 Found 99 hits with Last Name = 'khan' and Initial = 'si'
Found 99 hits with Last Name = 'khan' and Initial = 'si' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase
(Homo sapiens (Human)) | BDBM50481553
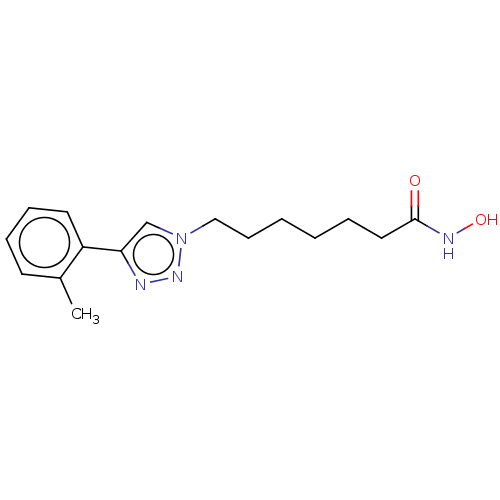
(7-(2-Tolyl)Triazolylheptahydroxamamic Acid | CHEMB...)Show InChI InChI=1S/C16H22N4O2/c1-13-8-5-6-9-14(13)15-12-20(19-17-15)11-7-3-2-4-10-16(21)18-22/h5-6,8-9,12,22H,2-4,7,10-11H2,1H3,(H,18,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extracts after 15 mins by fluorescence assay |
Bioorg Med Chem 18: 415-25 (2010)
Article DOI: 10.1016/j.bmc.2009.10.042
BindingDB Entry DOI: 10.7270/Q2RV0RJB |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extracts after 15 mins by fluorescence assay |
Bioorg Med Chem 18: 415-25 (2010)
Article DOI: 10.1016/j.bmc.2009.10.042
BindingDB Entry DOI: 10.7270/Q2RV0RJB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase
(Plasmodium falciparum (isolate 3D7)) | BDBM50324111
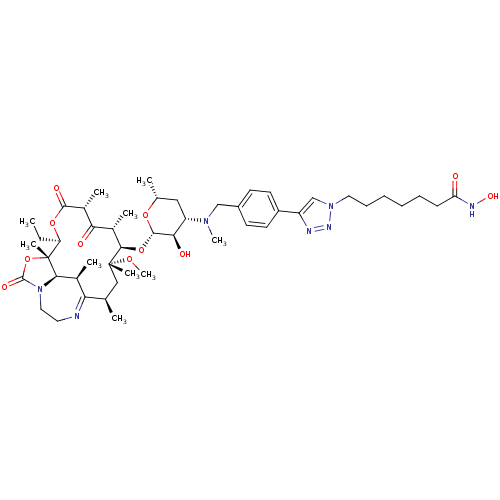
(CHEMBL1214760)Show SMILES CC[C@H]1OC(=O)[C@H](C)C(=O)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2O)N(C)Cc2ccc(cc2)-c2cn(CCCCCCC(=O)NO)nn2)[C@@](C)(C[C@@H](C)C2=NCCN3[C@H]([C@H]2C)[C@]1(C)OC3=O)OC |r,t:54| Show InChI InChI=1S/C48H73N7O11/c1-11-37-48(8)42-30(4)39(49-21-23-55(42)46(60)66-48)28(2)25-47(7,62-10)43(31(5)40(57)32(6)44(59)64-37)65-45-41(58)36(24-29(3)63-45)53(9)26-33-17-19-34(20-18-33)35-27-54(52-50-35)22-15-13-12-14-16-38(56)51-61/h17-20,27-32,36-37,41-43,45,58,61H,11-16,21-26H2,1-10H3,(H,51,56)/t28-,29-,30+,31+,32-,36+,37-,41-,42-,43-,45+,47-,48-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum HDAC1 |
J Med Chem 53: 6100-11 (2010)
Article DOI: 10.1021/jm100507q
BindingDB Entry DOI: 10.7270/Q29Z954B |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50481551

(CHEMBL590567)Show InChI InChI=1S/C18H27N5O2/c1-22(2)16-11-9-15(10-12-16)17-14-23(21-19-17)13-7-5-3-4-6-8-18(24)20-25/h9-12,14,25H,3-8,13H2,1-2H3,(H,20,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extracts after 15 mins by fluorescence assay |
Bioorg Med Chem 18: 415-25 (2010)
Article DOI: 10.1016/j.bmc.2009.10.042
BindingDB Entry DOI: 10.7270/Q2RV0RJB |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Plasmodium falciparum (isolate 3D7)) | BDBM50324110
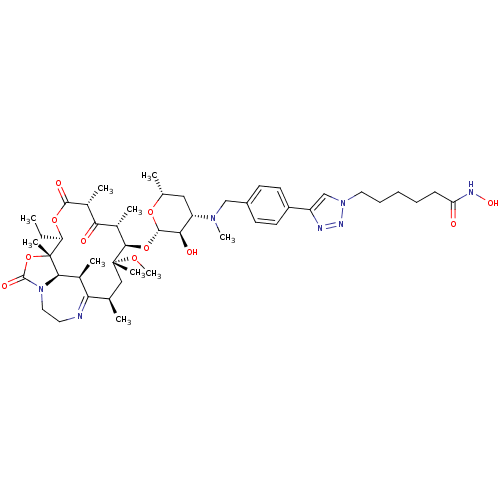
(CHEMBL1214759)Show SMILES CC[C@H]1OC(=O)[C@H](C)C(=O)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2O)N(C)Cc2ccc(cc2)-c2cn(CCCCCC(=O)NO)nn2)[C@@](C)(C[C@@H](C)C2=NCCN3[C@H]([C@H]2C)[C@]1(C)OC3=O)OC |r,t:53| Show InChI InChI=1S/C47H71N7O11/c1-11-36-47(8)41-29(4)38(48-20-22-54(41)45(59)65-47)27(2)24-46(7,61-10)42(30(5)39(56)31(6)43(58)63-36)64-44-40(57)35(23-28(3)62-44)52(9)25-32-16-18-33(19-17-32)34-26-53(51-49-34)21-14-12-13-15-37(55)50-60/h16-19,26-31,35-36,40-42,44,57,60H,11-15,20-25H2,1-10H3,(H,50,55)/t27-,28-,29+,30+,31-,35+,36-,40-,41-,42-,44+,46-,47-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum HDAC1 |
J Med Chem 53: 6100-11 (2010)
Article DOI: 10.1021/jm100507q
BindingDB Entry DOI: 10.7270/Q29Z954B |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Plasmodium falciparum (isolate 3D7)) | BDBM50324114

(CHEMBL1214761)Show SMILES CC[C@H]1OC(=O)[C@H](C)C(=O)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2O)N(C)Cc2ccc(cc2)-c2cn(CCCCCCCC(=O)NO)nn2)[C@@](C)(C[C@@H](C)C2=NCCN3[C@H]([C@H]2C)[C@]1(C)OC3=O)OC |r,t:55| Show InChI InChI=1S/C49H75N7O11/c1-11-38-49(8)43-31(4)40(50-22-24-56(43)47(61)67-49)29(2)26-48(7,63-10)44(32(5)41(58)33(6)45(60)65-38)66-46-42(59)37(25-30(3)64-46)54(9)27-34-18-20-35(21-19-34)36-28-55(53-51-36)23-16-14-12-13-15-17-39(57)52-62/h18-21,28-33,37-38,42-44,46,59,62H,11-17,22-27H2,1-10H3,(H,52,57)/t29-,30-,31+,32+,33-,37+,38-,42-,43-,44-,46+,48-,49-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum HDAC1 |
J Med Chem 53: 6100-11 (2010)
Article DOI: 10.1021/jm100507q
BindingDB Entry DOI: 10.7270/Q29Z954B |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50481556
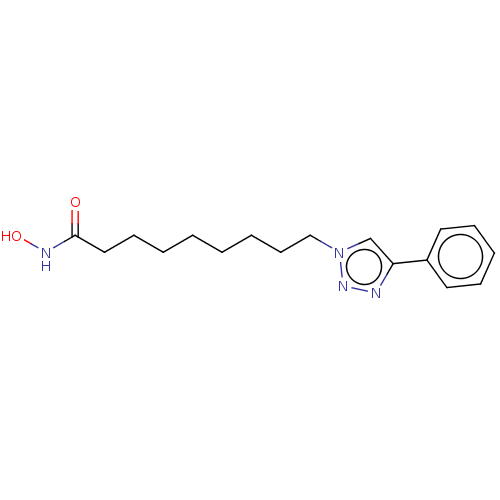
