

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50276903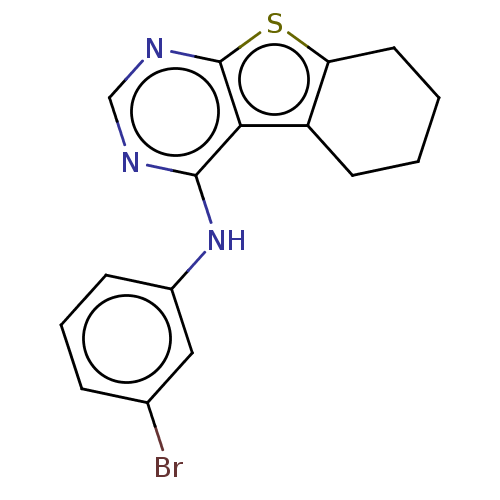 (CHEMBL4171764) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Inhibition of EGFR1 (unknown origin) after 1 hr in presence of adenosine 5'[gamma-33P]triphosphate by microbeta microplate counting method | Eur J Med Chem 138: 1053-1065 (2017) Article DOI: 10.1016/j.ejmech.2017.07.028 BindingDB Entry DOI: 10.7270/Q2794760 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2939 (4-(2-cyclopropylethynyl)-5-fluoro-4-(trifluorometh...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
S.G.S.I.T.S. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | Bioorg Med Chem Lett 14: 6089-94 (2004) Article DOI: 10.1016/j.bmcl.2004.09.068 BindingDB Entry DOI: 10.7270/Q2XS5TVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 5 activator 1 (Homo sapiens (Human)) | BDBM7401 ((2 Z,3 E)-6-Bromoindirubin-3 -oxime | (3E)-6-bromo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Rajiv Gandhi Proudyogiki Vishwavidyalaya Curated by ChEMBL | Assay Description inhibition of human recombinant CDK5/p25 using histone H1 as substrate by scintillation counting in presence of [gamma-33P]ATP | Bioorg Med Chem 22: 1909-15 (2014) Article DOI: 10.1016/j.bmc.2014.01.044 BindingDB Entry DOI: 10.7270/Q26D5VHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2942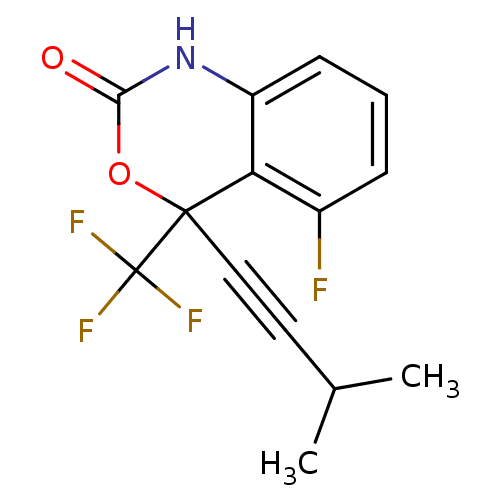 (5-fluoro-4-(3-methylbut-1-yn-1-yl)-4-(trifluoromet...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
S.G.S.I.T.S. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | Bioorg Med Chem Lett 14: 6089-94 (2004) Article DOI: 10.1016/j.bmcl.2004.09.068 BindingDB Entry DOI: 10.7270/Q2XS5TVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2940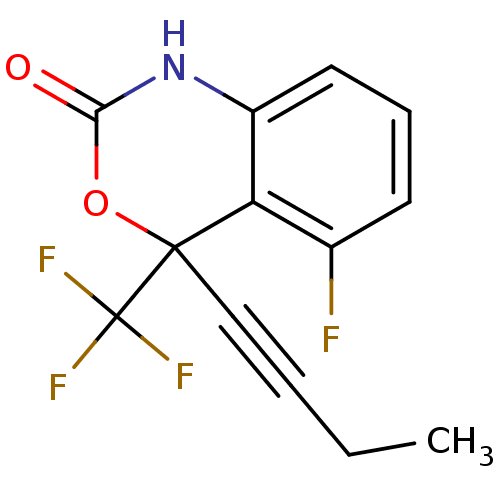 (4-(but-1-yn-1-yl)-5-fluoro-4-(trifluoromethyl)-2,4...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
S.G.S.I.T.S. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | Bioorg Med Chem Lett 14: 6089-94 (2004) Article DOI: 10.1016/j.bmcl.2004.09.068 BindingDB Entry DOI: 10.7270/Q2XS5TVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2941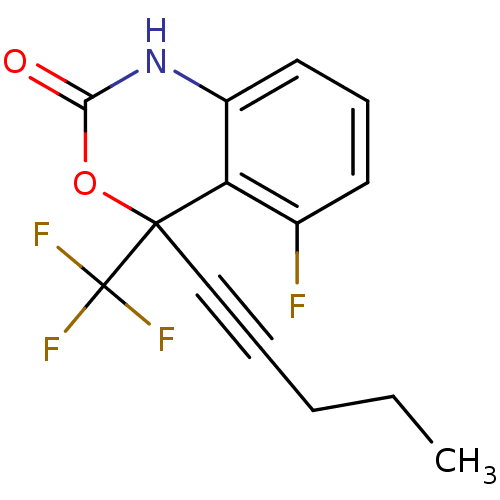 (5-fluoro-4-(pent-1-yn-1-yl)-4-(trifluoromethyl)-2,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 156 | n/a | n/a | n/a | n/a | n/a | n/a |
S.G.S.I.T.S. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | Bioorg Med Chem Lett 14: 6089-94 (2004) Article DOI: 10.1016/j.bmcl.2004.09.068 BindingDB Entry DOI: 10.7270/Q2XS5TVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2946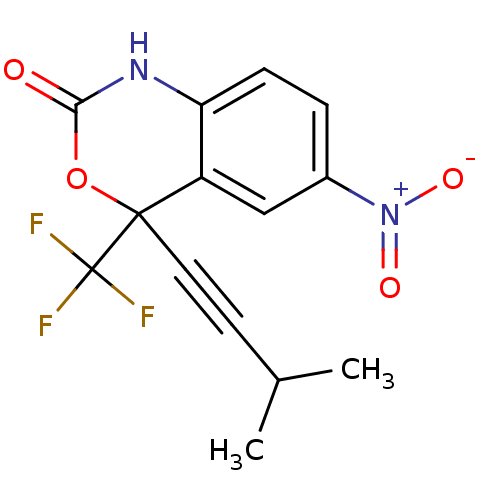 (4-(3-methylbut-1-yn-1-yl)-6-nitro-4-(trifluorometh...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 199 | n/a | n/a | n/a | n/a | n/a | n/a |
