

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serum paraoxonase/arylesterase 1 (Homo sapiens (Human)) | BDBM50139137 (CHEMBL3759162) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
In£n£ University Curated by ChEMBL | Assay Description Competitive inhibition of human serum PON1 using paraoxon as substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem 24: 1392-401 (2016) Article DOI: 10.1016/j.bmc.2016.02.012 BindingDB Entry DOI: 10.7270/Q2FT8NWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serum paraoxonase/arylesterase 1 (Homo sapiens (Human)) | BDBM86068 (PPC) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
In£n£ University Curated by ChEMBL | Assay Description Inhibition of human serum PON1 using paraoxon as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1392-401 (2016) Article DOI: 10.1016/j.bmc.2016.02.012 BindingDB Entry DOI: 10.7270/Q2FT8NWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serum paraoxonase/arylesterase 1 (Homo sapiens (Human)) | BDBM50139137 (CHEMBL3759162) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
In£n£ University Curated by ChEMBL | Assay Description Inhibition of human serum PON1 using paraoxon as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1392-401 (2016) Article DOI: 10.1016/j.bmc.2016.02.012 BindingDB Entry DOI: 10.7270/Q2FT8NWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serum paraoxonase/arylesterase 1 (Homo sapiens (Human)) | BDBM50144692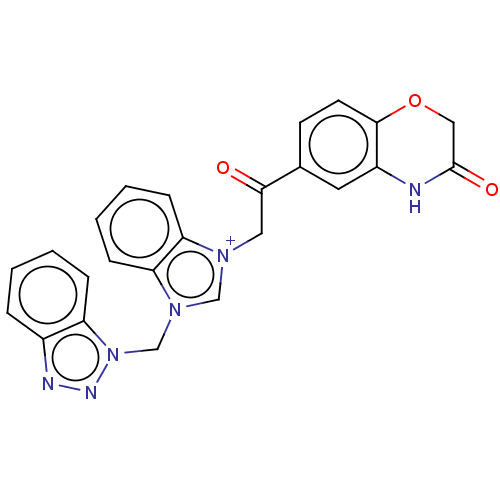 (CHEMBL3759251) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
In£n£ University Curated by ChEMBL | Assay Description Inhibition of human serum PON1 using paraoxon as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1392-401 (2016) Article DOI: 10.1016/j.bmc.2016.02.012 BindingDB Entry DOI: 10.7270/Q2FT8NWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serum paraoxonase/arylesterase 1 (Homo sapiens (Human)) | BDBM50144693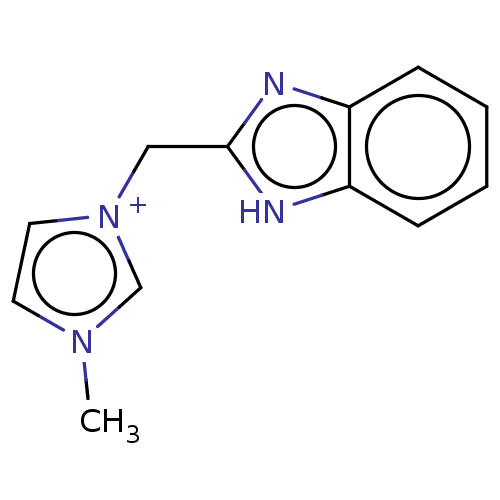 (CHEMBL3758983) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
In£n£ University Curated by ChEMBL | Assay Description Inhibition of human serum PON1 using paraoxon as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1392-401 (2016) Article DOI: 10.1016/j.bmc.2016.02.012 BindingDB Entry DOI: 10.7270/Q2FT8NWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM237365 (1-(3,4,5-Trimethoxybenzyl)-3-(4-methyl-7,8-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.03E+4 | n/a | n/a | n/a | n/a | 10.0 | n/a |