(9-(Phenyl)Triazolylnonahydroxamic Acid | CHEMBL591...)Show InChI InChI=1S/C17H24N4O2/c22-17(19-23)12-8-3-1-2-4-9-13-21-14-16(18-20-21)15-10-6-5-7-11-15/h5-7,10-11,14,23H,1-4,8-9,12-13H2,(H,19,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extracts after 15 mins by fluorescence assay |
Bioorg Med Chem 18: 415-25 (2010)
Article DOI: 10.1016/j.bmc.2009.10.042
BindingDB Entry DOI: 10.7270/Q2RV0RJB |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extracts after 15 mins by fluorescence assay |
Bioorg Med Chem 18: 415-25 (2010)
Article DOI: 10.1016/j.bmc.2009.10.042
BindingDB Entry DOI: 10.7270/Q2RV0RJB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 85.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 |
J Med Chem 53: 6100-11 (2010)
Article DOI: 10.1021/jm100507q
BindingDB Entry DOI: 10.7270/Q29Z954B |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50481554

(8-(3-Biphenyl)Triazolyloctahydroxamic Acid | CHEMB...)Show SMILES ONC(=O)CCCCCCCn1cc(nn1)-c1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C22H26N4O2/c27-22(24-28)14-7-2-1-3-8-15-26-17-21(23-25-26)20-13-9-12-19(16-20)18-10-5-4-6-11-18/h4-6,9-13,16-17,28H,1-3,7-8,14-15H2,(H,24,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extracts after 15 mins by fluorescence assay |
Bioorg Med Chem 18: 415-25 (2010)
Article DOI: 10.1016/j.bmc.2009.10.042
BindingDB Entry DOI: 10.7270/Q2RV0RJB |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Plasmodium falciparum (isolate 3D7)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum HDAC1 |
J Med Chem 53: 6100-11 (2010)
Article DOI: 10.1021/jm100507q
BindingDB Entry DOI: 10.7270/Q29Z954B |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50481560
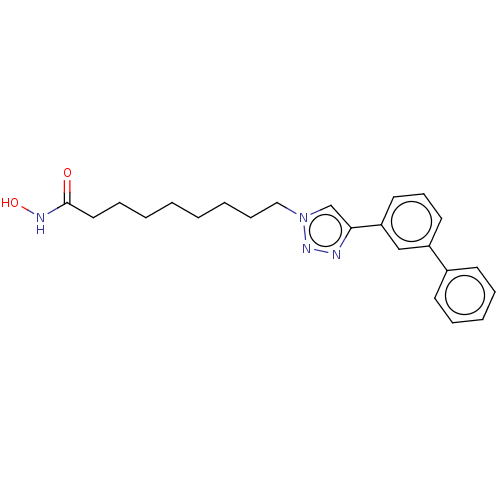
(9-(3-Biphenyl)Triazolylnonahydroxamic Acid | CHEMB...)Show SMILES ONC(=O)CCCCCCCCn1cc(nn1)-c1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C23H28N4O2/c28-23(25-29)15-8-3-1-2-4-9-16-27-18-22(24-26-27)21-14-10-13-20(17-21)19-11-6-5-7-12-19/h5-7,10-14,17-18,29H,1-4,8-9,15-16H2,(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extracts after 15 mins by fluorescence assay |
Bioorg Med Chem 18: 415-25 (2010)
Article DOI: 10.1016/j.bmc.2009.10.042
BindingDB Entry DOI: 10.7270/Q2RV0RJB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50324111

(CHEMBL1214760)Show SMILES CC[C@H]1OC(=O)[C@H](C)C(=O)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2O)N(C)Cc2ccc(cc2)-c2cn(CCCCCCC(=O)NO)nn2)[C@@](C)(C[C@@H](C)C2=NCCN3[C@H]([C@H]2C)[C@]1(C)OC3=O)OC |r,t:54| Show InChI InChI=1S/C48H73N7O11/c1-11-37-48(8)42-30(4)39(49-21-23-55(42)46(60)66-48)28(2)25-47(7,62-10)43(31(5)40(57)32(6)44(59)64-37)65-45-41(58)36(24-29(3)63-45)53(9)26-33-17-19-34(20-18-33)35-27-54(52-50-35)22-15-13-12-14-16-38(56)51-61/h17-20,27-32,36-37,41-43,45,58,61H,11-16,21-26H2,1-10H3,(H,51,56)/t28-,29-,30+,31+,32-,36+,37-,41-,42-,43-,45+,47-,48-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 |
J Med Chem 53: 6100-11 (2010)
Article DOI: 10.1021/jm100507q
BindingDB Entry DOI: 10.7270/Q29Z954B |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50481558
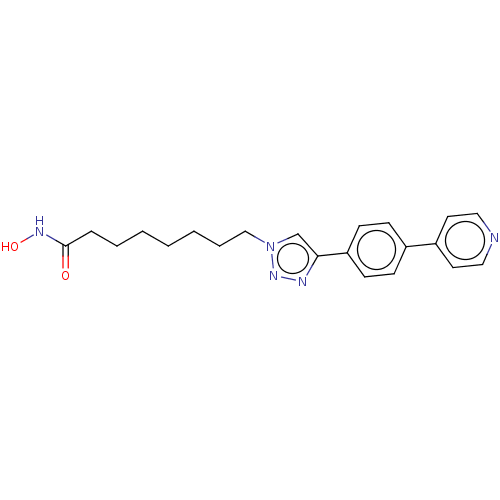
(CHEMBL589664)Show SMILES ONC(=O)CCCCCCCn1cc(nn1)-c1ccc(cc1)-c1ccncc1 Show InChI InChI=1S/C21H25N5O2/c27-21(24-28)6-4-2-1-3-5-15-26-16-20(23-25-26)19-9-7-17(8-10-19)18-11-13-22-14-12-18/h7-14,16,28H,1-6,15H2,(H,24,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 213 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extracts after 15 mins by fluorescence assay |
Bioorg Med Chem 18: 415-25 (2010)
Article DOI: 10.1016/j.bmc.2009.10.042
BindingDB Entry DOI: 10.7270/Q2RV0RJB |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50481555
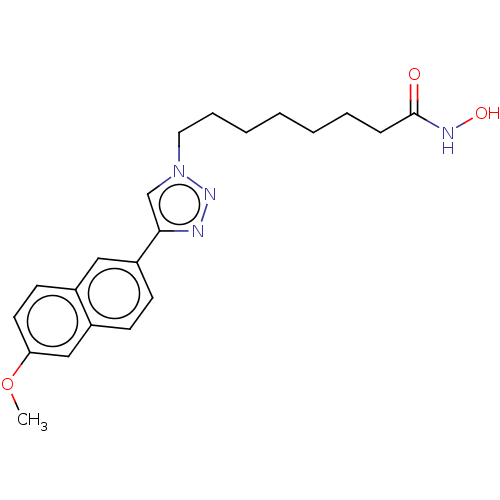
(CHEMBL590872)Show InChI InChI=1S/C21H26N4O3/c1-28-19-11-10-16-13-18(9-8-17(16)14-19)20-15-25(24-22-20)12-6-4-2-3-5-7-21(26)23-27/h8-11,13-15,27H,2-7,12H2,1H3,(H,23,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 226 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extracts after 15 mins by fluorescence assay |
Bioorg Med Chem 18: 415-25 (2010)
Article DOI: 10.1016/j.bmc.2009.10.042
