 Found 371 hits with Last Name = 'kawatkar' and Initial = 'sp'
Found 371 hits with Last Name = 'kawatkar' and Initial = 'sp' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84465
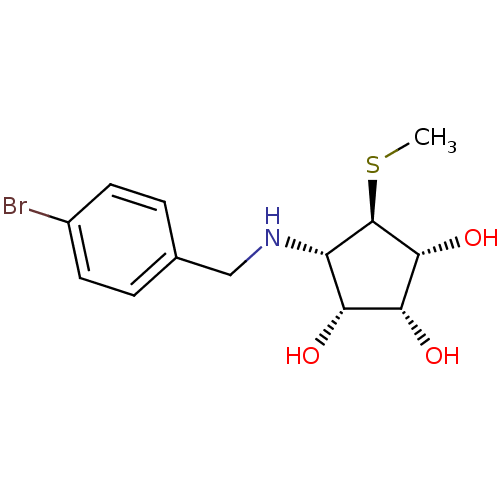
(Benzylation of mannostatin A, 1e)Show SMILES CS[C@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H]1NCc1ccc(Br)cc1 |r| Show InChI InChI=1S/C13H18BrNO3S/c1-19-13-9(10(16)11(17)12(13)18)15-6-7-2-4-8(14)5-3-7/h2-5,9-13,15-18H,6H2,1H3/t9-,10+,11+,12+,13+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | -43.3 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM50078117
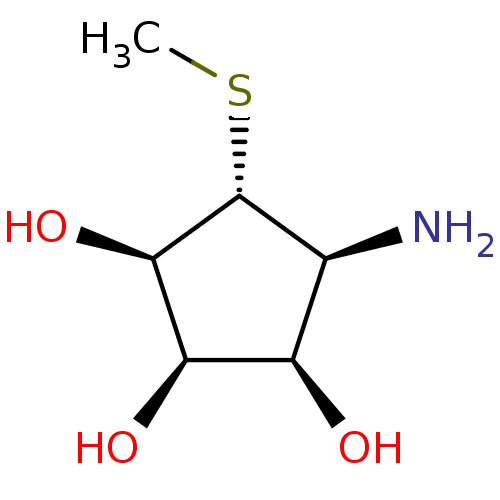
((1R,2R,3R,4S,5R)-4-AMINO-5-(METHYLTHIO)CYCLOPENTAN...)Show SMILES CS[C@@H]1[C@@H](N)[C@@H](O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C6H13NO3S/c1-11-6-2(7)3(8)4(9)5(6)10/h2-6,8-10H,7H2,1H3/t2-,3+,4+,5+,6+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 90 | -41.8 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84464
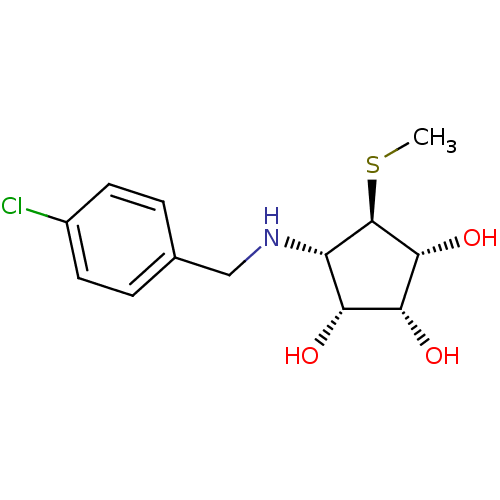
(Benzylation of mannostatin A, 1d)Show SMILES CS[C@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H]1NCc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C13H18ClNO3S/c1-19-13-9(10(16)11(17)12(13)18)15-6-7-2-4-8(14)5-3-7/h2-5,9-13,15-18H,6H2,1H3/t9-,10+,11+,12+,13+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | -41.6 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84466
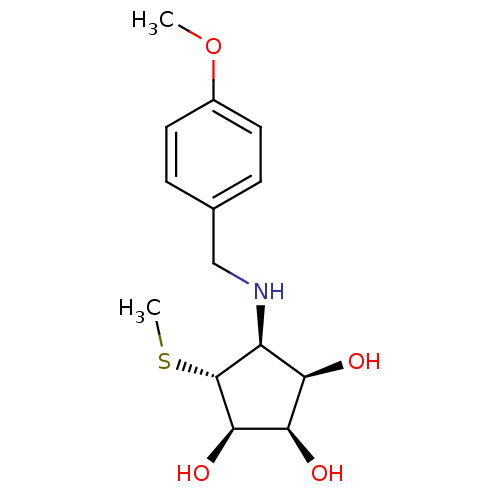
(Benzylation of mannostatin A, 1f)Show SMILES COc1ccc(CN[C@H]2[C@@H](O)[C@@H](O)[C@@H](O)[C@@H]2SC)cc1 |r| Show InChI InChI=1S/C14H21NO4S/c1-19-9-5-3-8(4-6-9)7-15-10-11(16)12(17)13(18)14(10)20-2/h3-6,10-18H,7H2,1-2H3/t10-,11+,12+,13+,14+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | -41.6 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84462
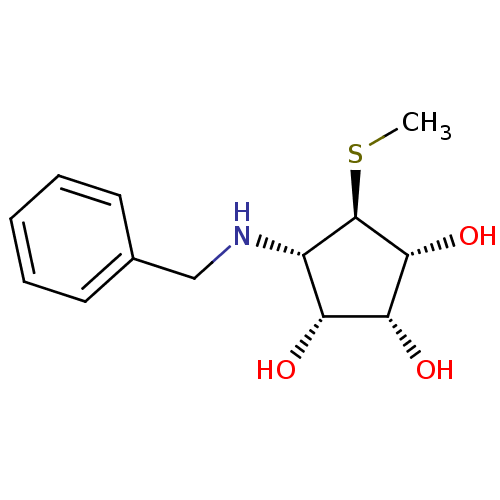
(Benzylation of mannostatin A, 1b)Show SMILES CS[C@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H]1NCc1ccccc1 |r| Show InChI InChI=1S/C13H19NO3S/c1-18-13-9(10(15)11(16)12(13)17)14-7-8-5-3-2-4-6-8/h2-6,9-17H,7H2,1H3/t9-,10+,11+,12+,13+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 110 | -41.3 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84467