Inonu University | Assay Description CA activity was measured by the Maren method which is based on determination of the time required for the pH to decrease from 10.0 to 7.4 due to CO2 ... | J Enzyme Inhib Med Chem 28: 299-304 (2013) Article DOI: 10.3109/14756366.2012.677838 BindingDB Entry DOI: 10.7270/Q2K0734K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serum paraoxonase/arylesterase 1 (Homo sapiens (Human)) | BDBM50139133 (CHEMBL3759178) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
In£n£ University Curated by ChEMBL | Assay Description Inhibition of human serum PON1 using paraoxon as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1392-401 (2016) Article DOI: 10.1016/j.bmc.2016.02.012 BindingDB Entry DOI: 10.7270/Q2FT8NWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM237362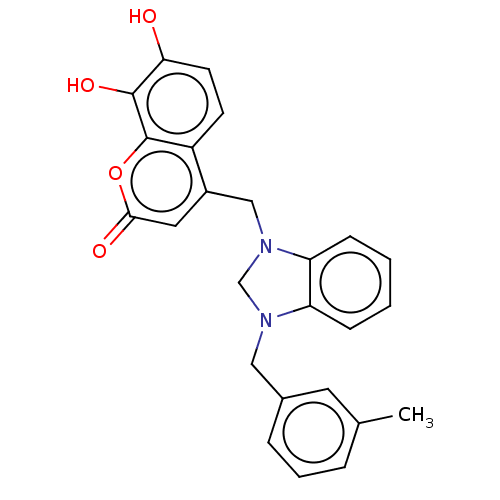 (1-(3-Methylbenzyl)-3-(4-methyl-7,8-dihydroxy-2H-ch...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.21E+4 | n/a | n/a | n/a | n/a | 10.0 | n/a |
Inonu University | Assay Description CA activity was measured by the Maren method which is based on determination of the time required for the pH to decrease from 10.0 to 7.4 due to CO2 ... | J Enzyme Inhib Med Chem 28: 299-304 (2013) Article DOI: 10.3109/14756366.2012.677838 BindingDB Entry DOI: 10.7270/Q2K0734K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serum paraoxonase/arylesterase 1 (Homo sapiens (Human)) | BDBM50139135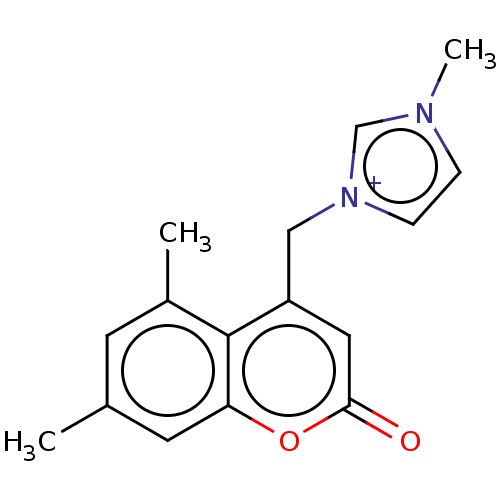 (CHEMBL3759775) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
In£n£ University Curated by ChEMBL | Assay Description Inhibition of human serum PON1 using paraoxon as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1392-401 (2016) Article DOI: 10.1016/j.bmc.2016.02.012 BindingDB Entry DOI: 10.7270/Q2FT8NWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM237361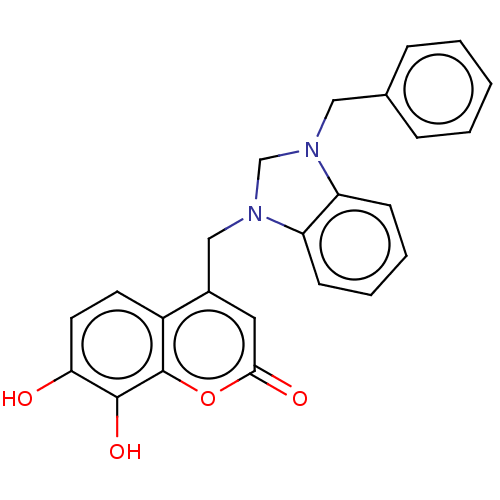 (1-Benzyl-3-(4-methyl-7,8-dihydroxy-2H-chromen-2-on...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.56E+4 | n/a | n/a | n/a | n/a | 10.0 | n/a |
Inonu University | Assay Description CA activity was measured by the Maren method which is based on determination of the time required for the pH to decrease from 10.0 to 7.4 due to CO2 ... | J Enzyme Inhib Med Chem 28: 299-304 (2013) Article DOI: 10.3109/14756366.2012.677838 BindingDB Entry DOI: 10.7270/Q2K0734K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM237356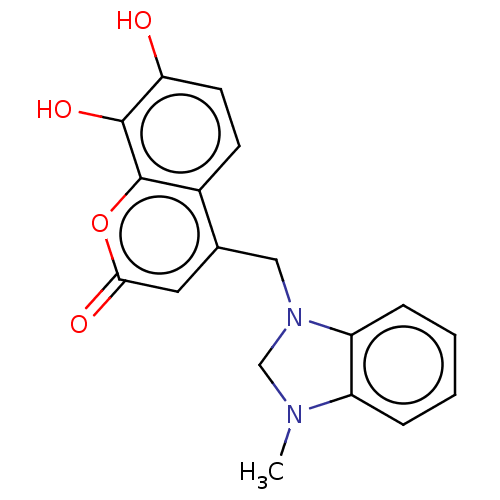 (1-Methyl-3-(4-methyl-7, 8-dihydroxy-2H-chromen-2-o...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.86E+4 | n/a | n/a | n/a | n/a | 10.0 | n/a |
Inonu University | Assay Description CA activity was measured by the Maren method which is based on determination of the time required for the pH to decrease from 10.0 to 7.4 due to CO2 ... | J Enzyme Inhib Med Chem 28: 299-304 (2013) Article DOI: 10.3109/14756366.2012.677838 BindingDB Entry DOI: 10.7270/Q2K0734K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM237362 (1-(3-Methylbenzyl)-3-(4-methyl-7,8-dihydroxy-2H-ch...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.89E+4 | n/a | n/a | n/a | n/a | 10.0 | n/a |