S.G.S.I.T.S. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | Bioorg Med Chem Lett 14: 6089-94 (2004) Article DOI: 10.1016/j.bmcl.2004.09.068 BindingDB Entry DOI: 10.7270/Q2XS5TVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2943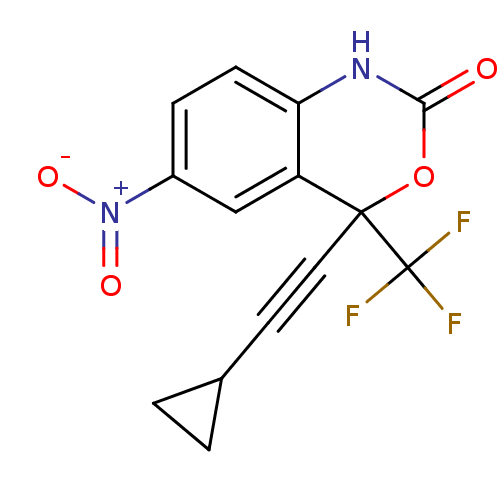 (4-(2-cyclopropylethynyl)-6-nitro-4-(trifluoromethy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 209 | n/a | n/a | n/a | n/a | n/a | n/a |
S.G.S.I.T.S. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | Bioorg Med Chem Lett 14: 6089-94 (2004) Article DOI: 10.1016/j.bmcl.2004.09.068 BindingDB Entry DOI: 10.7270/Q2XS5TVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2944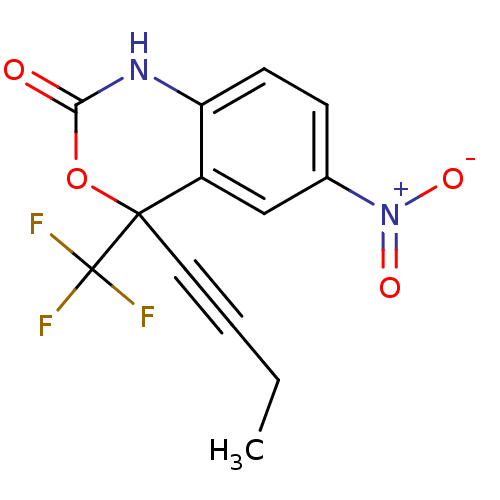 (4-(but-1-yn-1-yl)-6-nitro-4-(trifluoromethyl)-2,4-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 276 | n/a | n/a | n/a | n/a | n/a | n/a |
S.G.S.I.T.S. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | Bioorg Med Chem Lett 14: 6089-94 (2004) Article DOI: 10.1016/j.bmcl.2004.09.068 BindingDB Entry DOI: 10.7270/Q2XS5TVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2945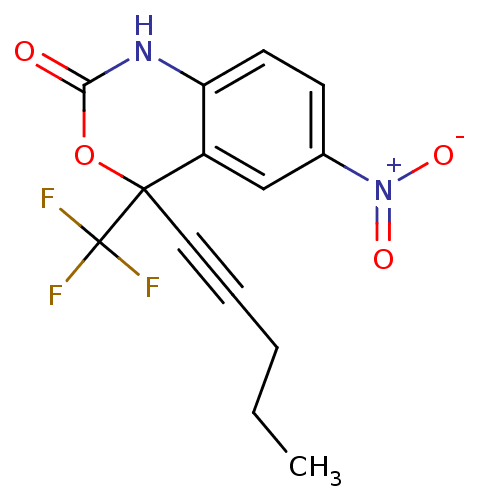 (6-nitro-4-(pent-1-yn-1-yl)-4-(trifluoromethyl)-2,4...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 304 | n/a | n/a | n/a | n/a | n/a | n/a |
S.G.S.I.T.S. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | Bioorg Med Chem Lett 14: 6089-94 (2004) Article DOI: 10.1016/j.bmcl.2004.09.068 BindingDB Entry DOI: 10.7270/Q2XS5TVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2952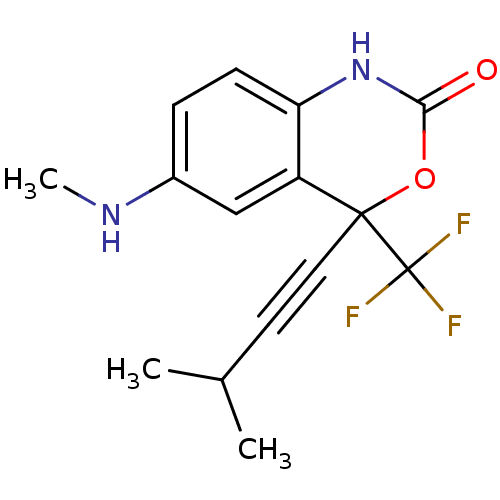 (6-(methylamino)-4-(3-methylbut-1-yn-1-yl)-4-(trifl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 473 | n/a | n/a | n/a | n/a | n/a | n/a |
S.G.S.I.T.S. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | Bioorg Med Chem Lett 14: 6089-94 (2004) Article DOI: 10.1016/j.bmcl.2004.09.068 BindingDB Entry DOI: 10.7270/Q2XS5TVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (RAT) | BDBM7401 ((2 Z,3 E)-6-Bromoindirubin-3 -oxime | (3E)-6-bromo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Rajiv Gandhi Proudyogiki Vishwavidyalaya Curated by ChEMBL | Assay Description Inhibition of rat recombinant GST-fused DYRK1A expressed in Escherichia coli using KKISGRLSPIMTEQ as substrate by scintillation counting in presence ... | Bioorg Med Chem 22: 1909-15 (2014) Article DOI: 10.1016/j.bmc.2014.01.044 BindingDB Entry DOI: 10.7270/Q26D5VHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2951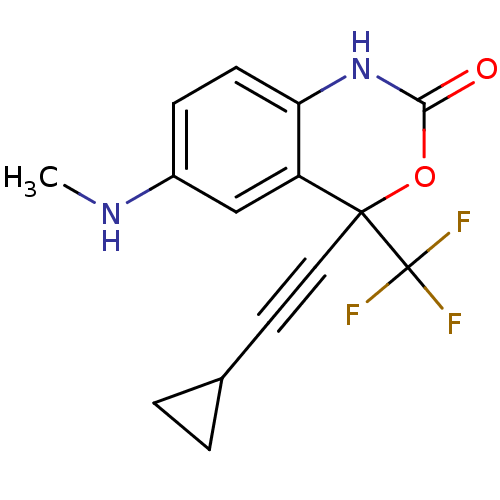 (4-(2-cyclopropylethynyl)-6-(methylamino)-4-(triflu...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 608 | n/a | n/a | n/a | n/a | n/a | n/a |
S.G.S.I.T.S. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | Bioorg Med Chem Lett 14: 6089-94 (2004) Article DOI: 10.1016/j.bmcl.2004.09.068 BindingDB Entry DOI: 10.7270/Q2XS5TVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2947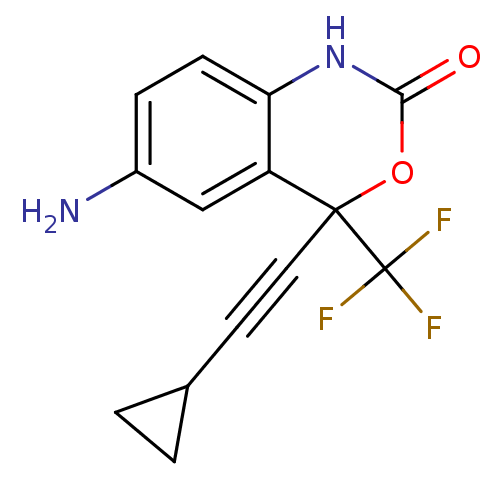 (6-amino-4-(2-cyclopropylethynyl)-4-(trifluoromethy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 802 | n/a | n/a | n/a | n/a | n/a | n/a |
