 Found 71 hits with Last Name = 'kim' and Initial = 'hp'
Found 71 hits with Last Name = 'kim' and Initial = 'hp' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085048
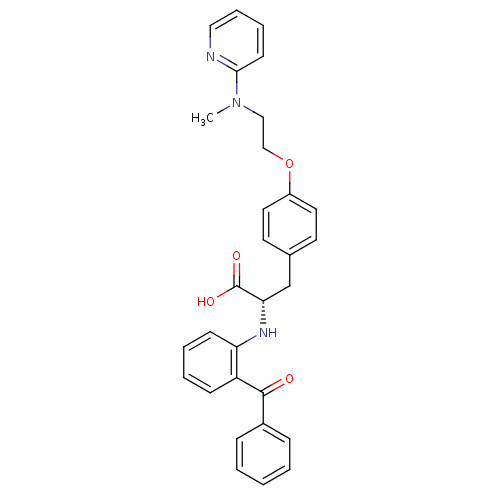
((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(methyl-pyri...)Show SMILES CN(CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)c1ccccn1 Show InChI InChI=1S/C30H29N3O4/c1-33(28-13-7-8-18-31-28)19-20-37-24-16-14-22(15-17-24)21-27(30(35)36)32-26-12-6-5-11-25(26)29(34)23-9-3-2-4-10-23/h2-18,27,32H,19-21H2,1H3,(H,35,36)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to PPARgamma (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2019.05.028
BindingDB Entry DOI: 10.7270/Q2930XSX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM28661
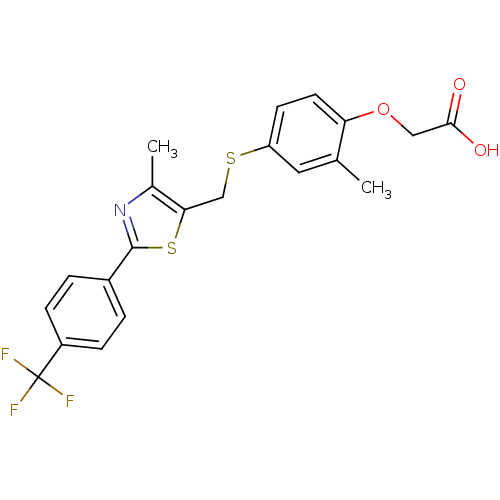
(2-{2-methyl-4-[({4-methyl-2-[4-(trifluoromethyl)ph...)Show SMILES Cc1nc(sc1CSc1ccc(OCC(O)=O)c(C)c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C21H18F3NO3S2/c1-12-9-16(7-8-17(12)28-10-19(26)27)29-11-18-13(2)25-20(30-18)14-3-5-15(6-4-14)21(22,23)24/h3-9H,10-11H2,1-2H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to PPARdelta (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2019.05.028
BindingDB Entry DOI: 10.7270/Q2930XSX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50099491
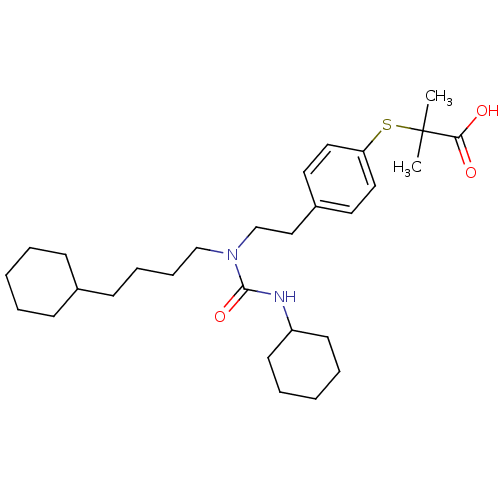
(2-(4-(2-(3-cyclohexyl-1-(4-cyclohexylbutyl)ureido)...)Show SMILES CC(C)(Sc1ccc(CCN(CCCCC2CCCCC2)C(=O)NC2CCCCC2)cc1)C(O)=O Show InChI InChI=1S/C29H46N2O3S/c1-29(2,27(32)33)35-26-18-16-24(17-19-26)20-22-31(28(34)30-25-14-7-4-8-15-25)21-10-9-13-23-11-5-3-6-12-23/h16-19,23,25H,3-15,20-22H2,1-2H3,(H,30,34)(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to PPARalpha (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2019.05.028
BindingDB Entry DOI: 10.7270/Q2930XSX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50103521
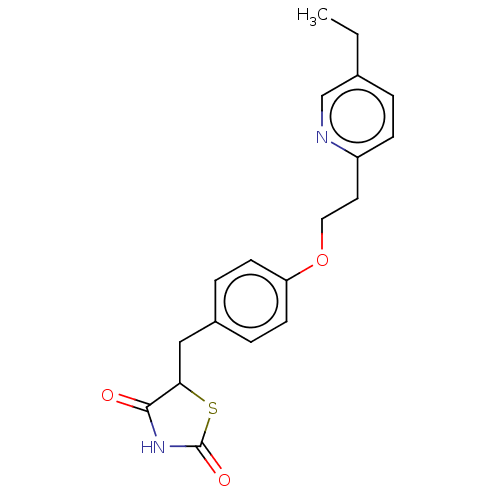
(Actos | CHEBI:8228 | Duetact | Pioglitazone | US10...)Show InChI InChI=1S/C19H20N2O3S/c1-2-13-3-6-15(20-12-13)9-10-24-16-7-4-14(5-8-16)11-17-18(22)21-19(23)25-17/h3-8,12,17H,2,9-11H2,1H3,(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to PPARgamma (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2019.05.028
BindingDB Entry DOI: 10.7270/Q2930XSX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50546080