(Benzylation of mannostatin A, 1g)Show SMILES CS[C@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H]1NCc1ccc(cc1)C(=O)OCC=C |r| Show InChI InChI=1S/C17H23NO5S/c1-3-8-23-17(22)11-6-4-10(5-7-11)9-18-12-13(19)14(20)15(21)16(12)24-2/h3-7,12-16,18-21H,1,8-9H2,2H3/t12-,13+,14+,15+,16+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 140 | -40.7 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84474
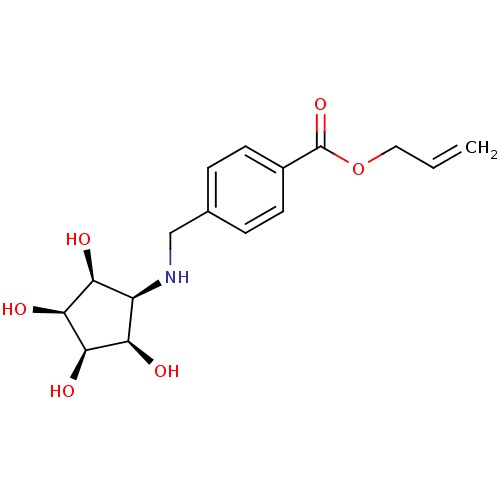
(Aminocyclopentitetrol, 2g)Show SMILES O[C@H]1[C@@H](O)[C@@H](O)[C@@H](NCc2ccc(cc2)C(=O)OCC=C)[C@H]1O |r| Show InChI InChI=1S/C16H21NO6/c1-2-7-23-16(22)10-5-3-9(4-6-10)8-17-11-12(18)14(20)15(21)13(11)19/h2-6,11-15,17-21H,1,7-8H2/t11-,12+,13-,14+,15- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 160 | -40.3 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84463
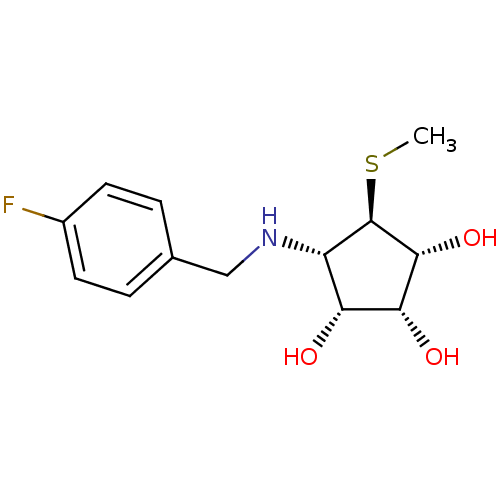
(Benzylation of mannostatin A, 1c)Show SMILES CS[C@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H]1NCc1ccc(F)cc1 |r| Show InChI InChI=1S/C13H18FNO3S/c1-19-13-9(10(16)11(17)12(13)18)15-6-7-2-4-8(14)5-3-7/h2-5,9-13,15-18H,6H2,1H3/t9-,10+,11+,12+,13+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | -40.2 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM50078117