S.G.S.I.T.S. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | Bioorg Med Chem Lett 14: 6089-94 (2004) Article DOI: 10.1016/j.bmcl.2004.09.068 BindingDB Entry DOI: 10.7270/Q2XS5TVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2950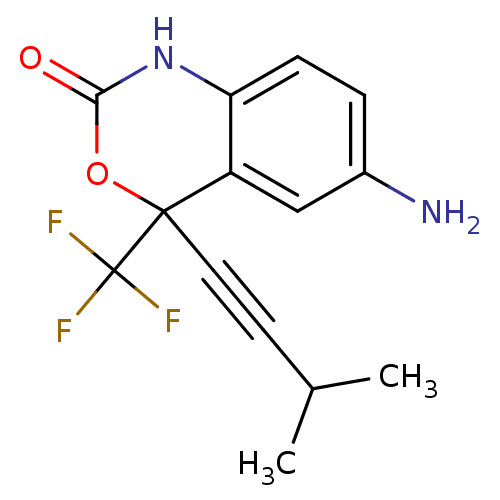 (6-amino-4-(3-methylbut-1-yn-1-yl)-4-(trifluorometh...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 896 | n/a | n/a | n/a | n/a | n/a | n/a |
S.G.S.I.T.S. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | Bioorg Med Chem Lett 14: 6089-94 (2004) Article DOI: 10.1016/j.bmcl.2004.09.068 BindingDB Entry DOI: 10.7270/Q2XS5TVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK1 (Homo sapiens (Human)) | BDBM50290835 ((3,4-Dimethoxy-phenyl)-(6,7-dimethoxy-quinazolin-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rajiv Gandhi Proudyogiki Vishwavidyalaya Curated by ChEMBL | Assay Description Inhibition of human recombinant GST-fused CLK1 expressed in Escherichia coli using GRSRSRSRSRSR as substrate | Bioorg Med Chem 22: 1909-15 (2014) Article DOI: 10.1016/j.bmc.2014.01.044 BindingDB Entry DOI: 10.7270/Q26D5VHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2949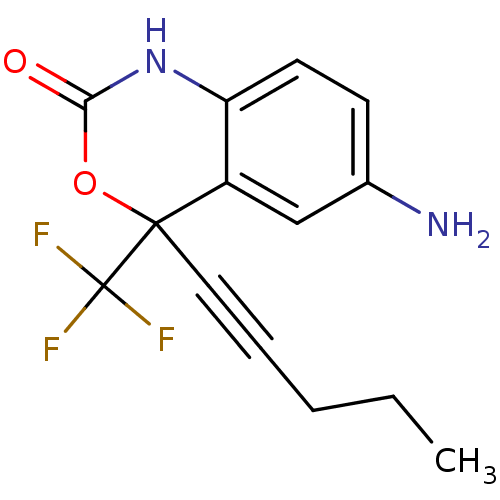 (6-amino-4-(pent-1-yn-1-yl)-4-(trifluoromethyl)-2,4...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
S.G.S.I.T.S. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | Bioorg Med Chem Lett 14: 6089-94 (2004) Article DOI: 10.1016/j.bmcl.2004.09.068 BindingDB Entry DOI: 10.7270/Q2XS5TVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2948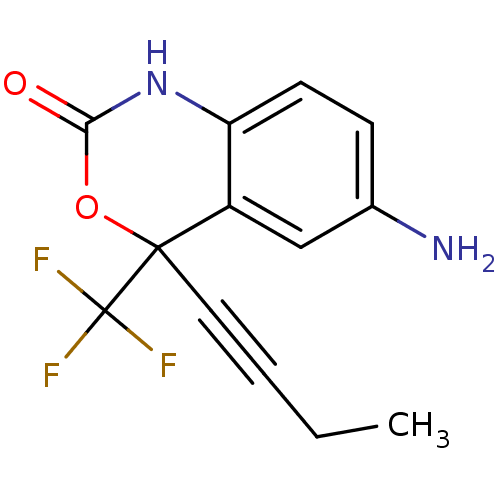 (6-amino-4-(but-1-yn-1-yl)-4-(trifluoromethyl)-2,4-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
S.G.S.I.T.S. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | Bioorg Med Chem Lett 14: 6089-94 (2004) Article DOI: 10.1016/j.bmcl.2004.09.068 BindingDB Entry DOI: 10.7270/Q2XS5TVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2954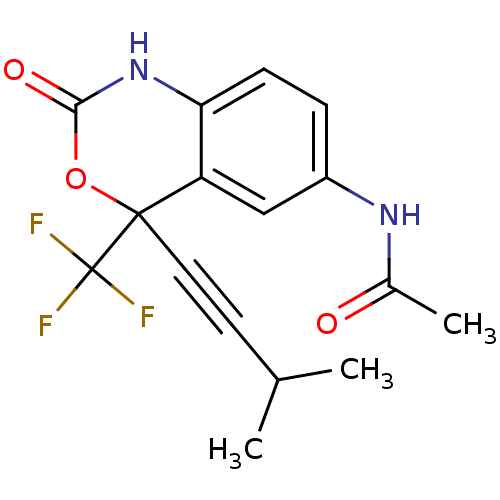 (Benzoxazinone deriv. 8b | CHEMBL111598 | N-[4-(3-m...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
S.G.S.I.T.S. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | Bioorg Med Chem Lett 14: 6089-94 (2004) Article DOI: 10.1016/j.bmcl.2004.09.068 BindingDB Entry DOI: 10.7270/Q2XS5TVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2953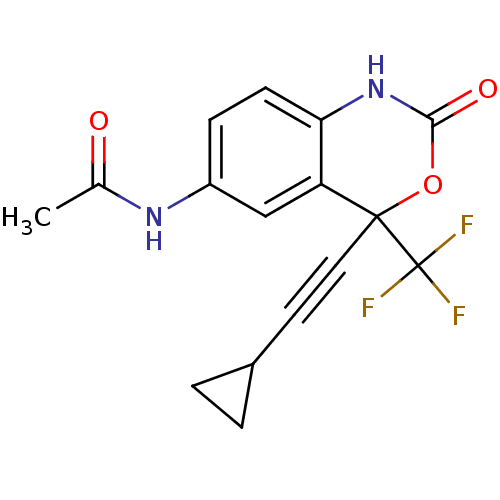 (Benzoxazinone deriv. 8a | CHEMBL111492 | N-[4-(2-c...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