(CHEMBL4746218)Show SMILES Oc1cc(O)c2c(oc(cc2=O)-c2ccccc2)c1C(C=C)c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to PPARalpha (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2019.05.028
BindingDB Entry DOI: 10.7270/Q2930XSX |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50546080

(CHEMBL4746218)Show SMILES Oc1cc(O)c2c(oc(cc2=O)-c2ccccc2)c1C(C=C)c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to PPARdelta (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2019.05.028
BindingDB Entry DOI: 10.7270/Q2930XSX |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50546080

(CHEMBL4746218)Show SMILES Oc1cc(O)c2c(oc(cc2=O)-c2ccccc2)c1C(C=C)c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to PPARgamma (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2019.05.028
BindingDB Entry DOI: 10.7270/Q2930XSX |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50546079

(CHEMBL4746258)Show SMILES COc1cc(ccc1O)C(C=C)c1c(O)cc(O)c2c1oc(cc2=O)-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to PPARalpha (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2019.05.028
BindingDB Entry DOI: 10.7270/Q2930XSX |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50546079

(CHEMBL4746258)Show SMILES COc1cc(ccc1O)C(C=C)c1c(O)cc(O)c2c1oc(cc2=O)-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to PPARdelta (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2019.05.028
BindingDB Entry DOI: 10.7270/Q2930XSX |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50546079

(CHEMBL4746258)Show SMILES COc1cc(ccc1O)C(C=C)c1c(O)cc(O)c2c1oc(cc2=O)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to PPARgamma (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2019.05.028
BindingDB Entry DOI: 10.7270/Q2930XSX |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM24566
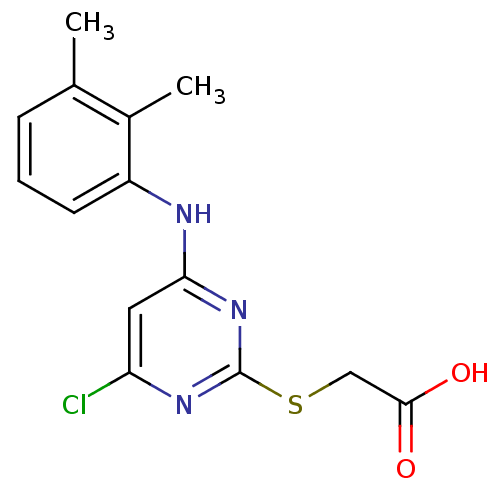
(2-({4-chloro-6-[(2,3-dimethylphenyl)amino]pyrimidi...)Show InChI InChI=1S/C14H14ClN3O2S/c1-8-4-3-5-10(9(8)2)16-12-6-11(15)17-14(18-12)21-7-13(19)20/h3-6H,7H2,1-2H3,(H,19,20)(H,16,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to PPARalpha (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2019.05.028
BindingDB Entry DOI: 10.7270/Q2930XSX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50029593