Inonu University | Assay Description CA activity was measured by the Maren method which is based on determination of the time required for the pH to decrease from 10.0 to 7.4 due to CO2 ... | J Enzyme Inhib Med Chem 28: 299-304 (2013) Article DOI: 10.3109/14756366.2012.677838 BindingDB Entry DOI: 10.7270/Q2K0734K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM237364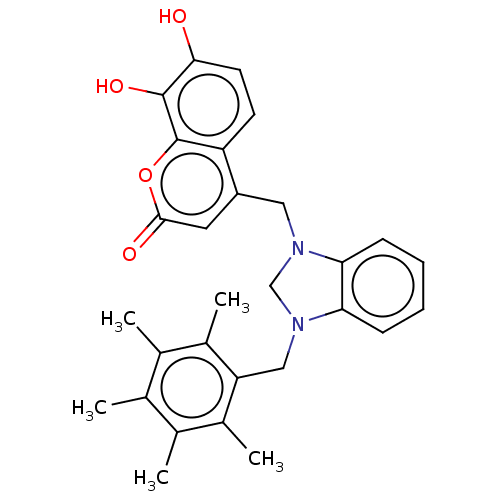 (1-(2,3,4,5,6-Pentamethylbenzyl)-3-(4-methyl-7,8-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | 10.0 | n/a |
Inonu University | Assay Description CA activity was measured by the Maren method which is based on determination of the time required for the pH to decrease from 10.0 to 7.4 due to CO2 ... | J Enzyme Inhib Med Chem 28: 299-304 (2013) Article DOI: 10.3109/14756366.2012.677838 BindingDB Entry DOI: 10.7270/Q2K0734K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serum paraoxonase/arylesterase 1 (Homo sapiens (Human)) | BDBM50144739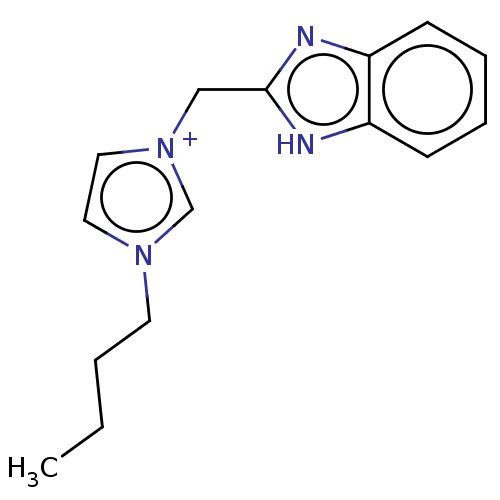 (CHEMBL3759516) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
In£n£ University Curated by ChEMBL | Assay Description Inhibition of human serum PON1 using paraoxon as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1392-401 (2016) Article DOI: 10.1016/j.bmc.2016.02.012 BindingDB Entry DOI: 10.7270/Q2FT8NWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM237364 (1-(2,3,4,5,6-Pentamethylbenzyl)-3-(4-methyl-7,8-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.95E+4 | n/a | n/a | n/a | n/a | 10.0 | n/a |
Inonu University | Assay Description CA activity was measured by the Maren method which is based on determination of the time required for the pH to decrease from 10.0 to 7.4 due to CO2 ... | J Enzyme Inhib Med Chem 28: 299-304 (2013) Article DOI: 10.3109/14756366.2012.677838 BindingDB Entry DOI: 10.7270/Q2K0734K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM237358 (1-Allyl-3-(4-methyl-7,8-dihydroxy-2H-chromen-2-one...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.97E+4 | n/a | n/a | n/a | n/a | 10.0 | n/a |
Inonu University | Assay Description CA activity was measured by the Maren method which is based on determination of the time required for the pH to decrease from 10.0 to 7.4 due to CO2 ... | J Enzyme Inhib Med Chem 28: 299-304 (2013) Article DOI: 10.3109/14756366.2012.677838 BindingDB Entry DOI: 10.7270/Q2K0734K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM237363 (1-(2,3,5,6-Tetramethylbenzyl)-3-(4-methyl-7,8-dihy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.12E+4 | n/a | n/a | n/a | n/a | 10.0 | n/a |