BindingDB Entry DOI: 10.7270/Q2RV0RJB |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50105335

(CHEMBL3597247)Show SMILES O=c1sc2ccccc2n1CCCCCCN1CCN(CCCCCCn2c3ccccc3sc2=O)CC1 Show InChI InChI=1S/C30H40N4O2S2/c35-29-33(25-13-5-7-15-27(25)37-29)19-11-3-1-9-17-31-21-23-32(24-22-31)18-10-2-4-12-20-34-26-14-6-8-16-28(26)38-30(34)36/h5-8,13-16H,1-4,9-12,17-24H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University
Curated by ChEMBL
| Assay Description
Antiinflammatory activity in mouse RAW264.7 cells assessed as inhibition of LPS-induced iNOS activity incubated for 30 mins prior to LPS challenge me... |
Bioorg Med Chem 23: 3248-59 (2015)
Article DOI: 10.1016/j.bmc.2015.04.057
BindingDB Entry DOI: 10.7270/Q26H4K5F |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Plasmodium falciparum (isolate 3D7)) | BDBM50324113
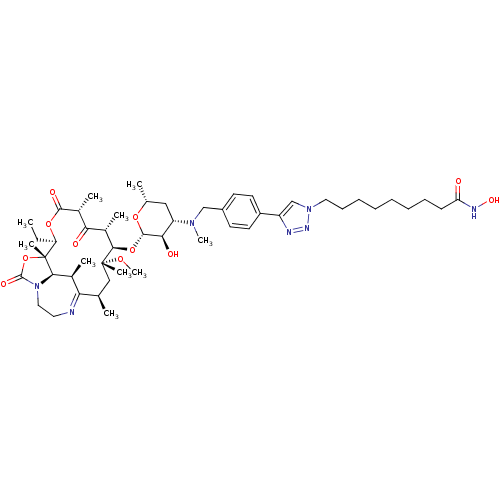
(CHEMBL1214762)Show SMILES CC[C@H]1OC(=O)[C@H](C)C(=O)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2O)N(C)Cc2ccc(cc2)-c2cn(CCCCCCCCC(=O)NO)nn2)[C@@](C)(C[C@@H](C)C2=NCCN3[C@H]([C@H]2C)[C@]1(C)OC3=O)OC |r,t:56| Show InChI InChI=1S/C50H77N7O11/c1-11-39-50(8)44-32(4)41(51-23-25-57(44)48(62)68-50)30(2)27-49(7,64-10)45(33(5)42(59)34(6)46(61)66-39)67-47-43(60)38(26-31(3)65-47)55(9)28-35-19-21-36(22-20-35)37-29-56(54-52-37)24-17-15-13-12-14-16-18-40(58)53-63/h19-22,29-34,38-39,43-45,47,60,63H,11-18,23-28H2,1-10H3,(H,53,58)/t30-,31-,32+,33+,34-,38+,39-,43-,44-,45-,47+,49-,50-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum HDAC1 |
J Med Chem 53: 6100-11 (2010)
Article DOI: 10.1021/jm100507q
BindingDB Entry DOI: 10.7270/Q29Z954B |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50105353
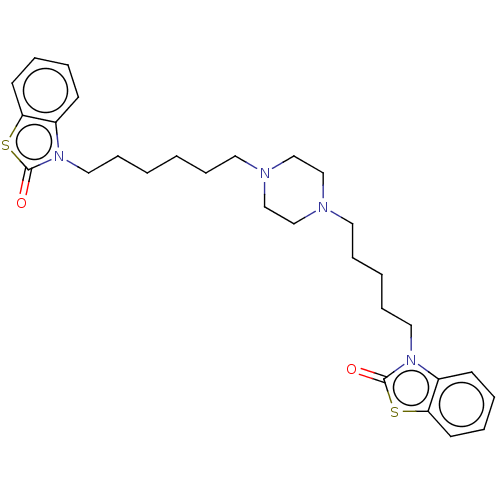
(CHEMBL3597266)Show SMILES O=c1sc2ccccc2n1CCCCCCN1CCN(CCCCCn2c3ccccc3sc2=O)CC1 Show InChI InChI=1S/C29H38N4O2S2/c34-28-32(24-12-4-6-14-26(24)36-28)18-10-2-1-8-16-30-20-22-31(23-21-30)17-9-3-11-19-33-25-13-5-7-15-27(25)37-29(33)35/h4-7,12-15H,1-3,8-11,16-23H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University
Curated by ChEMBL
| Assay Description
Antiinflammatory activity in mouse RAW264.7 cells assessed as inhibition of LPS-induced iNOS activity incubated for 30 mins prior to LPS challenge me... |
Bioorg Med Chem 23: 3248-59 (2015)
Article DOI: 10.1016/j.bmc.2015.04.057
BindingDB Entry DOI: 10.7270/Q26H4K5F |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Plasmodium falciparum (isolate 3D7)) | BDBM50324112
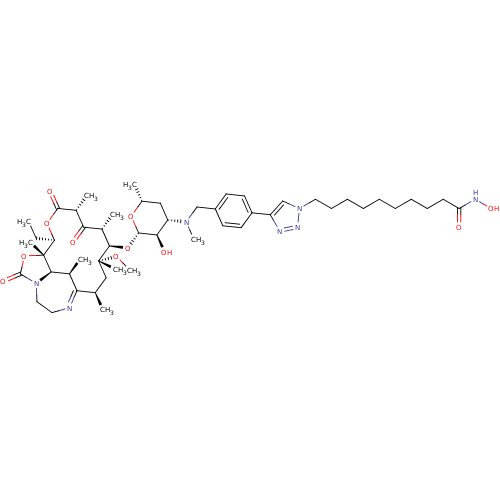
(CHEMBL1214763)Show SMILES CC[C@H]1OC(=O)[C@H](C)C(=O)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2O)N(C)Cc2ccc(cc2)-c2cn(CCCCCCCCCC(=O)NO)nn2)[C@@](C)(C[C@@H](C)C2=NCCN3[C@H]([C@H]2C)[C@]1(C)OC3=O)OC |r,t:57| Show InChI InChI=1S/C51H79N7O11/c1-11-40-51(8)45-33(4)42(52-24-26-58(45)49(63)69-51)31(2)28-50(7,65-10)46(34(5)43(60)35(6)47(62)67-40)68-48-44(61)39(27-32(3)66-48)56(9)29-36-20-22-37(23-21-36)38-30-57(55-53-38)25-18-16-14-12-13-15-17-19-41(59)54-64/h20-23,30-35,39-40,44-46,48,61,64H,11-19,24-29H2,1-10H3,(H,54,59)/t31-,32-,33+,34+,35-,39+,40-,44-,45-,46-,48+,50-,51-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 304 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum HDAC1 |
J Med Chem 53: 6100-11 (2010)
Article DOI: 10.1021/jm100507q
BindingDB Entry DOI: 10.7270/Q29Z954B |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50481550
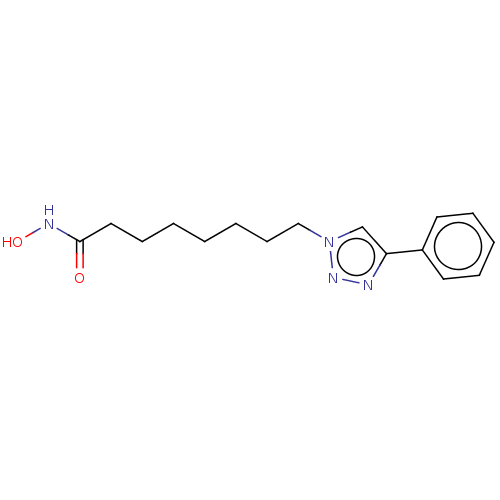
(8-(Phenyl)Triazolyloctahydroxamic Acid | CHEMBL591...)Show InChI InChI=1S/C16H22N4O2/c21-16(18-22)11-7-2-1-3-8-12-20-13-15(17-19-20)14-9-5-4-6-10-14/h4-6,9-10,13,22H,1-3,7-8,11-12H2,(H,18,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 364 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extracts after 15 mins by fluorescence assay |
Bioorg Med Chem 18: 415-25 (2010)
Article DOI: 10.1016/j.bmc.2009.10.042
BindingDB Entry DOI: 10.7270/Q2RV0RJB |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50481557
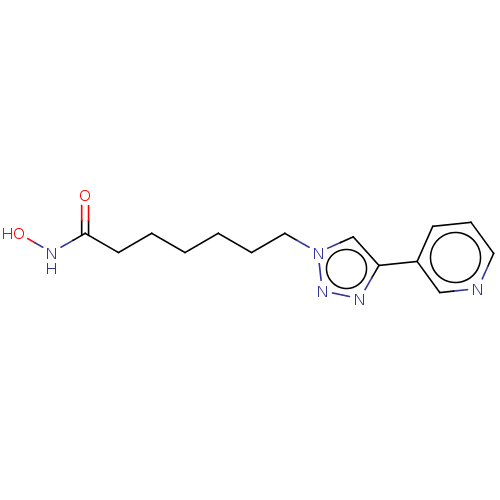