(CHEMBL7162 | N-(2-(cyclohexyloxy)-4-nitrophenyl)me...)Show InChI InChI=1S/C13H18N2O5S/c1-21(18,19)14-12-8-7-10(15(16)17)9-13(12)20-11-5-3-2-4-6-11/h7-9,11,14H,2-6H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Pharmacy at Ho Chi Minh City
Curated by ChEMBL
| Assay Description
Inhibition of COX2-mediated PGE2 production in LPS-stimulated mouse RAW264.7 cells by enzyme immunoassay |
Bioorg Med Chem Lett 19: 1650-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.001
BindingDB Entry DOI: 10.7270/Q28052HJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50140257
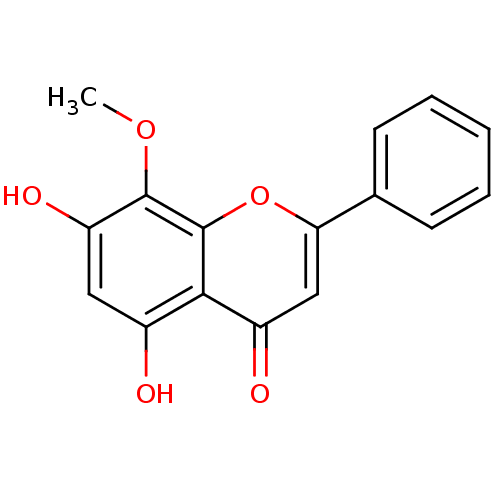
(5,7-Dihydroxy-8-methoxy-2-phenyl-chromen-4-one | 5...)Show InChI InChI=1S/C16H12O5/c1-20-15-12(19)7-10(17)14-11(18)8-13(21-16(14)15)9-5-3-2-4-6-9/h2-8,17,19H,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Pharmacy at Ho Chi Minh City
Curated by ChEMBL
| Assay Description
Inhibition of COX2-mediated PGE2 production in LPS-stimulated mouse RAW264.7 cells by enzyme immunoassay |
Bioorg Med Chem Lett 19: 1650-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.001
BindingDB Entry DOI: 10.7270/Q28052HJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50254327

(2-(2,4-dimethoxyphenyl)-6-(4-oxo-2-phenyl-4H-chrom...)Show SMILES COc1ccc(c(OC)c1)-c1cc(=O)c2cc(Oc3ccc4c(c3)oc(cc4=O)-c3ccccc3)ccc2o1 Show InChI InChI=1S/C32H22O7/c1-35-20-8-12-24(30(15-20)36-2)32-18-27(34)25-14-21(10-13-28(25)38-32)37-22-9-11-23-26(33)17-29(39-31(23)16-22)19-6-4-3-5-7-19/h3-18H,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of COX2-mediated PGE2 production in LPS-stimulated mouse RAW264.7 cells by ELISA |
Bioorg Med Chem Lett 19: 74-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.017
BindingDB Entry DOI: 10.7270/Q24B3153 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50254381

