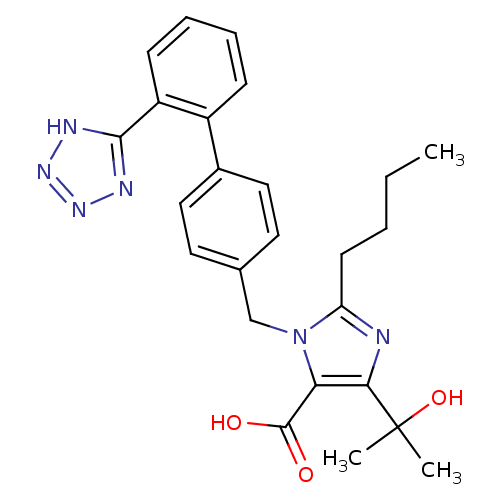 (2-Butyl-5-(1-hydroxy-1-methyl-ethyl)-3-[2'-(2H-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049124 (5-Methyl-2-propyl-3-[2'-(1H-tetrazol-5-yl)-bipheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049116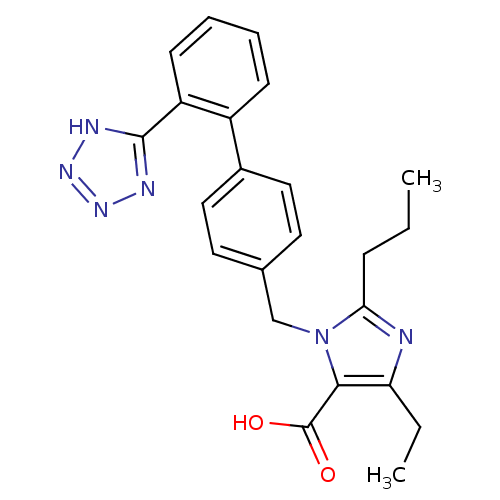 (5-Ethyl-2-propyl-3-(2'-tetrazol-1-yl-biphenyl-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50023302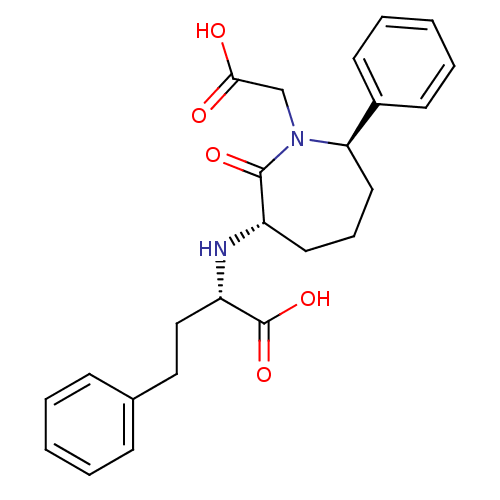 (2-(1-Carboxymethyl-2-oxo-7-phenyl-azepan-3-ylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of rabbit lung Angiotensin I converting enzyme with 5 mM hippuryl-histidyl-leucine as substrate | J Med Chem 31: 422-8 (1988) BindingDB Entry DOI: 10.7270/Q2S75GWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50006909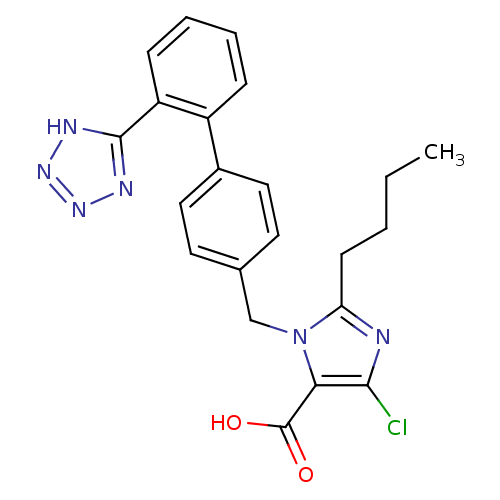 (2-Butyl-5-chloro-3-[2'-(1H-tetrazol-5-yl)-biphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50006909 (2-Butyl-5-chloro-3-[2'-(1H-tetrazol-5-yl)-biphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125l]-All binding to bovine adrenal cortex | Bioorg Med Chem Lett 4: 177-182 (1994) Article DOI: 10.1016/S0960-894X(01)81143-4 BindingDB Entry DOI: 10.7270/Q2BG2NZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049132 (3-(2'-Carboxy-biphenyl-4-ylmethyl)-5-(1-hydroxy-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049112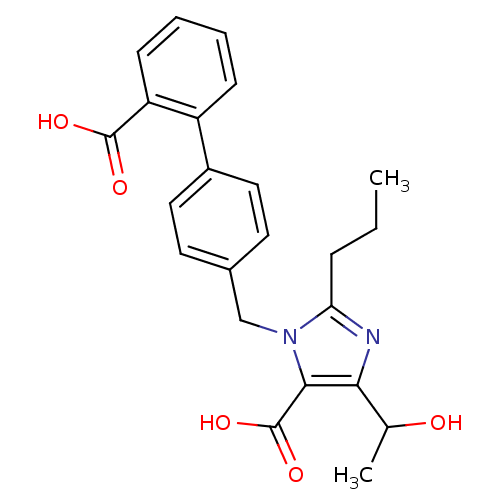 (3-(2'-Carboxy-biphenyl-4-ylmethyl)-5-(1-hydroxy-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049122 (2-Butyl-3-(2'-carboxy-biphenyl-4-ylmethyl)-5-(1-hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50023303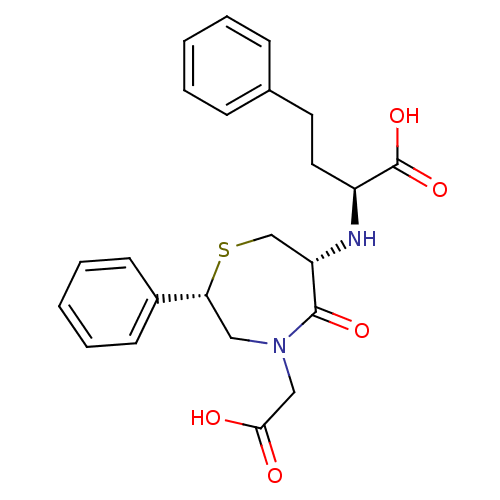 (2-(4-Carboxymethyl-5-oxo-2-phenyl-[1,4]thiazepan-6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of rabbit lung Angiotensin I converting enzyme with 5 mM hippuryl-histidyl-leucine as substrate | J Med Chem 31: 422-8 (1988) BindingDB Entry DOI: 10.7270/Q2S75GWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50023303 (2-(4-Carboxymethyl-5-oxo-2-phenyl-[1,4]thiazepan-6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate | J Med Chem 30: 1984-91 (1987) BindingDB Entry DOI: 10.7270/Q2Q52NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049117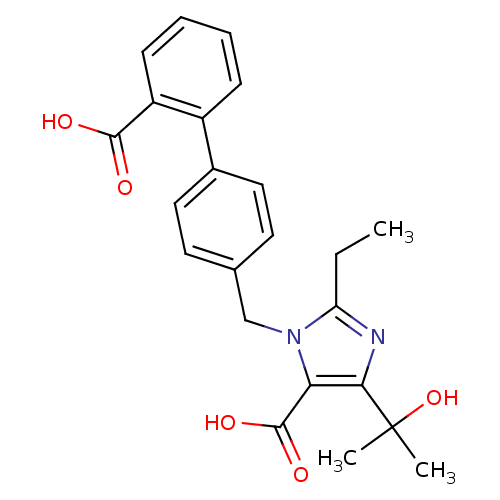 (3-(2'-Carboxy-biphenyl-4-ylmethyl)-2-ethyl-5-(1-hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049130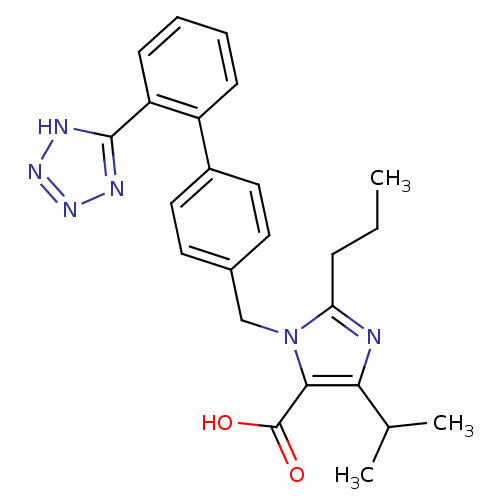 (5-Isopropyl-2-propyl-3-[2'-(1H-tetrazol-5-yl)-biph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049113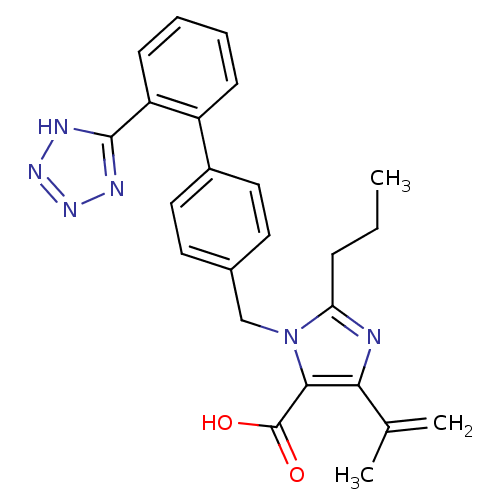 (5-Isopropenyl-2-propyl-3-[2'-(2H-tetrazol-5-yl)-bi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049106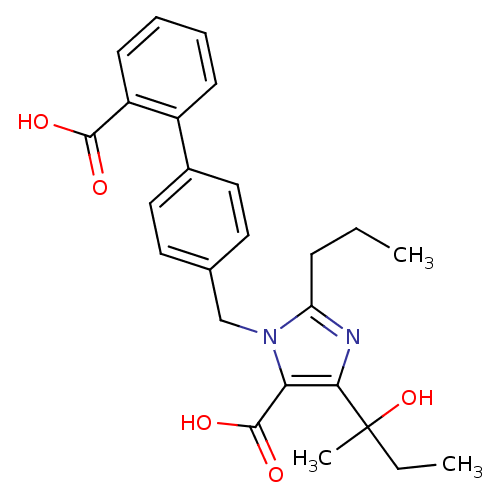 (3-(2'-Carboxy-biphenyl-4-ylmethyl)-5-(1-hydroxy-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049108 (3-(2'-Carboxy-biphenyl-4-ylmethyl)-5-hydroxymethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049108 (3-(2'-Carboxy-biphenyl-4-ylmethyl)-5-hydroxymethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125l]-All binding to bovine adrenal cortex | Bioorg Med Chem Lett 4: 177-182 (1994) Article DOI: 10.1016/S0960-894X(01)81143-4 BindingDB Entry DOI: 10.7270/Q2BG2NZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049126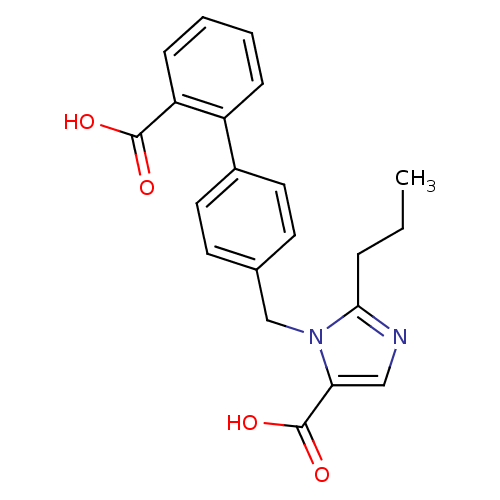 (3-(2'-Carboxy-biphenyl-4-ylmethyl)-2-propyl-3H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50024712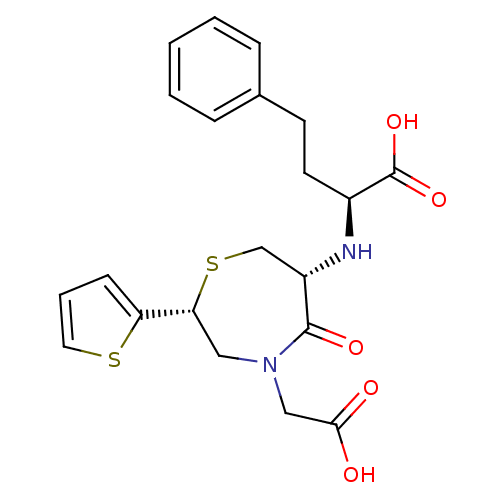 (2-(4-Carboxymethyl-5-oxo-2-thiophen-2-yl-[1,4]thia...