S.G.S.I.T.S. Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | Bioorg Med Chem Lett 14: 6089-94 (2004) Article DOI: 10.1016/j.bmcl.2004.09.068 BindingDB Entry DOI: 10.7270/Q2XS5TVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK1 (Homo sapiens (Human)) | BDBM7401 ((2 Z,3 E)-6-Bromoindirubin-3 -oxime | (3E)-6-bromo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rajiv Gandhi Proudyogiki Vishwavidyalaya Curated by ChEMBL | Assay Description Inhibition of human recombinant GST-fused CLK1 expressed in Escherichia coli using GRSRSRSRSRSR as substrate | Bioorg Med Chem 22: 1909-15 (2014) Article DOI: 10.1016/j.bmc.2014.01.044 BindingDB Entry DOI: 10.7270/Q26D5VHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK1 (Homo sapiens (Human)) | BDBM4626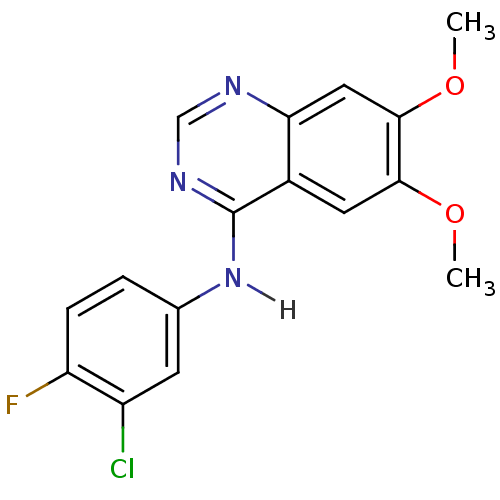 (Anilinoquinazoline deriv. 9 | CHEMBL301018 | N-(3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rajiv Gandhi Proudyogiki Vishwavidyalaya Curated by ChEMBL | Assay Description Inhibition of human recombinant GST-fused CLK1 expressed in Escherichia coli using GRSRSRSRSRSR as substrate | Bioorg Med Chem 22: 1909-15 (2014) Article DOI: 10.1016/j.bmc.2014.01.044 BindingDB Entry DOI: 10.7270/Q26D5VHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50443464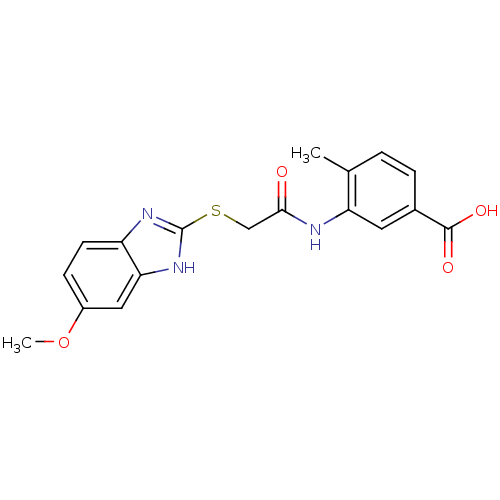 (CHEMBL259888) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B by malachite green assay | Eur J Med Chem 70: 469-76 (2013) Article DOI: 10.1016/j.ejmech.2013.10.030 BindingDB Entry DOI: 10.7270/Q20R9QWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50443463 (CHEMBL406596) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B by malachite green assay | Eur J Med Chem 70: 469-76 (2013) Article DOI: 10.1016/j.ejmech.2013.10.030 BindingDB Entry DOI: 10.7270/Q20R9QWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50443461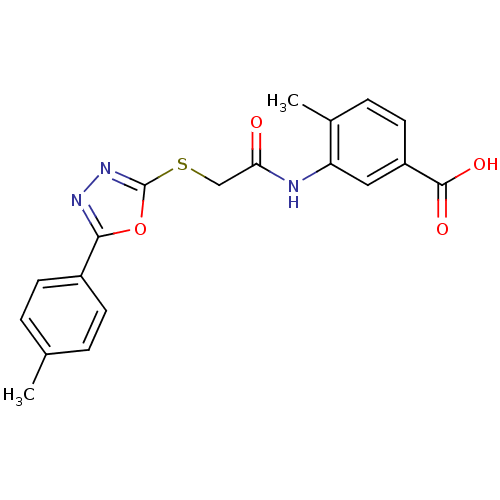 (CHEMBL3087456) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B by malachite green assay | Eur J Med Chem 70: 469-76 (2013) Article DOI: 10.1016/j.ejmech.2013.10.030 BindingDB Entry DOI: 10.7270/Q20R9QWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50443460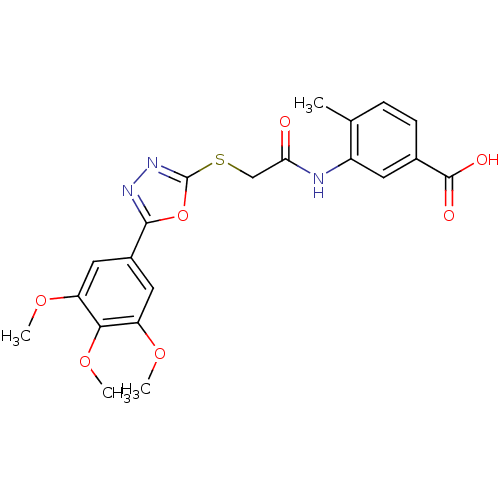 (CHEMBL3087457) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B by malachite green assay | Eur J Med Chem 70: 469-76 (2013) Article DOI: 10.1016/j.ejmech.2013.10.030 BindingDB Entry DOI: 10.7270/Q20R9QWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 5 activator 1 (Homo sapiens (Human)) | BDBM50449207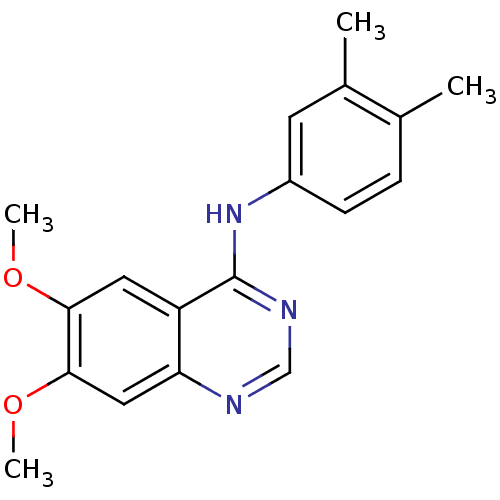 (CHEMBL3127768) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rajiv Gandhi Proudyogiki Vishwavidyalaya Curated by ChEMBL | Assay Description inhibition of human recombinant CDK5/p25 using histone H1 as substrate by scintillation counting in presence of [gamma-33P]ATP | Bioorg Med Chem 22: 1909-15 (2014) Article DOI: 10.1016/j.bmc.2014.01.044 BindingDB Entry DOI: 10.7270/Q26D5VHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 5 activator 1 (Homo sapiens (Human)) | BDBM3534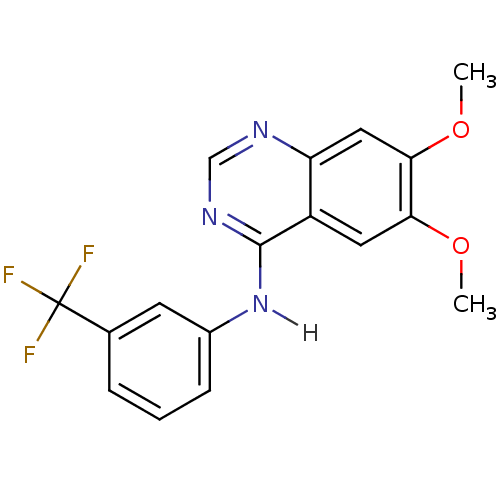 (6,7-dimethoxy-N-[3-(trifluoromethyl)phenyl]quinazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rajiv Gandhi Proudyogiki