(2-(4-methoxyphenyl)-6-(4-oxo-2-phenyl-4H-chromen-7...)Show SMILES COc1ccc(cc1)-c1cc(=O)c2cc(Oc3ccc4c(c3)oc(cc4=O)-c3ccccc3)ccc2o1 Show InChI InChI=1S/C31H20O6/c1-34-21-9-7-20(8-10-21)30-18-27(33)25-15-22(12-14-28(25)36-30)35-23-11-13-24-26(32)17-29(37-31(24)16-23)19-5-3-2-4-6-19/h2-18H,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of COX2-mediated PGE2 production in LPS-stimulated mouse RAW264.7 cells by ELISA |
Bioorg Med Chem Lett 19: 74-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.017
BindingDB Entry DOI: 10.7270/Q24B3153 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50257785
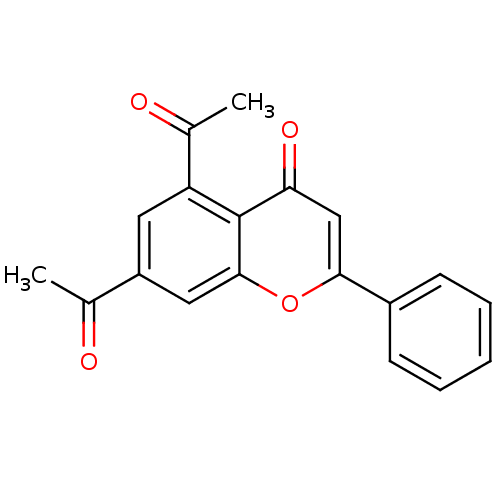
(5,7-diacetylflavone | CHEMBL493969)Show SMILES CC(=O)c1cc(C(C)=O)c2c(c1)oc(cc2=O)-c1ccccc1 Show InChI InChI=1S/C19H14O4/c1-11(20)14-8-15(12(2)21)19-16(22)10-17(23-18(19)9-14)13-6-4-3-5-7-13/h3-10H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Pharmacy at Ho Chi Minh City
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Bioorg Med Chem Lett 19: 1650-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.001
BindingDB Entry DOI: 10.7270/Q28052HJ |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50183248
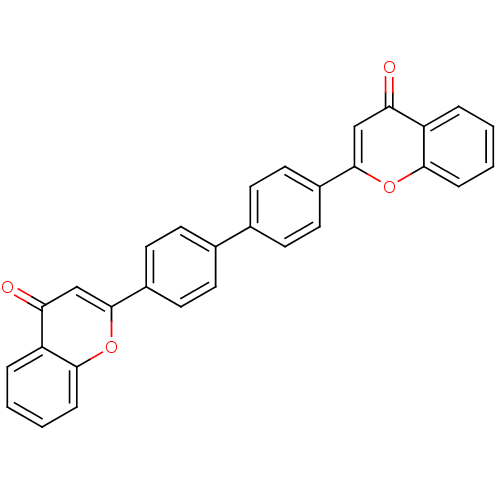
(2-{4-[4-(4-oxo-4H-chromen-2-yl)phenyl]phenyl}-4H-c...)Show SMILES O=c1cc(oc2ccccc12)-c1ccc(cc1)-c1ccc(cc1)-c1cc(=O)c2ccccc2o1 Show InChI InChI=1S/C30H18O4/c31-25-17-29(33-27-7-3-1-5-23(25)27)21-13-9-19(10-14-21)20-11-15-22(16-12-20)30-18-26(32)24-6-2-4-8-28(24)34-30/h1-18H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of sPLA2-2A in HEK293 cell |
Bioorg Med Chem Lett 16: 2373-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.117
BindingDB Entry DOI: 10.7270/Q20Z72VZ |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50183250