((1R,2R,3R,4S,5R)-4-AMINO-5-(METHYLTHIO)CYCLOPENTAN...)Show SMILES CS[C@@H]1[C@@H](N)[C@@H](O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C6H13NO3S/c1-11-6-2(7)3(8)4(9)5(6)10/h2-6,8-10H,7H2,1H3/t2-,3+,4+,5+,6+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 210 | -39.6 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84470
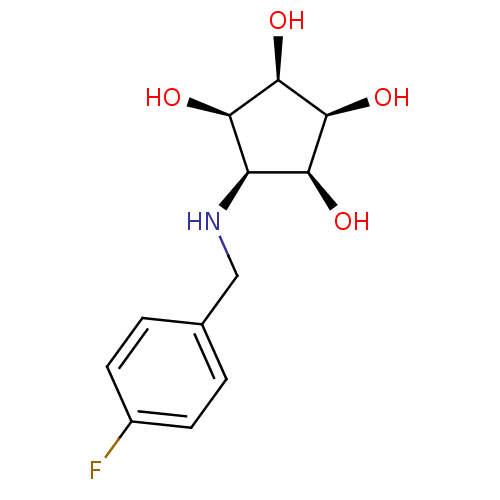
(Aminocyclopentitetrol, 2c)Show SMILES O[C@H]1[C@@H](O)[C@@H](O)[C@@H](NCc2ccc(F)cc2)[C@H]1O |r| Show InChI InChI=1S/C12H16FNO4/c13-7-3-1-6(2-4-7)5-14-8-9(15)11(17)12(18)10(8)16/h1-4,8-12,14-18H,5H2/t8-,9+,10-,11+,12- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 330 | -38.5 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84472
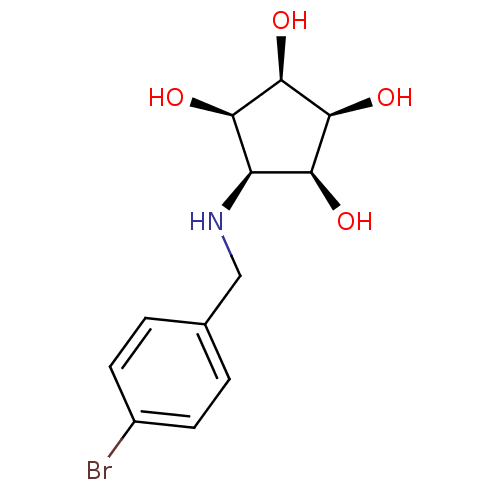
(Aminocyclopentitetrol, 2e)Show SMILES O[C@H]1[C@@H](O)[C@@H](O)[C@@H](NCc2ccc(Br)cc2)[C@H]1O |r| Show InChI InChI=1S/C12H16BrNO4/c13-7-3-1-6(2-4-7)5-14-8-9(15)11(17)12(18)10(8)16/h1-4,8-12,14-18H,5H2/t8-,9+,10-,11+,12- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 330 | -38.5 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84469
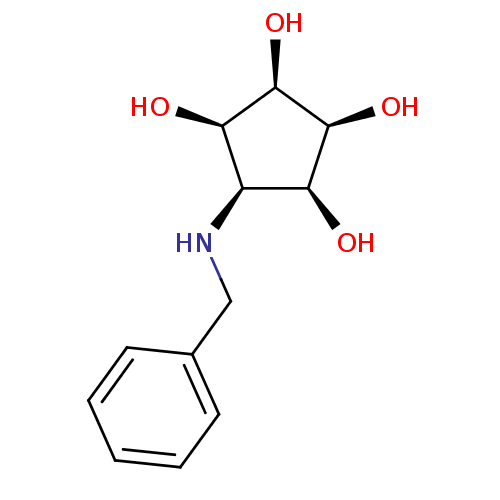
(Aminocyclopentitetrol, 2b)Show SMILES O[C@H]1[C@@H](O)[C@@H](O)[C@@H](NCc2ccccc2)[C@H]1O |r| Show InChI InChI=1S/C12H17NO4/c14-9-8(10(15)12(17)11(9)16)13-6-7-4-2-1-3-5-7/h1-5,8-17H,6H2/t8-,9+,10-,11+,12- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 450 | -37.7 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84473
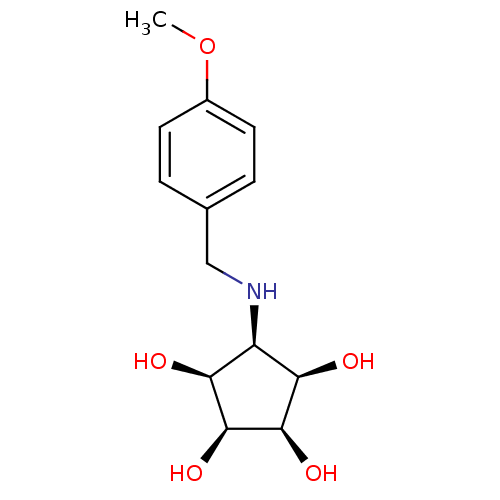
(Aminocyclopentitetrol, 2f)Show SMILES COc1ccc(CN[C@H]2[C@@H](O)[C@@H](O)[C@@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C13H19NO5/c1-19-8-4-2-7(3-5-8)6-14-9-10(15)12(17)13(18)11(9)16/h2-5,9-18H,6H2,1H3/t9-,10+,11-,12+,13- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 480 | -37.5 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84465

(Benzylation of mannostatin A, 1e)Show SMILES CS[C@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H]1NCc1ccc(Br)cc1 |r| Show InChI InChI=1S/C13H18BrNO3S/c1-19-13-9(10(16)11(17)12(13)18)15-6-7-2-4-8(14)5-3-7/h2-5,9-13,15-18H,6H2,1H3/t9-,10+,11+,12+,13+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 510 | -37.4 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84466

(Benzylation of mannostatin A, 1f)Show SMILES COc1ccc(CN[C@H]2[C@@H](O)[C@@H](O)[C@@H](O)[C@@H]2SC)cc1 |r| Show InChI InChI=1S/C14H21NO4S/c1-19-9-5-3-8(4-6-9)7-15-10-11(16)12(17)13(18)14(10)20-2/h3-6,10-18H,7H2,1-2H3/t10-,11+,12+,13+,14+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 520 | -37.3 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84463