(CHEMBL589605)Show InChI InChI=1S/C14H19N5O2/c20-14(17-21)7-3-1-2-4-9-19-11-13(16-18-19)12-6-5-8-15-10-12/h5-6,8,10-11,21H,1-4,7,9H2,(H,17,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 366 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extracts after 15 mins by fluorescence assay |
Bioorg Med Chem 18: 415-25 (2010)
Article DOI: 10.1016/j.bmc.2009.10.042
BindingDB Entry DOI: 10.7270/Q2RV0RJB |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50105355
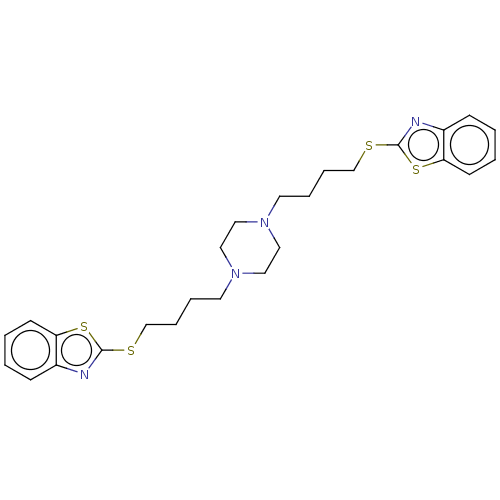
(CHEMBL3597268)Show SMILES C(CCN1CCN(CCCCSc2nc3ccccc3s2)CC1)CSc1nc2ccccc2s1 Show InChI InChI=1S/C26H32N4S4/c1-3-11-23-21(9-1)27-25(33-23)31-19-7-5-13-29-15-17-30(18-16-29)14-6-8-20-32-26-28-22-10-2-4-12-24(22)34-26/h1-4,9-12H,5-8,13-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University
Curated by ChEMBL
| Assay Description
Antiinflammatory activity in mouse RAW264.7 cells assessed as inhibition of LPS-induced iNOS activity incubated for 30 mins prior to LPS challenge me... |
Bioorg Med Chem 23: 3248-59 (2015)
Article DOI: 10.1016/j.bmc.2015.04.057
BindingDB Entry DOI: 10.7270/Q26H4K5F |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50105333
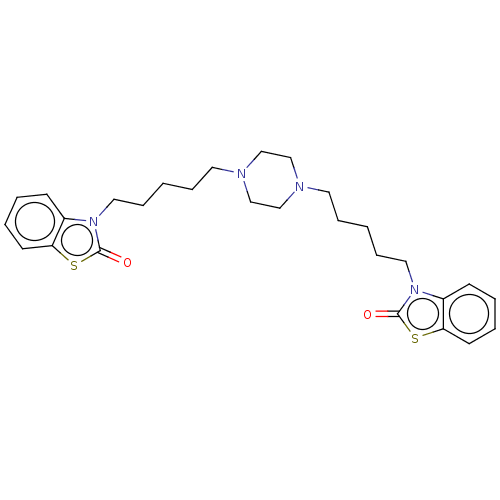
(CHEMBL3597245)Show SMILES O=c1sc2ccccc2n1CCCCCN1CCN(CCCCCn2c3ccccc3sc2=O)CC1 Show InChI InChI=1S/C28H36N4O2S2/c33-27-31(23-11-3-5-13-25(23)35-27)17-9-1-7-15-29-19-21-30(22-20-29)16-8-2-10-18-32-24-12-4-6-14-26(24)36-28(32)34/h3-6,11-14H,1-2,7-10,15-22H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University
Curated by ChEMBL
| Assay Description
Antiinflammatory activity in mouse RAW264.7 cells assessed as inhibition of LPS-induced iNOS activity incubated for 30 mins prior to LPS challenge me... |
Bioorg Med Chem 23: 3248-59 (2015)
Article DOI: 10.1016/j.bmc.2015.04.057
BindingDB Entry DOI: 10.7270/Q26H4K5F |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50481559
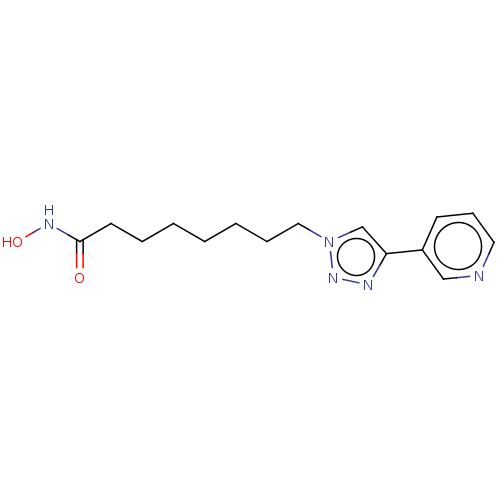
(8-(3-Pyridyl)Triazolyloctahydroxamic Acid | CHEMBL...)Show InChI InChI=1S/C15H21N5O2/c21-15(18-22)8-4-2-1-3-5-10-20-12-14(17-19-20)13-7-6-9-16-11-13/h6-7,9,11-12,22H,1-5,8,10H2,(H,18,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 426 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extracts after 15 mins by fluorescence assay |
Bioorg Med Chem 18: 415-25 (2010)
Article DOI: 10.1016/j.bmc.2009.10.042
BindingDB Entry DOI: 10.7270/Q2RV0RJB |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50105350
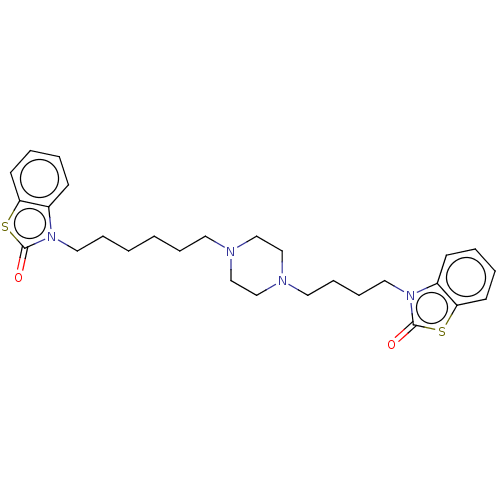
(CHEMBL3597264)Show SMILES O=c1sc2ccccc2n1CCCCCCN1CCN(CCCCn2c3ccccc3sc2=O)CC1 Show InChI InChI=1S/C28H36N4O2S2/c33-27-31(23-11-3-5-13-25(23)35-27)17-8-2-1-7-15-29-19-21-30(22-20-29)16-9-10-18-32-24-12-4-6-14-26(24)36-28(32)34/h3-6,11-14H,1-2,7-10,15-22H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University
Curated by ChEMBL
| Assay Description
Antiinflammatory activity in mouse RAW264.7 cells assessed as inhibition of LPS-induced iNOS activity incubated for 30 mins prior to LPS challenge me... |
Bioorg Med Chem 23: 3248-59 (2015)
Article DOI: 10.1016/j.bmc.2015.04.057
BindingDB Entry DOI: 10.7270/Q26H4K5F |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50324114

(CHEMBL1214761)Show SMILES CC[C@H]1OC(=O)[C@H](C)C(=O)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2O)N(C)Cc2ccc(cc2)-c2cn(CCCCCCCC(=O)NO)nn2)[C@@](C)(C[C@@H](C)C2=NCCN3[C@H]([C@H]2C)[C@]1(C)OC3=O)OC |r,t:55| Show InChI InChI=1S/C49H75N7O11/c1-11-38-49(8)43-31(4)40(50-22-24-56(43)47(61)67-49)29(2)26-48(7,63-10)44(32(5)41(58)33(6)45(60)65-38)66-46-42(59)37(25-30(3)64-46)54(9)27-34-18-20-35(21-19-34)36-28-55(53-51-36)23-16-14-12-13-15-17-39(57)52-62/h18-21,28-33,37-38,42-44,46,59,62H,11-17,22-27H2,1-10H3,(H,52,57)/t29-,30-,31+,32+,33-,37+,38-,42-,43-,44-,46+,48-,49-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 |
J Med Chem 53: 6100-11 (2010)
Article DOI: 10.1021/jm100507q
BindingDB Entry DOI: 10.7270/Q29Z954B |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50481552
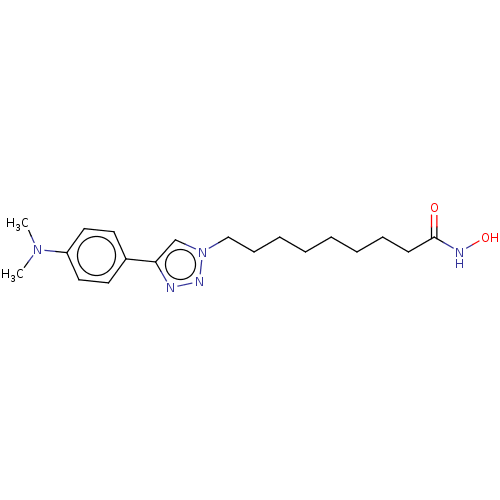