(2-(3-(4-(5,7-dihydroxy-4-oxo-4H-chromen-2-yl)pheno...)Show SMILES Oc1cc2oc(cc(O)c2c(=O)c1)-c1ccc(Oc2cc(ccc2O)-c2cc(O)c3c(cc(O)cc3=O)o2)cc1 Show InChI InChI=1S/C30H18O10/c31-16-8-20(34)29-22(36)12-24(39-27(29)10-16)14-1-4-18(5-2-14)38-26-7-15(3-6-19(26)33)25-13-23(37)30-21(35)9-17(32)11-28(30)40-25/h1-13,31-33,36-37H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of sPLA2-2A in HEK293 cell |
Bioorg Med Chem Lett 16: 2373-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.117
BindingDB Entry DOI: 10.7270/Q20Z72VZ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50257725
(2,3-dimethoxy-2'-hydroxychalcone | CHEMBL492959)Show InChI InChI=1S/C17H16O4/c1-20-16-9-5-6-12(17(16)21-2)10-11-15(19)13-7-3-4-8-14(13)18/h3-11,18H,1-2H3/b11-10+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Pharmacy at Ho Chi Minh City
Curated by ChEMBL
| Assay Description
Inhibition of COX2-mediated PGE2 production in LPS-stimulated mouse RAW264.7 cells by enzyme immunoassay |
Bioorg Med Chem Lett 19: 1650-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.001
BindingDB Entry DOI: 10.7270/Q28052HJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50241204

((E)-3-(4-(benzyloxy)phenyl)-1-(2-hydroxyphenyl)pro...)Show InChI InChI=1S/C22H18O3/c23-21-9-5-4-8-20(21)22(24)15-12-17-10-13-19(14-11-17)25-16-18-6-2-1-3-7-18/h1-15,23H,16H2/b15-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Pharmacy at Ho Chi Minh City
Curated by ChEMBL
| Assay Description
Inhibition of COX2-mediated PGE2 production in LPS-stimulated mouse RAW264.7 cells by enzyme immunoassay |
Bioorg Med Chem Lett 19: 1650-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.001
BindingDB Entry DOI: 10.7270/Q28052HJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50257724
(3,4-dibenzyloxy-2'-hydroxychalcone | CHEMBL492554)Show SMILES Oc1ccccc1C(=O)\C=C\c1ccc(OCc2ccccc2)c(OCc2ccccc2)c1 Show InChI InChI=1S/C29H24O4/c30-26-14-8-7-13-25(26)27(31)17-15-22-16-18-28(32-20-23-9-3-1-4-10-23)29(19-22)33-21-24-11-5-2-6-12-24/h1-19,30H,20-21H2/b17-15+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Pharmacy at Ho Chi Minh City
Curated by ChEMBL
| Assay Description
Inhibition of COX2-mediated PGE2 production in LPS-stimulated mouse RAW264.7 cells by enzyme immunoassay |
Bioorg Med Chem Lett 19: 1650-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.001
BindingDB Entry DOI: 10.7270/Q28052HJ |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50183248

(2-{4-[4-(4-oxo-4H-chromen-2-yl)phenyl]phenyl}-4H-c...)Show SMILES O=c1cc(oc2ccccc12)-c1ccc(cc1)-c1ccc(cc1)-c1cc(=O)c2ccccc2o1 Show InChI InChI=1S/C30H18O4/c31-25-17-29(33-27-7-3-1-5-23(25)27)21-13-9-19(10-14-21)20-11-15-22(16-12-20)30-18-26(32)24-6-2-4-8-28(24)34-30/h1-18H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yeungnam University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned cPLA2 expressed in HEK293 cells |
Bioorg Med Chem 15: 7138-43 (2007)
Article DOI: 10.1016/j.bmc.2007.07.054
BindingDB Entry DOI: 10.7270/Q2WM1D5G |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50257726