(Benzylation of mannostatin A, 1c)Show SMILES CS[C@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H]1NCc1ccc(F)cc1 |r| Show InChI InChI=1S/C13H18FNO3S/c1-19-13-9(10(16)11(17)12(13)18)15-6-7-2-4-8(14)5-3-7/h2-5,9-13,15-18H,6H2,1H3/t9-,10+,11+,12+,13+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 530 | -37.3 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84471
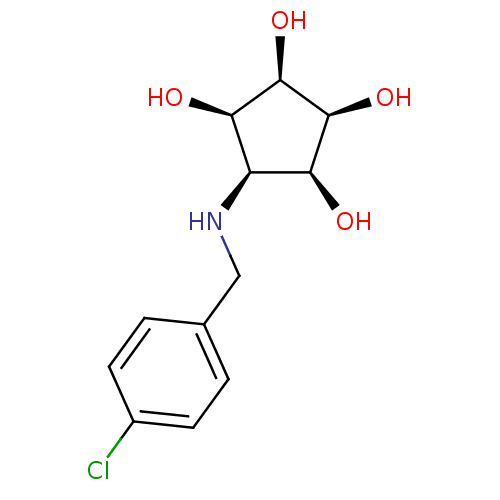
(Aminocyclopentitetrol, 2d)Show SMILES O[C@H]1[C@@H](O)[C@@H](O)[C@@H](NCc2ccc(Cl)cc2)[C@H]1O |r| Show InChI InChI=1S/C12H16ClNO4/c13-7-3-1-6(2-4-7)5-14-8-9(15)11(17)12(18)10(8)16/h1-4,8-12,14-18H,5H2/t8-,9+,10-,11+,12- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 670 | -36.7 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84462

(Benzylation of mannostatin A, 1b)Show SMILES CS[C@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H]1NCc1ccccc1 |r| Show InChI InChI=1S/C13H19NO3S/c1-18-13-9(10(15)11(16)12(13)17)14-7-8-5-3-2-4-6-8/h2-6,9-17H,7H2,1H3/t9-,10+,11+,12+,13+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 880 | -36.0 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84464

(Benzylation of mannostatin A, 1d)Show SMILES CS[C@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H]1NCc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C13H18ClNO3S/c1-19-13-9(10(16)11(17)12(13)18)15-6-7-2-4-8(14)5-3-7/h2-5,9-13,15-18H,6H2,1H3/t9-,10+,11+,12+,13+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 910 | -35.9 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84467

(Benzylation of mannostatin A, 1g)Show SMILES CS[C@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H]1NCc1ccc(cc1)C(=O)OCC=C |r| Show InChI InChI=1S/C17H23NO5S/c1-3-8-23-17(22)11-6-4-10(5-7-11)9-18-12-13(19)14(20)15(21)16(12)24-2/h3-7,12-16,18-21H,1,8-9H2,2H3/t12-,13+,14+,15+,16+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.22E+3 | -32.6 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84474

(Aminocyclopentitetrol, 2g)Show SMILES O[C@H]1[C@@H](O)[C@@H](O)[C@@H](NCc2ccc(cc2)C(=O)OCC=C)[C@H]1O |r| Show InChI InChI=1S/C16H21NO6/c1-2-7-23-16(22)10-5-3-9(4-6-10)8-17-11-12(18)14(20)15(21)13(11)19/h2-6,11-15,17-21H,1,7-8H2/t11-,12+,13-,14+,15- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.40E+3 | -31.8 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84470

(Aminocyclopentitetrol, 2c)Show SMILES O[C@H]1[C@@H](O)[C@@H](O)[C@@H](NCc2ccc(F)cc2)[C@H]1O |r| Show InChI InChI=1S/C12H16FNO4/c13-7-3-1-6(2-4-7)5-14-8-9(15)11(17)12(18)10(8)16/h1-4,8-12,14-18H,5H2/t8-,9+,10-,11+,12- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.00E+3 | -31.0 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84473

(Aminocyclopentitetrol, 2f)Show SMILES COc1ccc(CN[C@H]2[C@@H](O)[C@@H](O)[C@@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C13H19NO5/c1-19-8-4-2-7(3-5-8)6-14-9-10(15)12(17)13(18)11(9)16/h2-5,9-18H,6H2,1H3/t9-,10+,11-,12+,13- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.60E+3 | -30.8 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84468
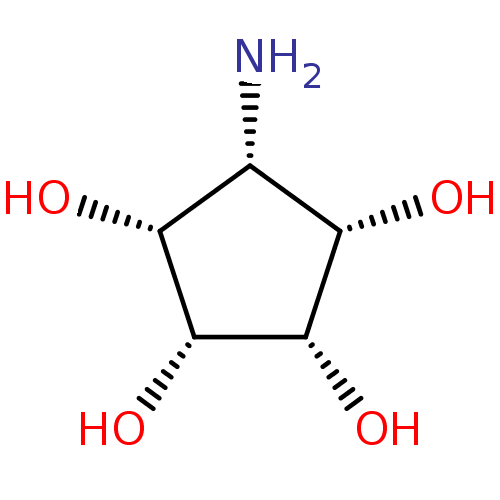
(Mannostatin analogue, 4b | Meso-aminocyclopentitet...)Show SMILES N[C@@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C5H11NO4/c6-1-2(7)4(9)5(10)3(1)8/h1-5,7-10H,6H2/t1-,2+,3-,4+,5- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 6.60E+3 | -30.8 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84472