(CHEMBL590566)Show InChI InChI=1S/C19H29N5O2/c1-23(2)17-12-10-16(11-13-17)18-15-24(22-20-18)14-8-6-4-3-5-7-9-19(25)21-26/h10-13,15,26H,3-9,14H2,1-2H3,(H,21,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 597 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extracts after 15 mins by fluorescence assay |
Bioorg Med Chem 18: 415-25 (2010)
Article DOI: 10.1016/j.bmc.2009.10.042
BindingDB Entry DOI: 10.7270/Q2RV0RJB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50324113

(CHEMBL1214762)Show SMILES CC[C@H]1OC(=O)[C@H](C)C(=O)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2O)N(C)Cc2ccc(cc2)-c2cn(CCCCCCCCC(=O)NO)nn2)[C@@](C)(C[C@@H](C)C2=NCCN3[C@H]([C@H]2C)[C@]1(C)OC3=O)OC |r,t:56| Show InChI InChI=1S/C50H77N7O11/c1-11-39-50(8)44-32(4)41(51-23-25-57(44)48(62)68-50)30(2)27-49(7,64-10)45(33(5)42(59)34(6)46(61)66-39)67-47-43(60)38(26-31(3)65-47)55(9)28-35-19-21-36(22-20-35)37-29-56(54-52-37)24-17-15-13-12-14-16-18-40(58)53-63/h19-22,29-34,38-39,43-45,47,60,63H,11-18,23-28H2,1-10H3,(H,53,58)/t30-,31-,32+,33+,34-,38+,39-,43-,44-,45-,47+,49-,50-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 |
J Med Chem 53: 6100-11 (2010)
Article DOI: 10.1021/jm100507q
BindingDB Entry DOI: 10.7270/Q29Z954B |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50324111

(CHEMBL1214760)Show SMILES CC[C@H]1OC(=O)[C@H](C)C(=O)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2O)N(C)Cc2ccc(cc2)-c2cn(CCCCCCC(=O)NO)nn2)[C@@](C)(C[C@@H](C)C2=NCCN3[C@H]([C@H]2C)[C@]1(C)OC3=O)OC |r,t:54| Show InChI InChI=1S/C48H73N7O11/c1-11-37-48(8)42-30(4)39(49-21-23-55(42)46(60)66-48)28(2)25-47(7,62-10)43(31(5)40(57)32(6)44(59)64-37)65-45-41(58)36(24-29(3)63-45)53(9)26-33-17-19-34(20-18-33)35-27-54(52-50-35)22-15-13-12-14-16-38(56)51-61/h17-20,27-32,36-37,41-43,45,58,61H,11-16,21-26H2,1-10H3,(H,51,56)/t28-,29-,30+,31+,32-,36+,37-,41-,42-,43-,45+,47-,48-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 729 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 |
J Med Chem 53: 6100-11 (2010)
Article DOI: 10.1021/jm100507q
BindingDB Entry DOI: 10.7270/Q29Z954B |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50324110

(CHEMBL1214759)Show SMILES CC[C@H]1OC(=O)[C@H](C)C(=O)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2O)N(C)Cc2ccc(cc2)-c2cn(CCCCCC(=O)NO)nn2)[C@@](C)(C[C@@H](C)C2=NCCN3[C@H]([C@H]2C)[C@]1(C)OC3=O)OC |r,t:53| Show InChI InChI=1S/C47H71N7O11/c1-11-36-47(8)41-29(4)38(48-20-22-54(41)45(59)65-47)27(2)24-46(7,61-10)42(30(5)39(56)31(6)43(58)63-36)64-44-40(57)35(23-28(3)62-44)52(9)25-32-16-18-33(19-17-32)34-26-53(51-49-34)21-14-12-13-15-37(55)50-60/h16-19,26-31,35-36,40-42,44,57,60H,11-15,20-25H2,1-10H3,(H,50,55)/t27-,28-,29+,30+,31-,35+,36-,40-,41-,42-,44+,46-,47-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 796 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 |
J Med Chem 53: 6100-11 (2010)
Article DOI: 10.1021/jm100507q
BindingDB Entry DOI: 10.7270/Q29Z954B |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50324112

(CHEMBL1214763)Show SMILES CC[C@H]1OC(=O)[C@H](C)C(=O)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2O)N(C)Cc2ccc(cc2)-c2cn(CCCCCCCCCC(=O)NO)nn2)[C@@](C)(C[C@@H](C)C2=NCCN3[C@H]([C@H]2C)[C@]1(C)OC3=O)OC |r,t:57| Show InChI InChI=1S/C51H79N7O11/c1-11-40-51(8)45-33(4)42(52-24-26-58(45)49(63)69-51)31(2)28-50(7,65-10)46(34(5)43(60)35(6)47(62)67-40)68-48-44(61)39(27-32(3)66-48)56(9)29-36-20-22-37(23-21-36)38-30-57(55-53-38)25-18-16-14-12-13-15-17-19-41(59)54-64/h20-23,30-35,39-40,44-46,48,61,64H,11-19,24-29H2,1-10H3,(H,54,59)/t31-,32-,33+,34+,35-,39+,40-,44-,45-,46-,48+,50-,51-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 825 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 |
J Med Chem 53: 6100-11 (2010)
Article DOI: 10.1021/jm100507q
BindingDB Entry DOI: 10.7270/Q29Z954B |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Homo sapiens (Human)) | BDBM50009248
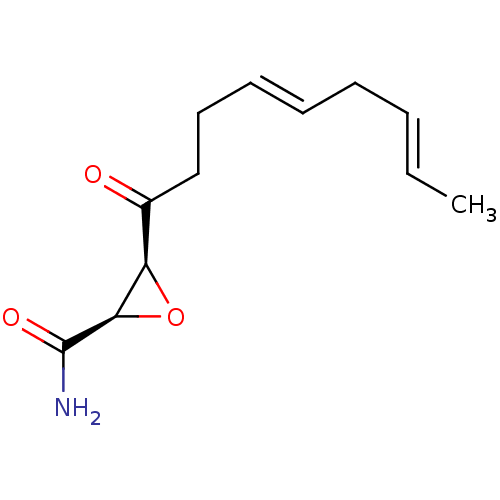
((2R,3S)-3-((4E,7E)-Nona-4,7-dienoyl)-oxirane-2-car...)Show InChI InChI=1S/C12H17NO3/c1-2-3-4-5-6-7-8-9(14)10-11(16-10)12(13)15/h2-3,5-6,10-11H,4,7-8H2,1H3,(H2,13,15)/b3-2+,6-5+/t10-,11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi
Curated by ChEMBL
| Assay Description
Inhibition of fatty acid synthase |