Inonu University | Assay Description CA activity was measured by the Maren method which is based on determination of the time required for the pH to decrease from 10.0 to 7.4 due to CO2 ... | J Enzyme Inhib Med Chem 28: 299-304 (2013) Article DOI: 10.3109/14756366.2012.677838 BindingDB Entry DOI: 10.7270/Q2K0734K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serum paraoxonase/arylesterase 1 (Homo sapiens (Human)) | BDBM50139132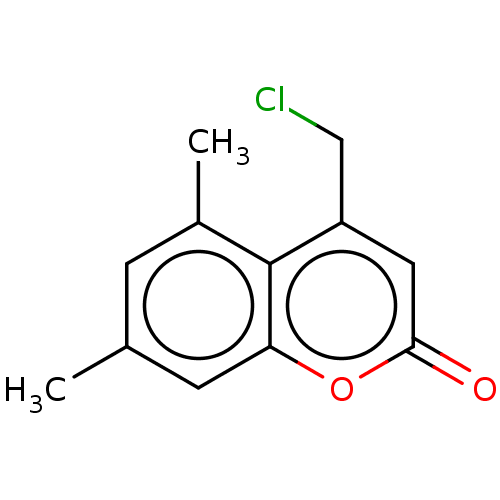 (CHEMBL1462074) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
In£n£ University Curated by ChEMBL | Assay Description Inhibition of human serum PON1 using paraoxon as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1392-401 (2016) Article DOI: 10.1016/j.bmc.2016.02.012 BindingDB Entry DOI: 10.7270/Q2FT8NWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM237359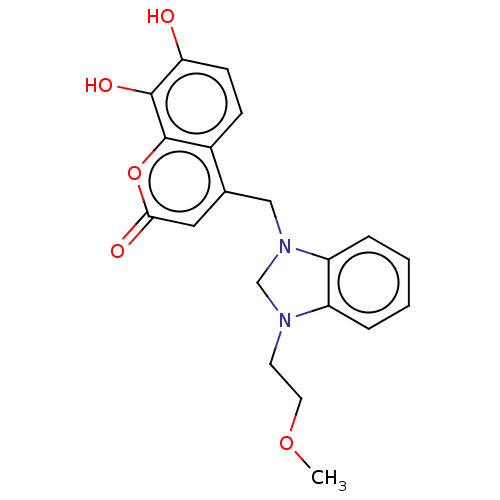 (1-(2-Methoxyethyl)-3-(4-methyl-7,8-dihydroxy-2H-ch...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.26E+4 | n/a | n/a | n/a | n/a | 10.0 | n/a |
Inonu University | Assay Description CA activity was measured by the Maren method which is based on determination of the time required for the pH to decrease from 10.0 to 7.4 due to CO2 ... | J Enzyme Inhib Med Chem 28: 299-304 (2013) Article DOI: 10.3109/14756366.2012.677838 BindingDB Entry DOI: 10.7270/Q2K0734K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM237360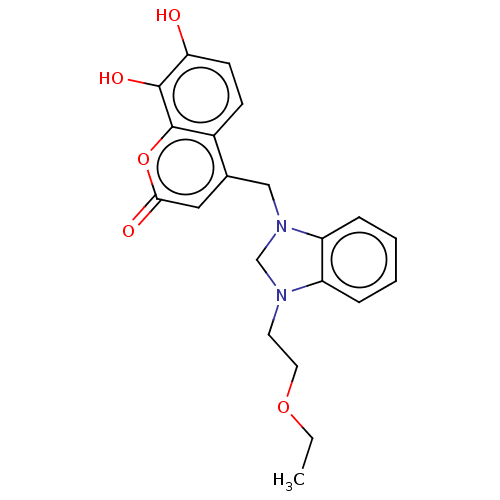 (1-(2-Ethoxyethyl)-3-(4-methyl-7,8-dihydroxy-2H-chr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.29E+4 | n/a | n/a | n/a | n/a | 10.0 | n/a |
Inonu University | Assay Description CA activity was measured by the Maren method which is based on determination of the time required for the pH to decrease from 10.0 to 7.4 due to CO2 ... | J Enzyme Inhib Med Chem 28: 299-304 (2013) Article DOI: 10.3109/14756366.2012.677838 BindingDB Entry DOI: 10.7270/Q2K0734K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM237365 (1-(3,4,5-Trimethoxybenzyl)-3-(4-methyl-7,8-dihydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.31E+4 | n/a | n/a | n/a | n/a | 10.0 | n/a |
Inonu University | Assay Description CA activity was measured by the Maren method which is based on determination of the time required for the pH to decrease from 10.0 to 7.4 due to CO2 ... | J Enzyme Inhib Med Chem 28: 299-304 (2013) Article DOI: 10.3109/14756366.2012.677838 BindingDB Entry DOI: 10.7270/Q2K0734K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM237360 (1-(2-Ethoxyethyl)-3-(4-methyl-7,8-dihydroxy-2H-chr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.32E+4 | n/a | n/a | n/a | n/a | 10.0 | n/a |