Vishwavidyalaya Curated by ChEMBL | Assay Description inhibition of human recombinant CDK5/p25 using histone H1 as substrate by scintillation counting in presence of [gamma-33P]ATP | Bioorg Med Chem 22: 1909-15 (2014) Article DOI: 10.1016/j.bmc.2014.01.044 BindingDB Entry DOI: 10.7270/Q26D5VHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 5 activator 1 (Homo sapiens (Human)) | BDBM3530 (6,7-dimethoxy-N-phenylquinazolin-4-amine | CHEMBL5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rajiv Gandhi Proudyogiki Vishwavidyalaya Curated by ChEMBL | Assay Description inhibition of human recombinant CDK5/p25 using histone H1 as substrate by scintillation counting in presence of [gamma-33P]ATP | Bioorg Med Chem 22: 1909-15 (2014) Article DOI: 10.1016/j.bmc.2014.01.044 BindingDB Entry DOI: 10.7270/Q26D5VHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK1 (Homo sapiens (Human)) | BDBM50449204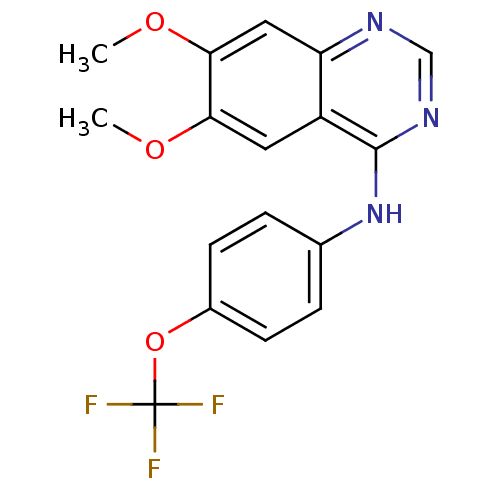 (CHEMBL1719330) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rajiv Gandhi Proudyogiki Vishwavidyalaya Curated by ChEMBL | Assay Description Inhibition of human recombinant GST-fused CLK1 expressed in Escherichia coli using GRSRSRSRSRSR as substrate | Bioorg Med Chem 22: 1909-15 (2014) Article DOI: 10.1016/j.bmc.2014.01.044 BindingDB Entry DOI: 10.7270/Q26D5VHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK1 (Homo sapiens (Human)) | BDBM50449205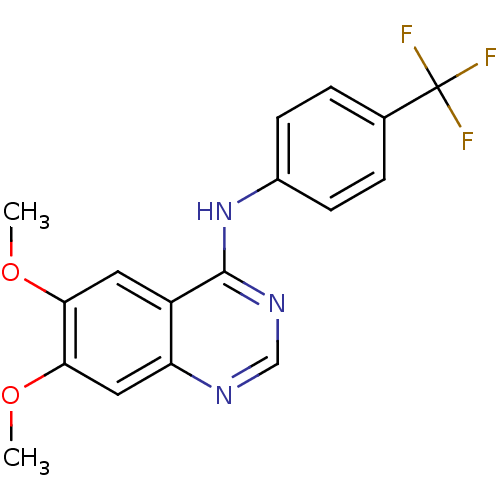 (CHEMBL1277433) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rajiv Gandhi Proudyogiki Vishwavidyalaya Curated by ChEMBL | Assay Description Inhibition of human recombinant GST-fused CLK1 expressed in Escherichia coli using GRSRSRSRSRSR as substrate | Bioorg Med Chem 22: 1909-15 (2014) Article DOI: 10.1016/j.bmc.2014.01.044 BindingDB Entry DOI: 10.7270/Q26D5VHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK1 (Homo sapiens (Human)) | BDBM50340921 (CHEMBL1761927 | N-(4-(6,7-dimethoxyquinazolin-4-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rajiv Gandhi Proudyogiki Vishwavidyalaya Curated by ChEMBL | Assay Description Inhibition of human recombinant GST-fused CLK1 expressed in Escherichia coli using GRSRSRSRSRSR as substrate | Bioorg Med Chem 22: 1909-15 (2014) Article DOI: 10.1016/j.bmc.2014.01.044 BindingDB Entry DOI: 10.7270/Q26D5VHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK1 (Homo sapiens (Human)) | BDBM50291090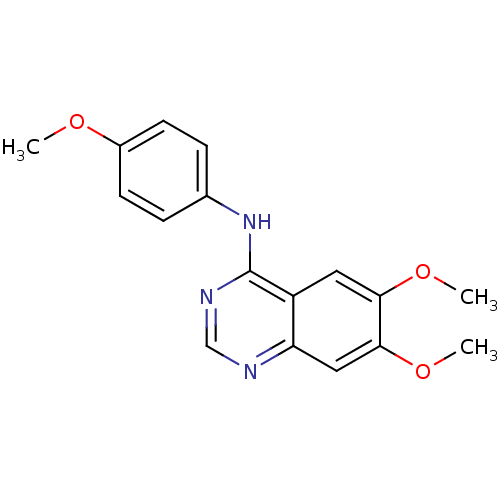 ((6,7-Dimethoxy-quinazolin-4-yl)-p-tolyl-amine; hyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rajiv Gandhi Proudyogiki Vishwavidyalaya Curated by ChEMBL | Assay Description Inhibition of human recombinant GST-fused CLK1 expressed in Escherichia coli using GRSRSRSRSRSR as substrate | Bioorg Med Chem 22: 1909-15 (2014) Article DOI: 10.1016/j.bmc.2014.01.044 BindingDB Entry DOI: 10.7270/Q26D5VHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK1 (Homo sapiens (Human)) | BDBM50449207 (CHEMBL3127768) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rajiv Gandhi Proudyogiki Vishwavidyalaya Curated by ChEMBL | Assay Description Inhibition of human recombinant GST-fused CLK1 expressed in Escherichia coli using GRSRSRSRSRSR as substrate | Bioorg Med Chem 22: 1909-15 (2014) Article DOI: 10.1016/j.bmc.2014.01.044 BindingDB Entry DOI: 10.7270/Q26D5VHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK1 (Homo sapiens (Human)) | BDBM3534 (6,7-dimethoxy-N-[3-(trifluoromethyl)phenyl]quinazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rajiv Gandhi Proudyogiki Vishwavidyalaya Curated by ChEMBL | Assay Description Inhibition of human recombinant GST-fused CLK1 expressed in Escherichia coli using GRSRSRSRSRSR as substrate | Bioorg Med Chem 22: 1909-15 (2014) Article DOI: 10.1016/j.bmc.2014.01.044 BindingDB Entry DOI: 10.7270/Q26D5VHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (RAT) | BDBM50449204 (CHEMBL1719330) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rajiv Gandhi Proudyogiki Vishwavidyalaya Curated by ChEMBL | Assay Description Inhibition of rat recombinant GST-fused DYRK1A expressed in Escherichia coli using KKISGRLSPIMTEQ as substrate by scintillation counting in presence ... | Bioorg Med Chem 22: 1909-15 (2014) Article DOI: 10.1016/j.bmc.2014.01.044 BindingDB Entry DOI: 10.7270/Q26D5VHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (RAT) | BDBM50449205 (CHEMBL1277433) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rajiv Gandhi Proudyogiki Vishwavidyalaya Curated by ChEMBL | Assay Description Inhibition of rat recombinant GST-fused DYRK1A expressed in Escherichia coli using KKISGRLSPIMTEQ as substrate by scintillation counting in presence ... | Bioorg Med Chem 22: 1909-15 (2014) Article DOI: 10.1016/j.bmc.2014.01.044 BindingDB Entry DOI: 10.7270/Q26D5VHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (RAT) | BDBM4626 (Anilinoquinazoline deriv. 9 | CHEMBL301018 | N-(3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rajiv Gandhi Proudyogiki Vishwavidyalaya Curated by ChEMBL | Assay Description Inhibition of rat recombinant GST-fused DYRK1A expressed in Escherichia coli using KKISGRLSPIMTEQ as substrate by scintillation counting in presence ... | Bioorg Med Chem 22: 1909-15 (2014) Article DOI: 10.1016/j.bmc.2014.01.044 BindingDB Entry DOI: 10.7270/Q26D5VHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (RAT) | BDBM50340921 (CHEMBL1761927 | N-(4-(6,7-dimethoxyquinazolin-4-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rajiv Gandhi Proudyogiki Vishwavidyalaya Curated by ChEMBL | Assay Description Inhibition of rat recombinant GST-fused DYRK1A expressed in Escherichia coli using KKISGRLSPIMTEQ as substrate by scintillation counting in presence ... | Bioorg Med Chem 22: 1909-15 (2014) Article DOI: 10.1016/j.bmc.2014.01.044 