J Nat Prod 66: 39-41 (2003)
Article DOI: 10.1021/np020429z
BindingDB Entry DOI: 10.7270/Q2DN47VH |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50203110
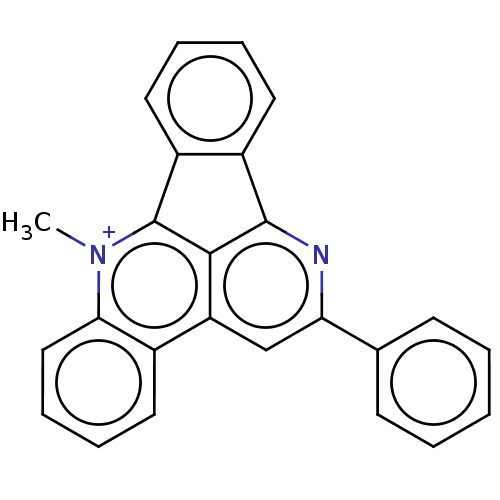
(CHEMBL3914791)Show SMILES [I-].C[n+]1c2-c3ccccc3-c3nc(cc(c23)c2ccccc12)-c1ccccc1 Show InChI InChI=1S/C25H17N2/c1-27-22-14-8-7-11-17(22)20-15-21(16-9-3-2-4-10-16)26-24-18-12-5-6-13-19(18)25(27)23(20)24/h2-15H,1H3/q+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human DNA topoisomerase 1 using pHOT1 as substrate after 30 mins by agarose gel electrophoresis |
Bioorg Med Chem 24: 6119-6130 (2016)
Article DOI: 10.1016/j.bmc.2016.02.028
BindingDB Entry DOI: 10.7270/Q2BG2QZJ |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50203111
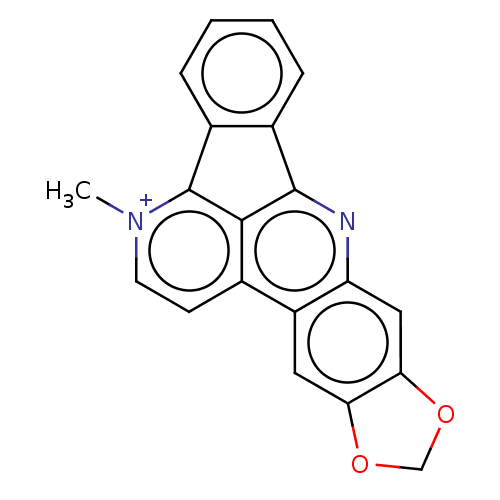
(CHEMBL3968023)Show SMILES [I-].C[n+]1ccc2c3c(nc4cc5OCOc5cc24)-c2ccccc2-c13 Show InChI InChI=1S/C20H13N2O2/c1-22-7-6-11-14-8-16-17(24-10-23-16)9-15(14)21-19-12-4-2-3-5-13(12)20(22)18(11)19/h2-9H,10H2,1H3/q+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human DNA topoisomerase 1 using pHOT1 as substrate after 30 mins by agarose gel electrophoresis |
Bioorg Med Chem 24: 6119-6130 (2016)
Article DOI: 10.1016/j.bmc.2016.02.028
BindingDB Entry DOI: 10.7270/Q2BG2QZJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50433441
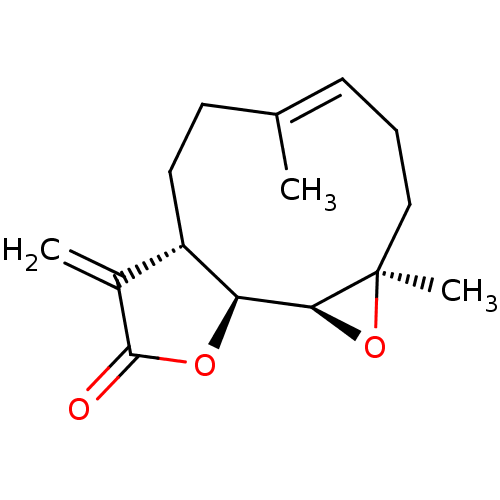
(PARTHENOLIDE)Show SMILES C\C1=C/CC[C@@]2(C)O[C@H]2[C@H]2OC(=O)C(=C)[C@@H]2CC1 |r,c:1| Show InChI InChI=1S/C15H20O3/c1-9-5-4-8-15(3)13(18-15)12-11(7-6-9)10(2)14(16)17-12/h5,11-13H,2,4,6-8H2,1,3H3/b9-5+/t11-,12-,13-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi
Curated by ChEMBL
| Assay Description
Inhibition of iNOS in mouse RAW264.7 cells assessed as LPS-induced nitric oxide production treated for 30 mins followed by LPS challenge measured aft... |
J Nat Prod 77: 509-15 (2014)
Article DOI: 10.1021/np400780n
BindingDB Entry DOI: 10.7270/Q2DF6SPK |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50203043
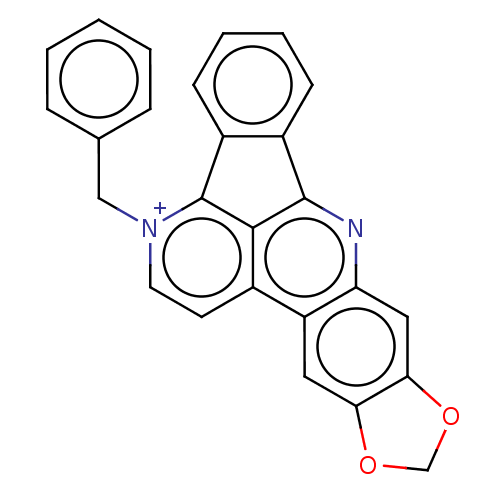
(CHEMBL3896867)Show SMILES [Br-].C(c1ccccc1)[n+]1ccc2c3c(nc4cc5OCOc5cc24)-c2ccccc2-c13 Show InChI InChI=1S/C26H17N2O2/c1-2-6-16(7-3-1)14-28-11-10-17-20-12-22-23(30-15-29-22)13-21(20)27-25-18-8-4-5-9-19(18)26(28)24(17)25/h1-13H,14-15H2/q+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human DNA topoisomerase 1 using pHOT1 as substrate after 30 mins by agarose gel electrophoresis |
Bioorg Med Chem 24: 6119-6130 (2016)
Article DOI: 10.1016/j.bmc.2016.02.028
BindingDB Entry DOI: 10.7270/Q2BG2QZJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50105354
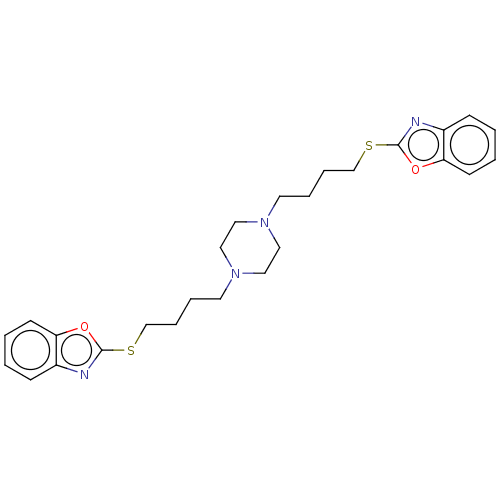
(CHEMBL3597267)Show SMILES C(CCN1CCN(CCCCSc2nc3ccccc3o2)CC1)CSc1nc2ccccc2o1 Show InChI InChI=1S/C26H32N4O2S2/c1-3-11-23-21(9-1)27-25(31-23)33-19-7-5-13-29-15-17-30(18-16-29)14-6-8-20-34-26-28-22-10-2-4-12-24(22)32-26/h1-4,9-12H,5-8,13-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University
Curated by ChEMBL
| Assay Description
Antiinflammatory activity in mouse RAW264.7 cells assessed as inhibition of LPS-induced iNOS activity incubated for 30 mins prior to LPS challenge me... |
Bioorg Med Chem 23: 3248-59 (2015)
Article DOI: 10.1016/j.bmc.2015.04.057
BindingDB Entry DOI: 10.7270/Q26H4K5F |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50324110

(CHEMBL1214759)Show SMILES CC[C@H]1OC(=O)[C@H](C)C(=O)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2O)N(C)Cc2ccc(cc2)-c2cn(CCCCCC(=O)NO)nn2)[C@@](C)(C[C@@H](C)C2=NCCN3[C@H]([C@H]2C)[C@]1(C)OC3=O)OC |r,t:53| Show InChI InChI=1S/C47H71N7O11/c1-11-36-47(8)41-29(4)38(48-20-22-54(41)45(59)65-47)27(2)24-46(7,61-10)42(30(5)39(56)31(6)43(58)63-36)64-44-40(57)35(23-28(3)62-44)52(9)25-32-16-18-33(19-17-32)34-26-53(51-49-34)21-14-12-13-15-37(55)50-60/h16-19,26-31,35-36,40-42,44,57,60H,11-15,20-25H2,1-10H3,(H,50,55)/t27-,28-,29+,30+,31-,35+,36-,40-,41-,42-,44+,46-,47-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 |
J Med Chem 53: 6100-11 (2010)
Article DOI: 10.1021/jm100507q
BindingDB Entry DOI: 10.7270/Q29Z954B |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50203113