Inonu University | Assay Description CA activity was measured by the Maren method which is based on determination of the time required for the pH to decrease from 10.0 to 7.4 due to CO2 ... | J Enzyme Inhib Med Chem 28: 299-304 (2013) Article DOI: 10.3109/14756366.2012.677838 BindingDB Entry DOI: 10.7270/Q2K0734K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM237363 (1-(2,3,5,6-Tetramethylbenzyl)-3-(4-methyl-7,8-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.41E+4 | n/a | n/a | n/a | n/a | 10.0 | n/a |
Inonu University | Assay Description CA activity was measured by the Maren method which is based on determination of the time required for the pH to decrease from 10.0 to 7.4 due to CO2 ... | J Enzyme Inhib Med Chem 28: 299-304 (2013) Article DOI: 10.3109/14756366.2012.677838 BindingDB Entry DOI: 10.7270/Q2K0734K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serum paraoxonase/arylesterase 1 (Homo sapiens (Human)) | BDBM50139134 (CHEMBL3758686) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
In£n£ University Curated by ChEMBL | Assay Description Inhibition of human serum PON1 using paraoxon as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1392-401 (2016) Article DOI: 10.1016/j.bmc.2016.02.012 BindingDB Entry DOI: 10.7270/Q2FT8NWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM237361 (1-Benzyl-3-(4-methyl-7,8-dihydroxy-2H-chromen-2-on...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | 10.0 | n/a |
Inonu University | Assay Description CA activity was measured by the Maren method which is based on determination of the time required for the pH to decrease from 10.0 to 7.4 due to CO2 ... | J Enzyme Inhib Med Chem 28: 299-304 (2013) Article DOI: 10.3109/14756366.2012.677838 BindingDB Entry DOI: 10.7270/Q2K0734K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM237359 (1-(2-Methoxyethyl)-3-(4-methyl-7,8-dihydroxy-2H-ch...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.14E+4 | n/a | n/a | n/a | n/a | 10.0 | n/a |
Inonu University | Assay Description CA activity was measured by the Maren method which is based on determination of the time required for the pH to decrease from 10.0 to 7.4 due to CO2 ... | J Enzyme Inhib Med Chem 28: 299-304 (2013) Article DOI: 10.3109/14756366.2012.677838 BindingDB Entry DOI: 10.7270/Q2K0734K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM237358 (1-Allyl-3-(4-methyl-7,8-dihydroxy-2H-chromen-2-one...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.81E+4 | n/a | n/a | n/a | n/a | 10.0 | n/a |