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate | J Med Chem 30: 1984-91 (1987) BindingDB Entry DOI: 10.7270/Q2Q52NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50282291 (2-Butyl-3-(2'-carboxy-biphenyl-4-ylmethyl)-5-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125l]-All binding to bovine adrenal cortex | Bioorg Med Chem Lett 4: 177-182 (1994) Article DOI: 10.1016/S0960-894X(01)81143-4 BindingDB Entry DOI: 10.7270/Q2BG2NZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50023301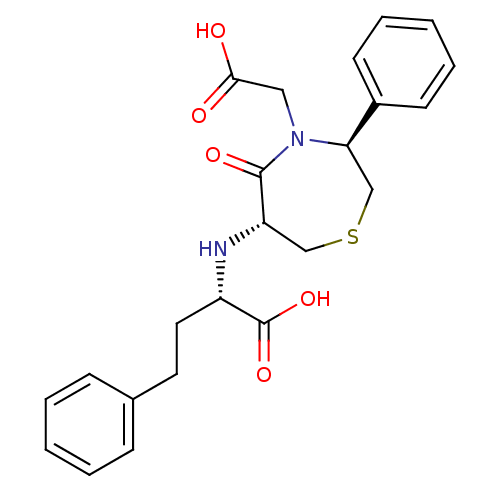 (2-(4-Carboxymethyl-5-oxo-3-phenyl-[1,4]thiazepan-6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate | J Med Chem 30: 1984-91 (1987) BindingDB Entry DOI: 10.7270/Q2Q52NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50023301 (2-(4-Carboxymethyl-5-oxo-3-phenyl-[1,4]thiazepan-6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of rabbit lung Angiotensin I converting enzyme with 5 mM hippuryl-histidyl-leucine as substrate | J Med Chem 31: 422-8 (1988) BindingDB Entry DOI: 10.7270/Q2S75GWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50024711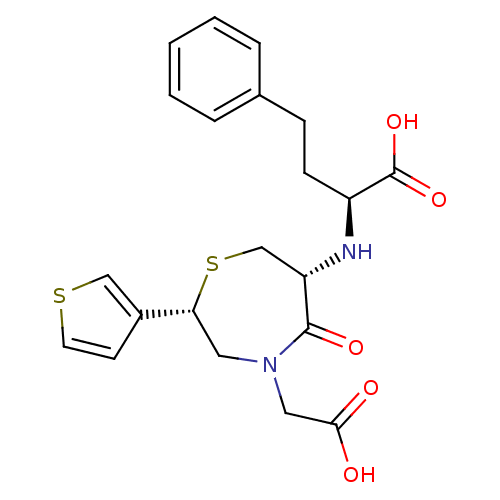 (2-(4-Carboxymethyl-5-oxo-2-thiophen-3-yl-[1,4]thia...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate | J Med Chem 30: 1984-91 (1987) BindingDB Entry DOI: 10.7270/Q2Q52NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049110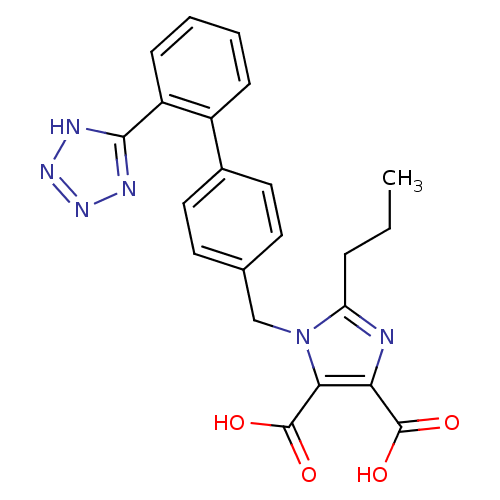 (2-Propyl-1-[2'-(2H-tetrazol-5-yl)-biphenyl-4-ylmet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049103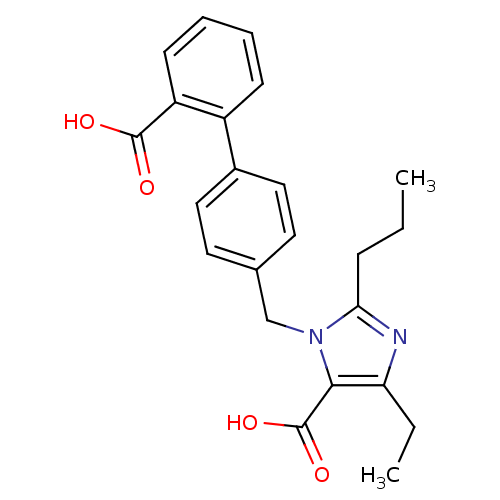 (3-(2'-Carboxy-biphenyl-4-ylmethyl)-5-ethyl-2-propy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049104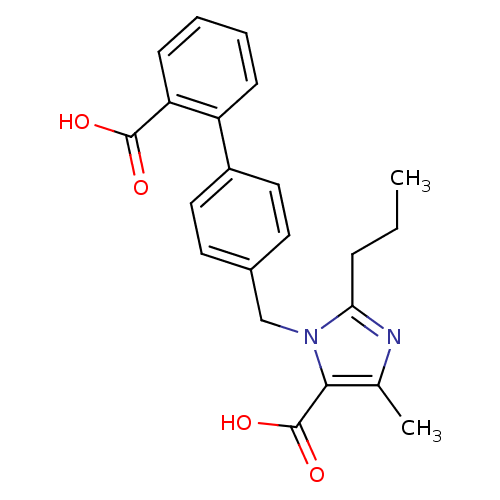 (3-(2'-Carboxy-biphenyl-4-ylmethyl)-5-methyl-2-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049129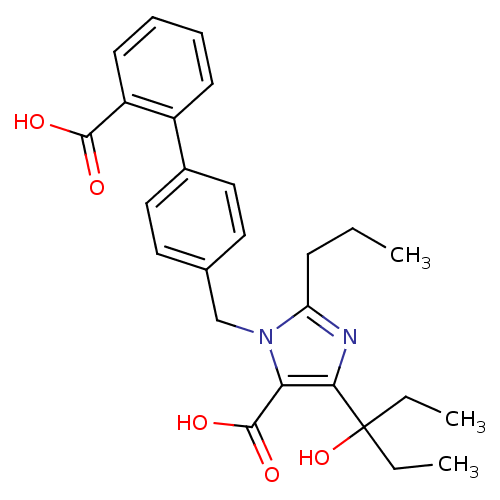 (3-(2'-Carboxy-biphenyl-4-ylmethyl)-5-(1-ethyl-1-hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50009714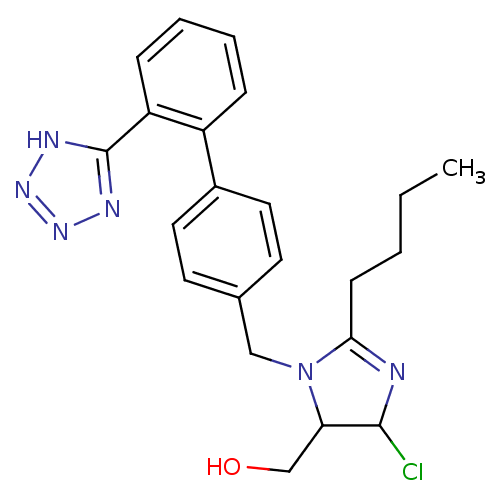 (CHEMBL191 | {2-Butyl-5-chloro-3-[2'-(2H-tetrazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50009714 (CHEMBL191 | {2-Butyl-5-chloro-3-[2'-(2H-tetrazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125l]-All binding to bovine adrenal cortex | Bioorg Med Chem Lett 4: 177-182 (1994) Article DOI: 10.1016/S0960-894X(01)81143-4 BindingDB Entry DOI: 10.7270/Q2BG2NZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50009505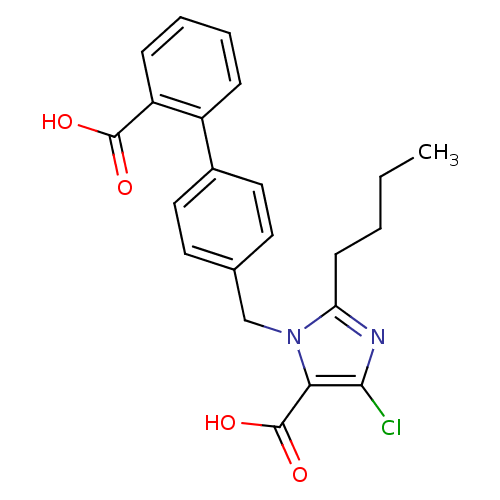 (2-Butyl-3-(2'-carboxy-biphenyl-4-ylmethyl)-5-chlor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125l]-All