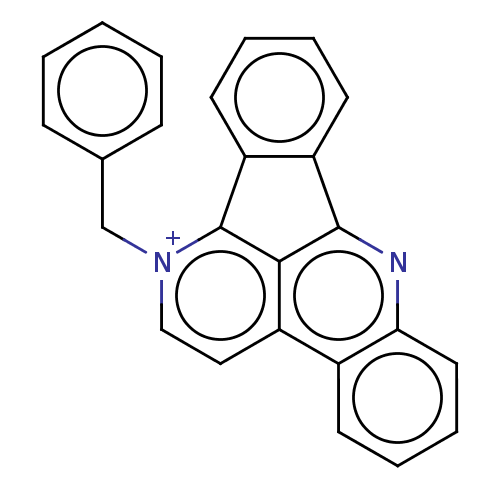
(CHEMBL3889816)Show SMILES [Br-].C(c1ccccc1)[n+]1ccc2c3c(nc4ccccc24)-c2ccccc2-c13 Show InChI InChI=1S/C25H17N2/c1-2-8-17(9-3-1)16-27-15-14-19-18-10-6-7-13-22(18)26-24-20-11-4-5-12-21(20)25(27)23(19)24/h1-15H,16H2/q+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human DNA topoisomerase 1 using pHOT1 as substrate after 30 mins by agarose gel electrophoresis |
Bioorg Med Chem 24: 6119-6130 (2016)
Article DOI: 10.1016/j.bmc.2016.02.028
BindingDB Entry DOI: 10.7270/Q2BG2QZJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50105348
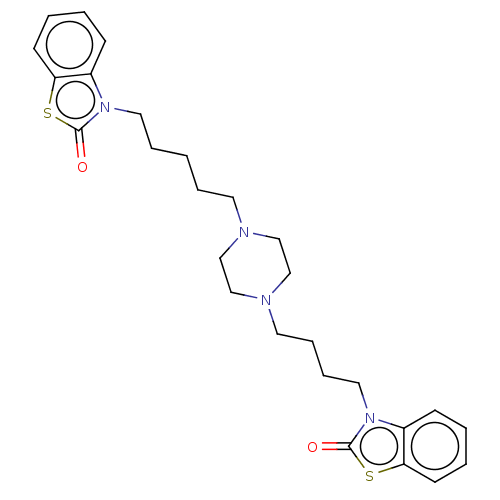
(CHEMBL3597262)Show SMILES O=c1sc2ccccc2n1CCCCCN1CCN(CCCCn2c3ccccc3sc2=O)CC1 Show InChI InChI=1S/C27H34N4O2S2/c32-26-30(22-10-2-4-12-24(22)34-26)16-7-1-6-14-28-18-20-29(21-19-28)15-8-9-17-31-23-11-3-5-13-25(23)35-27(31)33/h2-5,10-13H,1,6-9,14-21H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University
Curated by ChEMBL
| Assay Description
Antiinflammatory activity in mouse RAW264.7 cells assessed as inhibition of LPS-induced iNOS activity incubated for 30 mins prior to LPS challenge me... |
Bioorg Med Chem 23: 3248-59 (2015)
Article DOI: 10.1016/j.bmc.2015.04.057
BindingDB Entry DOI: 10.7270/Q26H4K5F |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50202837
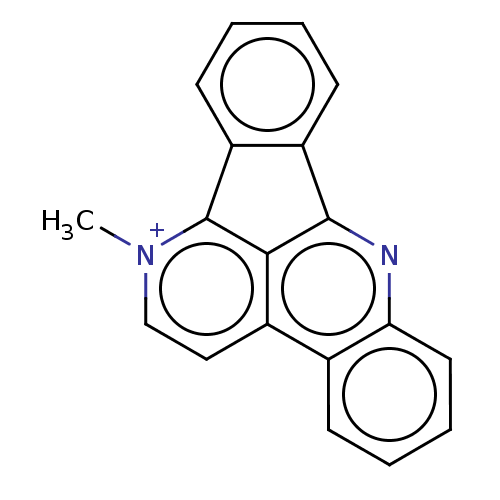
(CHEMBL3941751)Show SMILES Cc1ccc(cc1)S([O-])(=O)=O.C[n+]1ccc2c3c(nc4ccccc24)-c2ccccc2-c13 Show InChI InChI=1S/C19H13N2/c1-21-11-10-13-12-6-4-5-9-16(12)20-18-14-7-2-3-8-15(14)19(21)17(13)18/h2-11H,1H3/q+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human DNA topoisomerase 1 using pHOT1 as substrate after 30 mins by agarose gel electrophoresis |
Bioorg Med Chem 24: 6119-6130 (2016)
Article DOI: 10.1016/j.bmc.2016.02.028
BindingDB Entry DOI: 10.7270/Q2BG2QZJ |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50203048
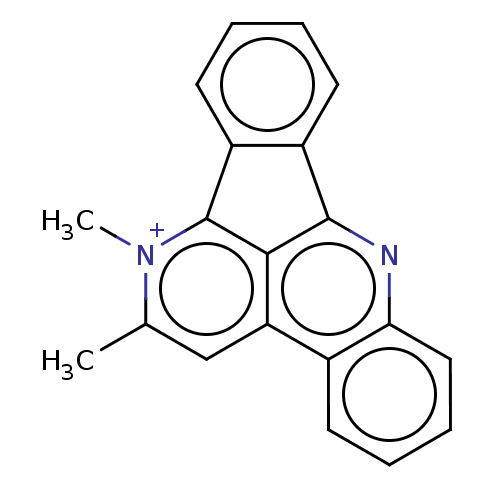
(CHEMBL3984603)Show SMILES [I-].Cc1cc2c3c(nc4ccccc24)-c2ccccc2-c3[n+]1C Show InChI InChI=1S/C20H15N2/c1-12-11-16-13-7-5-6-10-17(13)21-19-14-8-3-4-9-15(14)20(18(16)19)22(12)2/h3-11H,1-2H3/q+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human DNA topoisomerase 1 using pHOT1 as substrate after 30 mins by agarose gel electrophoresis |
Bioorg Med Chem 24: 6119-6130 (2016)
Article DOI: 10.1016/j.bmc.2016.02.028
BindingDB Entry DOI: 10.7270/Q2BG2QZJ |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50203040
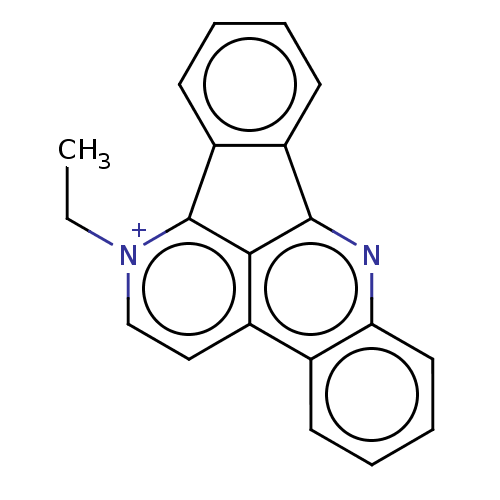
(CHEMBL3932774)Show SMILES [I-].CC[n+]1ccc2c3c(nc4ccccc24)-c2ccccc2-c13 Show InChI InChI=1S/C20H15N2/c1-2-22-12-11-14-13-7-5-6-10-17(13)21-19-15-8-3-4-9-16(15)20(22)18(14)19/h3-12H,2H2,1H3/q+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human DNA topoisomerase 1 using pHOT1 as substrate after 30 mins by agarose gel electrophoresis |
Bioorg Med Chem 24: 6119-6130 (2016)
Article DOI: 10.1016/j.bmc.2016.02.028
BindingDB Entry DOI: 10.7270/Q2BG2QZJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50324114

(CHEMBL1214761)Show SMILES CC[C@H]1OC(=O)[C@H](C)C(=O)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2O)N(C)Cc2ccc(cc2)-c2cn(CCCCCCCC(=O)NO)nn2)[C@@](C)(C[C@@H](C)C2=NCCN3[C@H]([C@H]2C)[C@]1(C)OC3=O)OC |r,t:55| Show InChI InChI=1S/C49H75N7O11/c1-11-38-49(8)43-31(4)40(50-22-24-56(43)47(61)67-49)29(2)26-48(7,63-10)44(32(5)41(58)33(6)45(60)65-38)66-46-42(59)37(25-30(3)64-46)54(9)27-34-18-20-35(21-19-34)36-28-55(53-51-36)23-16-14-12-13-15-17-39(57)52-62/h18-21,28-33,37-38,42-44,46,59,62H,11-17,22-27H2,1-10H3,(H,52,57)/t29-,30-,31+,32+,33-,37+,38-,42-,43-,44-,46+,48-,49-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 |
J Med Chem 53: 6100-11 (2010)
Article DOI: 10.1021/jm100507q
BindingDB Entry DOI: 10.7270/Q29Z954B |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50105349
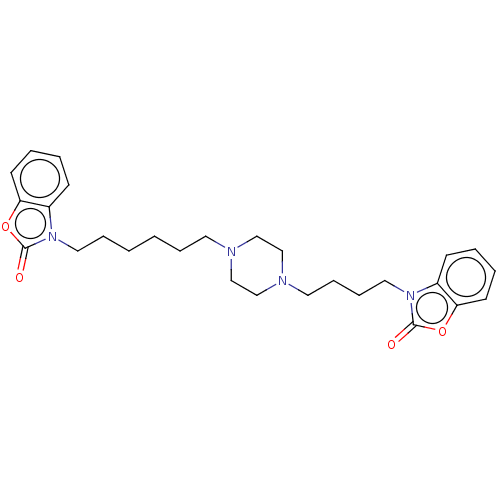
(CHEMBL3597263)Show SMILES O=c1oc2ccccc2n1CCCCCCN1CCN(CCCCn2c3ccccc3oc2=O)CC1 Show InChI InChI=1S/C28H36N4O4/c33-27-31(23-11-3-5-13-25(23)35-27)17-8-2-1-7-15-29-19-21-30(22-20-29)16-9-10-18-32-24-12-4-6-14-26(24)36-28(32)34/h3-6,11-14H,1-2,7-10,15-22H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University