(Aminocyclopentitetrol, 2e)Show SMILES O[C@H]1[C@@H](O)[C@@H](O)[C@@H](NCc2ccc(Br)cc2)[C@H]1O |r| Show InChI InChI=1S/C12H16BrNO4/c13-7-3-1-6(2-4-7)5-14-8-9(15)11(17)12(18)10(8)16/h1-4,8-12,14-18H,5H2/t8-,9+,10-,11+,12- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.60E+3 | -30.4 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84471

(Aminocyclopentitetrol, 2d)Show SMILES O[C@H]1[C@@H](O)[C@@H](O)[C@@H](NCc2ccc(Cl)cc2)[C@H]1O |r| Show InChI InChI=1S/C12H16ClNO4/c13-7-3-1-6(2-4-7)5-14-8-9(15)11(17)12(18)10(8)16/h1-4,8-12,14-18H,5H2/t8-,9+,10-,11+,12- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.10E+3 | -30.2 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84469

(Aminocyclopentitetrol, 2b)Show SMILES O[C@H]1[C@@H](O)[C@@H](O)[C@@H](NCc2ccccc2)[C@H]1O |r| Show InChI InChI=1S/C12H17NO4/c14-9-8(10(15)12(17)11(9)16)13-6-7-4-2-1-3-5-7/h1-5,8-17H,6H2/t8-,9+,10-,11+,12- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.00E+4 | -29.7 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Homo sapiens (Human)) | BDBM84468

(Mannostatin analogue, 4b | Meso-aminocyclopentitet...)Show SMILES N[C@@H]1[C@H](O)[C@H](O)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C5H11NO4/c6-1-2(7)4(9)5(10)3(1)8/h1-5,7-10H,6H2/t1-,2+,3-,4+,5- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 5.00E+4 | -25.5 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
University of Georgia
| |
Chembiochem 5: 1220-7 (2004)
Article DOI: 10.1002/cbic.200300842
BindingDB Entry DOI: 10.7270/Q2ZW1JF3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50546397

(CHEMBL4776352)Show SMILES CN(C)Cc1cnn(c1)-c1ccnc2[nH]c(nc12)-c1cn(C)c2ccc(cc12)S(C)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SYK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127393
BindingDB Entry DOI: 10.7270/Q28W3HVM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50546402
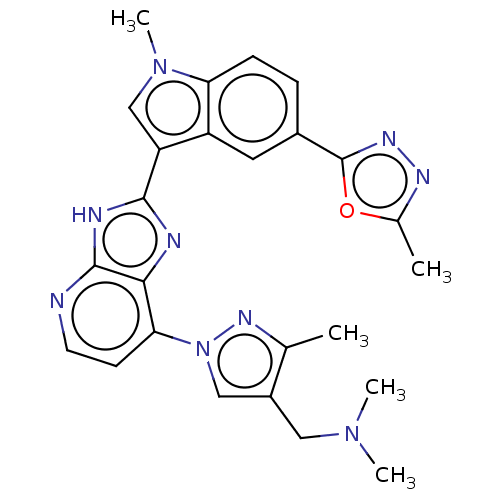
(CHEMBL4762995)Show SMILES CN(C)Cc1cn(nc1C)-c1ccnc2[nH]c(nc12)-c1cn(C)c2ccc(cc12)-c1nnc(C)o1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0980 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SYK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127393
BindingDB Entry DOI: 10.7270/Q28W3HVM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50546398