(2'-hydroxy-3,4,5-trimethoxychalcone | CHEMBL521653)Show InChI InChI=1S/C18H18O5/c1-21-16-10-12(11-17(22-2)18(16)23-3)8-9-15(20)13-6-4-5-7-14(13)19/h4-11,19H,1-3H3/b9-8+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Pharmacy at Ho Chi Minh City
Curated by ChEMBL
| Assay Description
Inhibition of COX2-mediated PGE2 production in LPS-stimulated mouse RAW264.7 cells by enzyme immunoassay |
Bioorg Med Chem Lett 19: 1650-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.001
BindingDB Entry DOI: 10.7270/Q28052HJ |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50183244
(3-(4-(4-oxo-4H-chromen-2-yl)phenyl)-2-phenyl-4H-ch...)Show SMILES O=c1cc(oc2ccccc12)-c1ccc(cc1)-c1c(oc2ccccc2c1=O)-c1ccccc1 Show InChI InChI=1S/C30H18O4/c31-24-18-27(33-25-12-6-4-10-22(24)25)19-14-16-20(17-15-19)28-29(32)23-11-5-7-13-26(23)34-30(28)21-8-2-1-3-9-21/h1-18H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yeungnam University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned cPLA2 expressed in HEK293 cells |
Bioorg Med Chem 15: 7138-43 (2007)
Article DOI: 10.1016/j.bmc.2007.07.054
BindingDB Entry DOI: 10.7270/Q2WM1D5G |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50254380
(6,7'-oxybis(2-(2,4-dihydroxyphenyl)-4H-chromen-4-o...)Show SMILES Oc1ccc(c(O)c1)-c1cc(=O)c2cc(Oc3ccc4c(c3)oc(cc4=O)-c3ccc(O)cc3O)ccc2o1 Show InChI InChI=1S/C30H18O9/c31-15-1-5-19(23(33)9-15)29-14-26(36)22-11-17(4-8-27(22)38-29)37-18-3-7-21-25(35)13-30(39-28(21)12-18)20-6-2-16(32)10-24(20)34/h1-14,31-34H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS-mediated NO production in LPS-stimulated mouse RAW264.7 cells by Griess method |
Bioorg Med Chem Lett 19: 74-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.017
BindingDB Entry DOI: 10.7270/Q24B3153 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50254380

(6,7'-oxybis(2-(2,4-dihydroxyphenyl)-4H-chromen-4-o...)Show SMILES Oc1ccc(c(O)c1)-c1cc(=O)c2cc(Oc3ccc4c(c3)oc(cc4=O)-c3ccc(O)cc3O)ccc2o1 Show InChI InChI=1S/C30H18O9/c31-15-1-5-19(23(33)9-15)29-14-26(36)22-11-17(4-8-27(22)38-29)37-18-3-7-21-25(35)13-30(39-28(21)12-18)20-6-2-16(32)10-24(20)34/h1-14,31-34H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of COX2-mediated PGE2 production in LPS-stimulated mouse RAW264.7 cells by ELISA |
Bioorg Med Chem Lett 19: 74-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.017
BindingDB Entry DOI: 10.7270/Q24B3153 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50253965

(2-(4-hydroxyphenyl)-7-(2-(2-hydroxyphenyl)-4-oxo-4...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2ccc(Oc3ccc4oc(cc(=O)c4c3)-c3ccccc3O)cc2o1 Show InChI InChI=1S/C30H18O7/c31-18-7-5-17(6-8-18)28-15-25(33)22-11-9-20(14-29(22)37-28)35-19-10-12-27-23(13-19)26(34)16-30(36-27)21-3-1-2-4-24(21)32/h1-16,31-32H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS-mediated NO production in LPS-stimulated mouse RAW264.7 cells by Griess method |
Bioorg Med Chem Lett 19: 74-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.017
BindingDB Entry DOI: 10.7270/Q24B3153 |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50183245

(6-[4-(4-Oxo-4H-chromen-2-yl)-phenyl]-2-phenyl-chro...)Show SMILES O=c1cc(oc2ccccc12)-c1ccc(cc1)-c1ccc2oc(cc(=O)c2c1)-c1ccccc1 Show InChI InChI=1S/C30H18O4/c31-25-17-30(33-27-9-5-4-8-23(25)27)21-12-10-19(11-13-21)22-14-15-28-24(16-22)26(32)18-29(34-28)20-6-2-1-3-7-20/h1-18H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yeungnam University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned cPLA2 expressed in HEK293 cells |
Bioorg Med Chem 15: 7138-43 (2007)
Article DOI: 10.1016/j.bmc.2007.07.054
BindingDB Entry DOI: 10.7270/Q2WM1D5G |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50183247