BindingDB Entry DOI: 10.7270/Q26D5VHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (RAT) | BDBM50291090 ((6,7-Dimethoxy-quinazolin-4-yl)-p-tolyl-amine; hyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rajiv Gandhi Proudyogiki Vishwavidyalaya Curated by ChEMBL | Assay Description Inhibition of rat recombinant GST-fused DYRK1A expressed in Escherichia coli using KKISGRLSPIMTEQ as substrate by scintillation counting in presence ... | Bioorg Med Chem 22: 1909-15 (2014) Article DOI: 10.1016/j.bmc.2014.01.044 BindingDB Entry DOI: 10.7270/Q26D5VHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (RAT) | BDBM50449208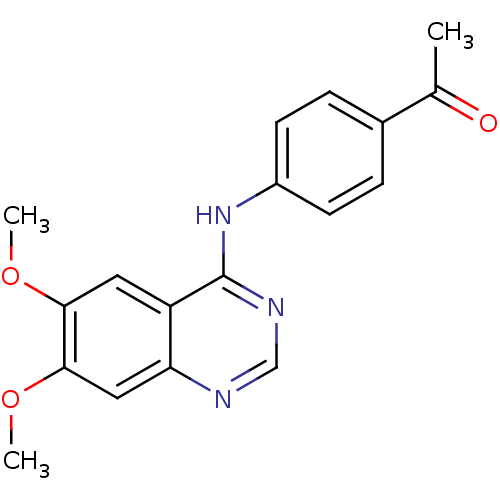 (CHEMBL3127769) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rajiv Gandhi Proudyogiki Vishwavidyalaya Curated by ChEMBL | Assay Description Inhibition of rat recombinant GST-fused DYRK1A expressed in Escherichia coli using KKISGRLSPIMTEQ as substrate by scintillation counting in presence ... | Bioorg Med Chem 22: 1909-15 (2014) Article DOI: 10.1016/j.bmc.2014.01.044 BindingDB Entry DOI: 10.7270/Q26D5VHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (RAT) | BDBM50449207 (CHEMBL3127768) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rajiv Gandhi Proudyogiki Vishwavidyalaya Curated by ChEMBL | Assay Description Inhibition of rat recombinant GST-fused DYRK1A expressed in Escherichia coli using KKISGRLSPIMTEQ as substrate by scintillation counting in presence ... | Bioorg Med Chem 22: 1909-15 (2014) Article DOI: 10.1016/j.bmc.2014.01.044 BindingDB Entry DOI: 10.7270/Q26D5VHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (RAT) | BDBM3534 (6,7-dimethoxy-N-[3-(trifluoromethyl)phenyl]quinazo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rajiv Gandhi Proudyogiki Vishwavidyalaya Curated by ChEMBL | Assay Description Inhibition of rat recombinant GST-fused DYRK1A expressed in Escherichia coli using KKISGRLSPIMTEQ as substrate by scintillation counting in presence ... | Bioorg Med Chem 22: 1909-15 (2014) Article DOI: 10.1016/j.bmc.2014.01.044 BindingDB Entry DOI: 10.7270/Q26D5VHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (RAT) | BDBM3530 (6,7-dimethoxy-N-phenylquinazolin-4-amine | CHEMBL5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rajiv Gandhi Proudyogiki Vishwavidyalaya Curated by ChEMBL | Assay Description Inhibition of rat recombinant GST-fused DYRK1A expressed in Escherichia coli using KKISGRLSPIMTEQ as substrate by scintillation counting in presence ... | Bioorg Med Chem 22: 1909-15 (2014) Article DOI: 10.1016/j.bmc.2014.01.044 BindingDB Entry DOI: 10.7270/Q26D5VHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 5 activator 1 (Homo sapiens (Human)) | BDBM50449204 (CHEMBL1719330) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rajiv Gandhi Proudyogiki Vishwavidyalaya Curated by ChEMBL | Assay Description inhibition of human recombinant