Inonu University | Assay Description CA activity was measured by the Maren method which is based on determination of the time required for the pH to decrease from 10.0 to 7.4 due to CO2 ... | J Enzyme Inhib Med Chem 28: 299-304 (2013) Article DOI: 10.3109/14756366.2012.677838 BindingDB Entry DOI: 10.7270/Q2K0734K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM237357 (1-(n-Butyl)-3-(4-methyl-7,8-dihydroxy-2H-chromen-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.93E+4 | n/a | n/a | n/a | n/a | 10.0 | n/a |
Inonu University | Assay Description CA activity was measured by the Maren method which is based on determination of the time required for the pH to decrease from 10.0 to 7.4 due to CO2 ... | J Enzyme Inhib Med Chem 28: 299-304 (2013) Article DOI: 10.3109/14756366.2012.677838 BindingDB Entry DOI: 10.7270/Q2K0734K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM237356 (1-Methyl-3-(4-methyl-7, 8-dihydroxy-2H-chromen-2-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.94E+4 | n/a | n/a | n/a | n/a | 10.0 | n/a |
Inonu University | Assay Description CA activity was measured by the Maren method which is based on determination of the time required for the pH to decrease from 10.0 to 7.4 due to CO2 ... | J Enzyme Inhib Med Chem 28: 299-304 (2013) Article DOI: 10.3109/14756366.2012.677838 BindingDB Entry DOI: 10.7270/Q2K0734K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM237357 (1-(n-Butyl)-3-(4-methyl-7,8-dihydroxy-2H-chromen-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.15E+4 | n/a | n/a | n/a | n/a | 10.0 | n/a |
Inonu University | Assay Description CA activity was measured by the Maren method which is based on determination of the time required for the pH to decrease from 10.0 to 7.4 due to CO2 ... | J Enzyme Inhib Med Chem 28: 299-304 (2013) Article DOI: 10.3109/14756366.2012.677838 BindingDB Entry DOI: 10.7270/Q2K0734K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serum paraoxonase/arylesterase 1 (Homo sapiens (Human)) | BDBM50144690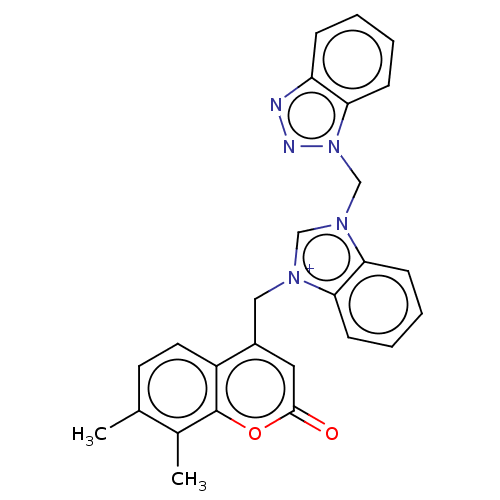 (CHEMBL3759001) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.38E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
In£n£ University Curated by ChEMBL | Assay Description Inhibition of human serum PON1 using paraoxon as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1392-401 (2016) Article DOI: 10.1016/j.bmc.2016.02.012 BindingDB Entry DOI: 10.7270/Q2FT8NWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serum paraoxonase/arylesterase 1 (Homo sapiens (Human)) | BDBM50139136 (CHEMBL3759337) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.17E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