(CHEMBL4754597)Show SMILES CNC(=O)c1ccc2n(C)cc(-c3nc4c(ccnc4[nH]3)-n3cc(CN(C)C)cn3)c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SYK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127393
BindingDB Entry DOI: 10.7270/Q28W3HVM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50546401
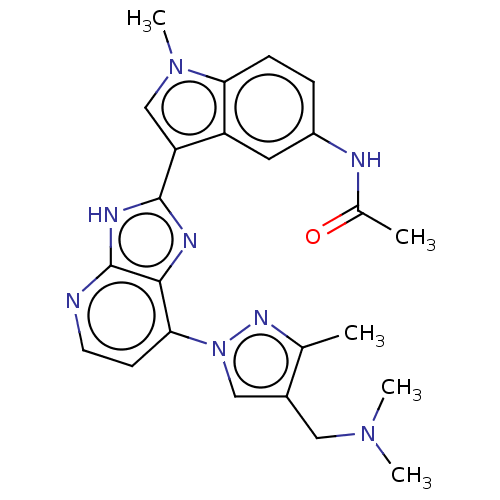
(CHEMBL4776842)Show SMILES CN(C)Cc1cn(nc1C)-c1ccnc2[nH]c(nc12)-c1cn(C)c2ccc(NC(C)=O)cc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SYK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127393
BindingDB Entry DOI: 10.7270/Q28W3HVM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50546400
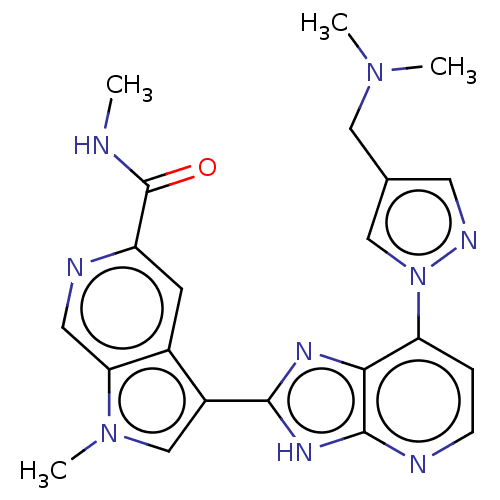
(CHEMBL4752127)Show SMILES CNC(=O)c1cc2c(cn(C)c2cn1)-c1nc2c(ccnc2[nH]1)-n1cc(CN(C)C)cn1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SYK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127393
BindingDB Entry DOI: 10.7270/Q28W3HVM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50546394

(CHEMBL4759636)Show SMILES CN(C)Cc1cc(nn1C)-c1ccnc(Nc2ccn3cnc(-c4nnc(C)o4)c3c2)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SYK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127393
BindingDB Entry DOI: 10.7270/Q28W3HVM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50546407

(CHEMBL4740065)Show SMILES Cn1ccc2c(cccc12)-c1nc2c(ccnc2[nH]1)-n1cc(N[C@@H]2CCOC[C@@H]2N)cn1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SYK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127393
BindingDB Entry DOI: 10.7270/Q28W3HVM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50546393

(CHEMBL4751083)Show SMILES CNC(=O)c1nn(C)c2ccc(Nc3nccc(n3)-n3cc(CN(C)C)c(C)n3)cc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SYK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127393
BindingDB Entry DOI: 10.7270/Q28W3HVM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50546403