CDK5/p25 using histone H1 as substrate by scintillation counting in presence of [gamma-33P]ATP | Bioorg Med Chem 22: 1909-15 (2014) Article DOI: 10.1016/j.bmc.2014.01.044 BindingDB Entry DOI: 10.7270/Q26D5VHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 5 activator 1 (Homo sapiens (Human)) | BDBM50449205 (CHEMBL1277433) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rajiv Gandhi Proudyogiki Vishwavidyalaya Curated by ChEMBL | Assay Description inhibition of human recombinant CDK5/p25 using histone H1 as substrate by scintillation counting in presence of [gamma-33P]ATP | Bioorg Med Chem 22: 1909-15 (2014) Article DOI: 10.1016/j.bmc.2014.01.044 BindingDB Entry DOI: 10.7270/Q26D5VHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 5 activator 1 (Homo sapiens (Human)) | BDBM4626 (Anilinoquinazoline deriv. 9 | CHEMBL301018 | N-(3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rajiv Gandhi Proudyogiki Vishwavidyalaya Curated by ChEMBL | Assay Description inhibition of human recombinant CDK5/p25 using histone H1 as substrate by scintillation counting in presence of [gamma-33P]ATP | Bioorg Med Chem 22: 1909-15 (2014) Article DOI: 10.1016/j.bmc.2014.01.044 BindingDB Entry DOI: 10.7270/Q26D5VHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 5 activator 1 (Homo sapiens (Human)) | BDBM50340921 (CHEMBL1761927 | N-(4-(6,7-dimethoxyquinazolin-4-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rajiv Gandhi Proudyogiki Vishwavidyalaya Curated by ChEMBL | Assay Description inhibition of human recombinant CDK5/p25 using histone H1 as substrate by scintillation counting in presence of [gamma-33P]ATP | Bioorg Med Chem 22: 1909-15 (2014) Article DOI: 10.1016/j.bmc.2014.01.044 BindingDB Entry DOI: 10.7270/Q26D5VHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 5 activator 1 (Homo sapiens (Human)) | BDBM50291090 ((6,7-Dimethoxy-quinazolin-4-yl)-p-tolyl-amine; hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rajiv Gandhi Proudyogiki Vishwavidyalaya Curated by ChEMBL | Assay Description inhibition of human recombinant CDK5/p25 using histone H1 as substrate by scintillation counting in presence of [gamma-33P]ATP | Bioorg Med Chem 22: 1909-15 (2014) Article DOI: 10.1016/j.bmc.2014.01.044 BindingDB Entry DOI: 10.7270/Q26D5VHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 5 activator 1 (Homo sapiens (Human)) | BDBM50290835 ((3,4-Dimethoxy-phenyl)-(6,7-dimethoxy-quinazolin-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rajiv Gandhi Proudyogiki Vishwavidyalaya Curated by ChEMBL | Assay Description inhibition of human recombinant CDK5/p25 using histone H1 as substrate by scintillation counting in presence of [gamma-33P]ATP | Bioorg Med Chem 22: 1909-15 (2014) Article DOI: 10.1016/j.bmc.2014.01.044 BindingDB Entry DOI: 10.7270/Q26D5VHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 90 total ) | Next | Last >> |