Curated by ChEMBL
| Assay Description
Antiinflammatory activity in mouse RAW264.7 cells assessed as inhibition of LPS-induced iNOS activity incubated for 30 mins prior to LPS challenge me... |
Bioorg Med Chem 23: 3248-59 (2015)
Article DOI: 10.1016/j.bmc.2015.04.057
BindingDB Entry DOI: 10.7270/Q26H4K5F |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 |
J Med Chem 53: 6100-11 (2010)
Article DOI: 10.1021/jm100507q
BindingDB Entry DOI: 10.7270/Q29Z954B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50324113

(CHEMBL1214762)Show SMILES CC[C@H]1OC(=O)[C@H](C)C(=O)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2O)N(C)Cc2ccc(cc2)-c2cn(CCCCCCCCC(=O)NO)nn2)[C@@](C)(C[C@@H](C)C2=NCCN3[C@H]([C@H]2C)[C@]1(C)OC3=O)OC |r,t:56| Show InChI InChI=1S/C50H77N7O11/c1-11-39-50(8)44-32(4)41(51-23-25-57(44)48(62)68-50)30(2)27-49(7,64-10)45(33(5)42(59)34(6)46(61)66-39)67-47-43(60)38(26-31(3)65-47)55(9)28-35-19-21-36(22-20-35)37-29-56(54-52-37)24-17-15-13-12-14-16-18-40(58)53-63/h19-22,29-34,38-39,43-45,47,60,63H,11-18,23-28H2,1-10H3,(H,53,58)/t30-,31-,32+,33+,34-,38+,39-,43-,44-,45-,47+,49-,50-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 |
J Med Chem 53: 6100-11 (2010)
Article DOI: 10.1021/jm100507q
BindingDB Entry DOI: 10.7270/Q29Z954B |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50499513
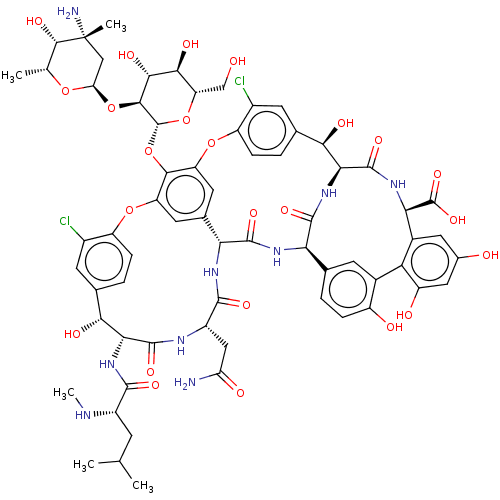
(CHEMBL4299436)Show SMILES [H][C@]1(C[C@@](C)(N)[C@@H](O)[C@@H](C)O1)O[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@@H]1Oc1c2Oc3ccc(cc3Cl)[C@@H](O)[C@@H](NC(=O)[C@H](CC(C)C)NC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@]3([H])c(c2)cc1Oc1ccc(cc1Cl)[C@@H](O)[C@]1([H])NC(=O)[C@]([H])(NC3=O)c2ccc(O)c(c2)-c2c(O)cc(O)cc2[C@@H](NC1=O)C(O)=O |r| Show InChI InChI=1S/C66H75Cl2N9O24/c1-23(2)12-34(71-5)58(88)76-49-51(83)26-7-10-38(32(67)14-26)97-40-16-28-17-41(55(40)101-65-56(54(86)53(85)42(22-78)99-65)100-44-21-66(4,70)57(87)24(3)96-44)98-39-11-8-27(15-33(39)68)52(84)50-63(93)75-48(64(94)95)31-18-29(79)19-37(81)45(31)30-13-25(6-9-36(30)80)46(60(90)77-50)74-61(91)47(28)73-59(89)35(20-43(69)82)72-62(49)92/h6-11,13-19,23-24,34-35,42,44,46-54,56-57,65,71,78-81,83-87H,12,20-22,70H2,1-5H3,(H2,69,82)(H,72,92)(H,73,89)(H,74,91)(H,75,93)(H,76,88)(H,77,90)(H,94,95)/t24-,34+,35+,42+,44-,46-,47-,48-,49-,50+,51-,52-,53+,54-,56+,57+,65-,66-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antibacterial activity against low level vancomycin-resistant Enterococcus faecalis ATCC 51299 by plate reader analysis |
J Nat Prod 78: 2748-53 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00721
BindingDB Entry DOI: 10.7270/Q2M61P8H |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50230993

((2S)-2-amino-5-[(N-methylcarbamimidoyl)amino]penta...)Show InChI InChI=1S/C7H16N4O2/c1-10-7(9)11-4-2-3-5(8)6(12)13/h5H,2-4,8H2,1H3,(H,12,13)(H3,9,10,11)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University
Curated by ChEMBL
| Assay Description
Antiinflammatory activity in mouse RAW264.7 cells assessed as inhibition of LPS-induced iNOS activity incubated for 30 mins prior to LPS challenge me... |
Bioorg Med Chem 23: 3248-59 (2015)
Article DOI: 10.1016/j.bmc.2015.04.057
BindingDB Entry DOI: 10.7270/Q26H4K5F |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50105344
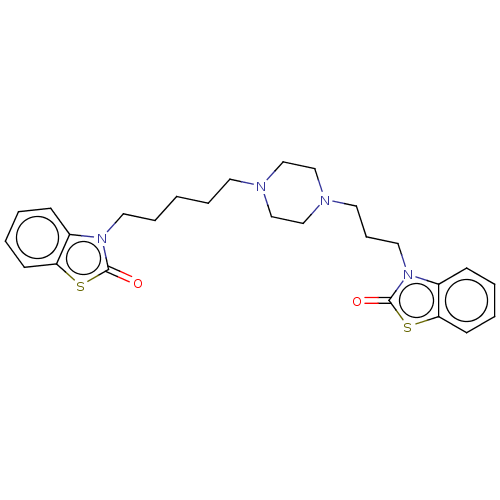
(CHEMBL3597258)Show SMILES O=c1sc2ccccc2n1CCCCCN1CCN(CCCn2c3ccccc3sc2=O)CC1 Show InChI InChI=1S/C26H32N4O2S2/c31-25-29(21-9-2-4-11-23(21)33-25)15-7-1-6-13-27-17-19-28(20-18-27)14-8-16-30-22-10-3-5-12-24(22)34-26(30)32/h2-5,9-12H,1,6-8,13-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University
Curated by ChEMBL
| Assay Description
Antiinflammatory activity in mouse RAW264.7 cells assessed as inhibition of LPS-induced iNOS activity incubated for 30 mins prior to LPS challenge me... |
Bioorg Med Chem 23: 3248-59 (2015)
Article DOI: 10.1016/j.bmc.2015.04.057
BindingDB Entry DOI: 10.7270/Q26H4K5F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data