(CHEMBL4779316)Show SMILES Cn1ccc2c(cccc12)-c1nc2c(ccnc2[nH]1)-n1cc(CN2CC[C@@H](O)C2)cn1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SYK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127393
BindingDB Entry DOI: 10.7270/Q28W3HVM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50546395
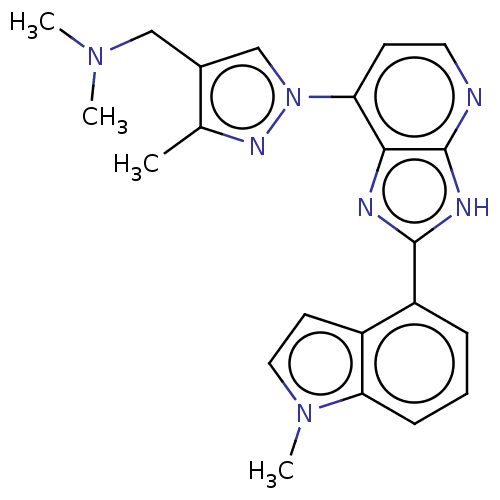
(CHEMBL4796370)Show SMILES CN(C)Cc1cn(nc1C)-c1ccnc2[nH]c(nc12)-c1cccc2n(C)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SYK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127393
BindingDB Entry DOI: 10.7270/Q28W3HVM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50546404
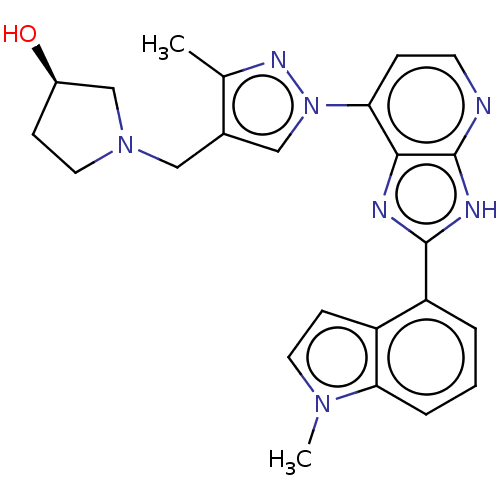
(CHEMBL4791746)Show SMILES Cc1nn(cc1CN1CC[C@@H](O)C1)-c1ccnc2[nH]c(nc12)-c1cccc2n(C)ccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SYK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127393
BindingDB Entry DOI: 10.7270/Q28W3HVM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50546405
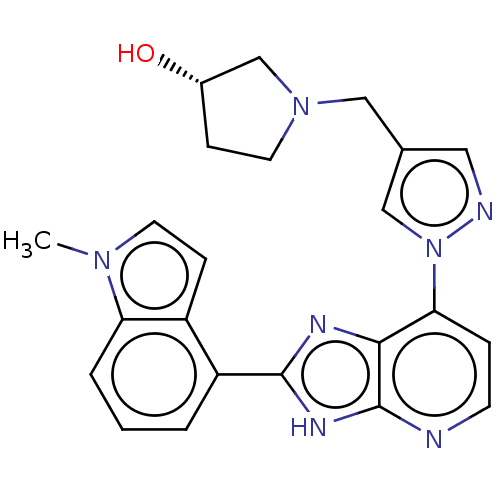
(CHEMBL4748597)Show SMILES Cn1ccc2c(cccc12)-c1nc2c(ccnc2[nH]1)-n1cc(CN2CC[C@H](O)C2)cn1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SYK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127393
BindingDB Entry DOI: 10.7270/Q28W3HVM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50546399
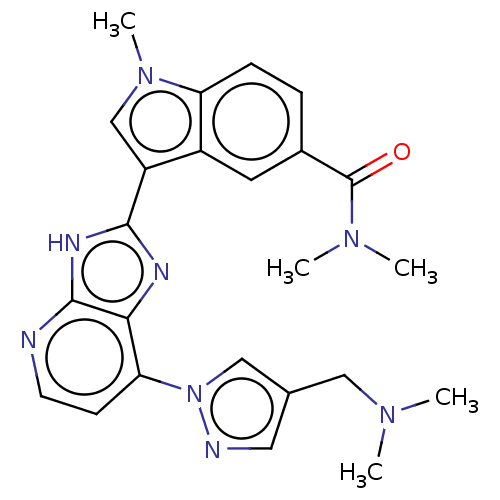
(CHEMBL4745530)Show SMILES CN(C)Cc1cnn(c1)-c1ccnc2[nH]c(nc12)-c1cn(C)c2ccc(cc12)C(=O)N(C)C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SYK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127393
BindingDB Entry DOI: 10.7270/Q28W3HVM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50135130
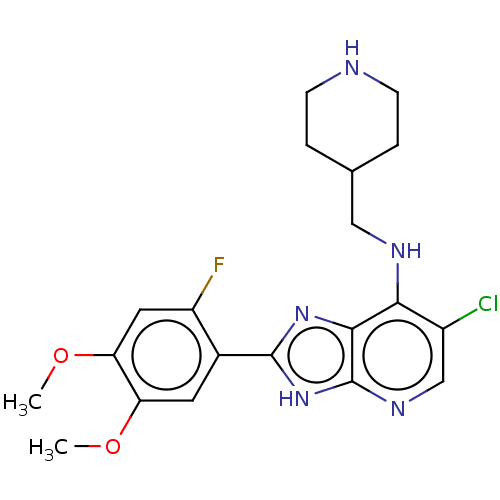
(CHEMBL3746463)Show SMILES COc1cc(F)c(cc1OC)-c1nc2c(NCC3CCNCC3)c(Cl)cnc2[nH]1 Show InChI InChI=1S/C20H23ClFN5O2/c1-28-15-7-12(14(22)8-16(15)29-2)19-26-18-17(13(21)10-25-20(18)27-19)24-9-11-3-5-23-6-4-11/h7-8,10-11,23H,3-6,9H2,1-2H3,(H2,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SYK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127393
BindingDB Entry DOI: 10.7270/Q28W3HVM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50546396
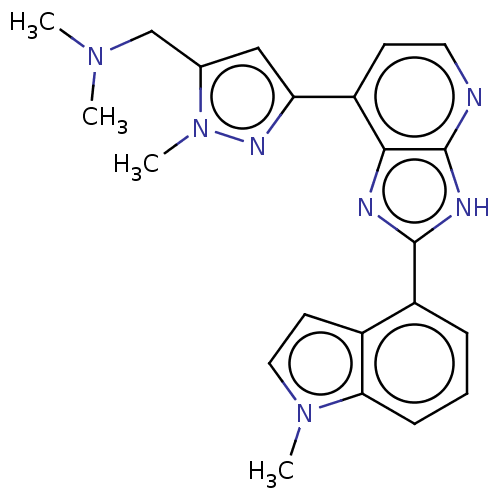
(CHEMBL4750942)Show SMILES CN(C)Cc1cc(nn1C)-c1ccnc2[nH]c(nc12)-c1cccc2n(C)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SYK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127393
BindingDB Entry DOI: 10.7270/Q28W3HVM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1 [866-1154]
(Homo sapiens (Human)) | BDBM488667
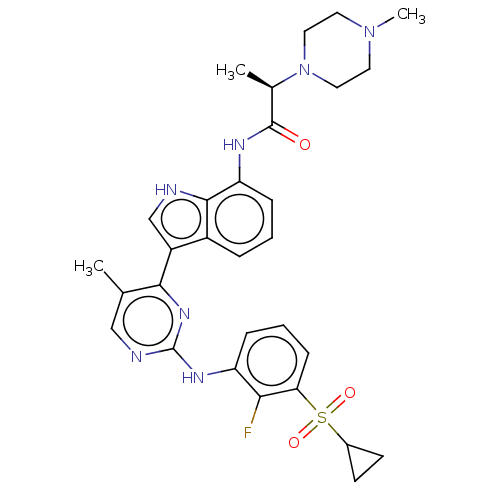
((R)-N-(3-(2-(3- cyclopropylsulfonyl)- 2-fluorophen...)Show SMILES C[C@@H](N1CCN(C)CC1)C(=O)Nc1cccc2c(c[nH]c12)-c1nc(Nc2cccc(c2F)S(=O)(=O)C2CC2)ncc1C |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB
US Patent
| Assay Description
Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... |
US Patent US10961228 (2021)
BindingDB Entry DOI: 10.7270/Q20G3P8F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1 [866-1154]
(Homo sapiens (Human)) | BDBM488663
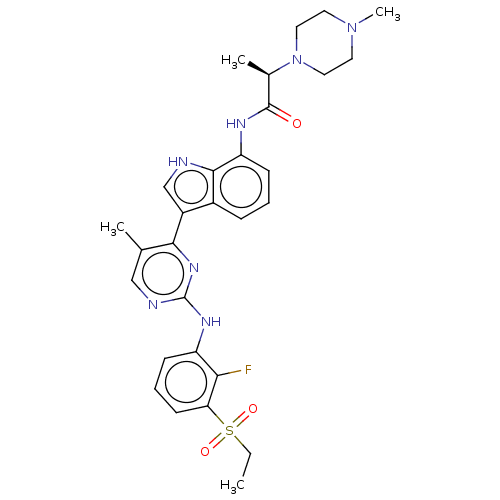
((R)-N-(3-(2-(3- (ethylsulfonyl)-2- fluorophenylami...)Show SMILES CCS(=O)(=O)c1cccc(Nc2ncc(C)c(n2)-c2c[nH]c3c(NC(=O)[C@@H](C)N4CCN(C)CC4)cccc23)c1F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB
US Patent
| Assay Description
Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... |
US Patent US10961228 (2021)
BindingDB Entry DOI: 10.7270/Q20G3P8F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1 [866-1154]
(Homo sapiens (Human)) | BDBM488662