binding to bovine adrenal cortex | Bioorg Med Chem Lett 4: 177-182 (1994) Article DOI: 10.1016/S0960-894X(01)81143-4 BindingDB Entry DOI: 10.7270/Q2BG2NZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50009505 (2-Butyl-3-(2'-carboxy-biphenyl-4-ylmethyl)-5-chlor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50282290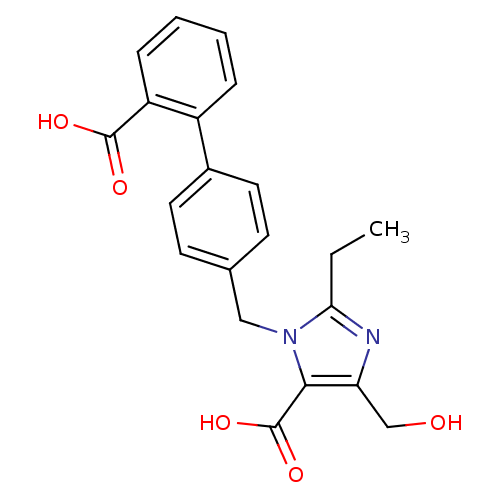 (3-(2'-Carboxy-biphenyl-4-ylmethyl)-2-ethyl-5-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125l]-All binding to bovine adrenal cortex | Bioorg Med Chem Lett 4: 177-182 (1994) Article DOI: 10.1016/S0960-894X(01)81143-4 BindingDB Entry DOI: 10.7270/Q2BG2NZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50049115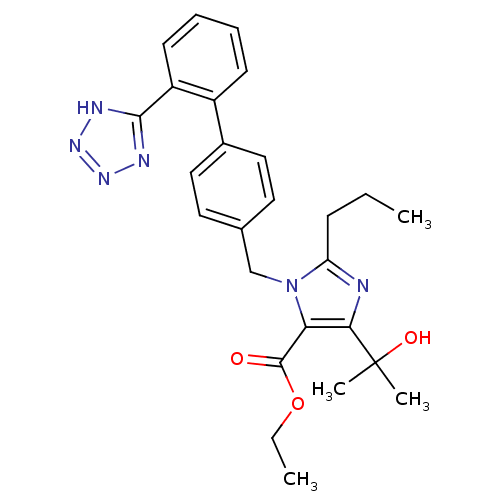 (5-(1-Hydroxy-1-methyl-ethyl)-2-propyl-3-[2'-(2H-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Company, Ltd. Curated by ChEMBL | Assay Description In vitro for inhibition of [125I]-angiotensin II (0.1 nM) binding to angiotensin II receptor type 1 in membrane fractions of bovine adrenal cortex | J Med Chem 39: 323-38 (1996) Article DOI: 10.1021/jm950450f BindingDB Entry DOI: 10.7270/Q28S4P18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 68 total ) | Next | Last >> |