In£n£ University Curated by ChEMBL | Assay Description Inhibition of human serum PON1 using paraoxon as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1392-401 (2016) Article DOI: 10.1016/j.bmc.2016.02.012 BindingDB Entry DOI: 10.7270/Q2FT8NWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serum paraoxonase/arylesterase 1 (Homo sapiens (Human)) | BDBM50144740 (CHEMBL3758357) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.64E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
In£n£ University Curated by ChEMBL | Assay Description Inhibition of human serum PON1 using paraoxon as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1392-401 (2016) Article DOI: 10.1016/j.bmc.2016.02.012 BindingDB Entry DOI: 10.7270/Q2FT8NWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serum paraoxonase/arylesterase 1 (Homo sapiens (Human)) | BDBM50144738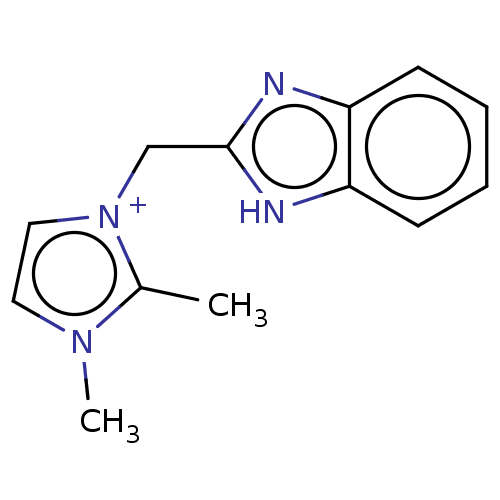 (CHEMBL3758838) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.26E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
In£n£ University Curated by ChEMBL | Assay Description Inhibition of human serum PON1 using paraoxon as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1392-401 (2016) Article DOI: 10.1016/j.bmc.2016.02.012 BindingDB Entry DOI: 10.7270/Q2FT8NWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serum paraoxonase/arylesterase 1 (Homo sapiens (Human)) | BDBM50144691 (CHEMBL3759697) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.27E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
In£n£ University Curated by ChEMBL | Assay Description Inhibition of human serum PON1 using paraoxon as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1392-401 (2016) Article DOI: 10.1016/j.bmc.2016.02.012 BindingDB Entry DOI: 10.7270/Q2FT8NWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serum paraoxonase/arylesterase 1 (Homo sapiens (Human)) | BDBM50144742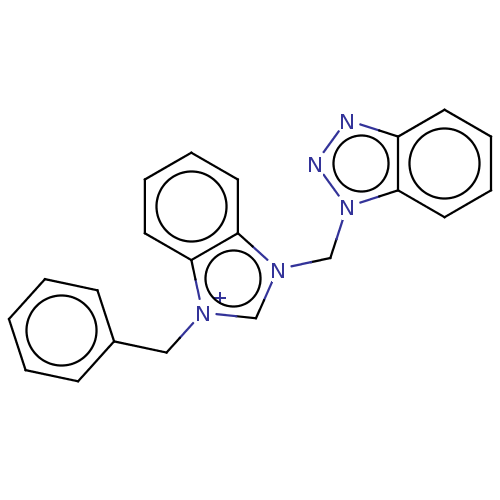 (CHEMBL3758332) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.06E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
In£n£ University Curated by ChEMBL | Assay Description Inhibition of human serum PON1 using paraoxon as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1392-401 (2016) Article DOI: 10.1016/j.bmc.2016.02.012 BindingDB Entry DOI: 10.7270/Q2FT8NWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serum paraoxonase/arylesterase 1 (Homo sapiens (Human)) | BDBM50144741 (CHEMBL3760112) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.33E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
In£n£ University Curated by ChEMBL | Assay Description Inhibition of human serum PON1 using paraoxon as substrate by spectrophotometric analysis | Bioorg Med Chem 24: 1392-401 (2016) Article DOI: 10.1016/j.bmc.2016.02.012 BindingDB Entry DOI: 10.7270/Q2FT8NWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||