((R)-N-(3-(2-(2-fluoro-3- (methylsulfonyl)phenyl am...)Show SMILES C[C@@H](N1CCN(C)CC1)C(=O)Nc1cccc2c(c[nH]c12)-c1nc(Nc2cccc(c2F)S(C)(=O)=O)ncc1C |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB
US Patent
| Assay Description
Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... |
US Patent US10961228 (2021)
BindingDB Entry DOI: 10.7270/Q20G3P8F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1 [866-1154]
(Homo sapiens (Human)) | BDBM488661

((R)-N-(3-(2-((2-fluoro- 3- (methylsulfonyl)phenyl)...)Show SMILES C[C@@H](N1CCN(CCO)CC1)C(=O)Nc1cccc2c(c[nH]c12)-c1nc(Nc2cccc(c2F)S(C)(=O)=O)ncc1C |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB
US Patent
| Assay Description
Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... |
US Patent US10961228 (2021)
BindingDB Entry DOI: 10.7270/Q20G3P8F |
More data for this
Ligand-Target Pair | |
Polycomb protein EED
(Homo sapiens (Human)) | BDBM50575300
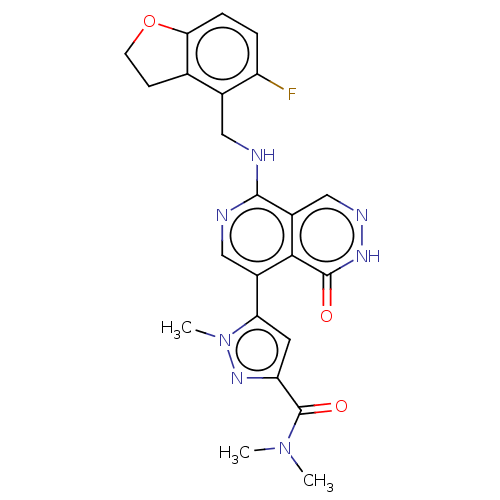
(CHEMBL4851414)Show SMILES CN(C)C(=O)c1cc(-c2cnc(NCc3c4CCOc4ccc3F)c3cn[nH]c(=O)c23)n(C)n1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EED in human G-401 cells assessed as reduction in H3K27 trimethylation incubated for 48 hrs by HTRF assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01161
BindingDB Entry DOI: 10.7270/Q2MP5737 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1 [866-1154]
(Homo sapiens (Human)) | BDBM489174

((R)-2-((R)-2,4- dimethylpiperazin-1- yl)-N-(3-(5-f...)Show SMILES COC[C@@H](N1CCN(C)C[C@H]1C)C(=O)Nc1cccc2c(c[nH]c12)-c1nc(Nc2cccc(c2F)S(C)(=O)=O)ncc1F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB
US Patent
| Assay Description
Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... |
US Patent US10961228 (2021)
BindingDB Entry DOI: 10.7270/Q20G3P8F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1 [866-1154]
(Homo sapiens (Human)) | BDBM488735

((S)-2-((3R,5R)-3,5- dimethylpiperazin-1- yl)-N-(3-...)Show SMILES CC[C@H](N1C[C@@H](C)N[C@H](C)C1)C(=O)Nc1cccc2c(c[nH]c12)-c1nc(Nc2cccc(c2F)S(C)(=O)=O)ncc1C |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB
US Patent
| Assay Description
Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... |
US Patent US10961228 (2021)
BindingDB Entry DOI: 10.7270/Q20G3P8F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data