(2-{4-[3-(4-oxo-4H-chromen-2-yl)phenyl]phenyl}-4H-c...)Show SMILES O=c1cc(oc2ccccc12)-c1ccc(cc1)-c1cccc(c1)-c1cc(=O)c2ccccc2o1 Show InChI InChI=1S/C30H18O4/c31-25-17-29(33-27-10-3-1-8-23(25)27)20-14-12-19(13-15-20)21-6-5-7-22(16-21)30-18-26(32)24-9-2-4-11-28(24)34-30/h1-18H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of sPLA2-2A in HEK293 cell |
Bioorg Med Chem Lett 16: 2373-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.117
BindingDB Entry DOI: 10.7270/Q20Z72VZ |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50183246
(6-[3-(4-Oxo-4H-chromen-2-yl)-phenyl]-2-phenyl-chro...)Show SMILES O=c1cc(oc2ccccc12)-c1cccc(c1)-c1ccc2oc(cc(=O)c2c1)-c1ccccc1 Show InChI InChI=1S/C30H18O4/c31-25-17-30(33-27-12-5-4-11-23(25)27)22-10-6-9-20(15-22)21-13-14-28-24(16-21)26(32)18-29(34-28)19-7-2-1-3-8-19/h1-18H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yeungnam University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned cPLA2 expressed in HEK293 cells |
Bioorg Med Chem 15: 7138-43 (2007)
Article DOI: 10.1016/j.bmc.2007.07.054
BindingDB Entry DOI: 10.7270/Q2WM1D5G |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50183246

(6-[3-(4-Oxo-4H-chromen-2-yl)-phenyl]-2-phenyl-chro...)Show SMILES O=c1cc(oc2ccccc12)-c1cccc(c1)-c1ccc2oc(cc(=O)c2c1)-c1ccccc1 Show InChI InChI=1S/C30H18O4/c31-25-17-30(33-27-12-5-4-11-23(25)27)22-10-6-9-20(15-22)21-13-14-28-24(16-21)26(32)18-29(34-28)19-7-2-1-3-8-19/h1-18H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of sPLA2-2A in HEK293 cell |
Bioorg Med Chem Lett 16: 2373-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.117
BindingDB Entry DOI: 10.7270/Q20Z72VZ |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50183244

(3-(4-(4-oxo-4H-chromen-2-yl)phenyl)-2-phenyl-4H-ch...)Show SMILES O=c1cc(oc2ccccc12)-c1ccc(cc1)-c1c(oc2ccccc2c1=O)-c1ccccc1 Show InChI InChI=1S/C30H18O4/c31-24-18-27(33-25-12-6-4-10-22(24)25)19-14-16-20(17-15-19)28-29(32)23-11-5-7-13-26(23)34-30(28)21-8-2-1-3-9-21/h1-18H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of sPLA2-2A in HEK293 cell |
Bioorg Med Chem Lett 16: 2373-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.117
BindingDB Entry DOI: 10.7270/Q20Z72VZ |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50129952

(2-(3-(5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-c...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3cc(ccc3O)-c3cc(O)c4c(cc(O)cc4=O)o3)c2o1 |(13.25,-26.01,;14.59,-25.24,;14.59,-23.7,;15.92,-22.93,;17.26,-23.7,;17.26,-25.24,;15.93,-26.01,;18.58,-22.93,;19.91,-23.69,;21.23,-22.92,;22.57,-23.69,;21.22,-21.4,;22.56,-20.64,;23.88,-21.41,;22.55,-19.1,;21.22,-18.33,;21.22,-16.79,;19.9,-19.11,;18.57,-18.34,;17.23,-19.1,;15.9,-18.33,;15.9,-16.79,;17.23,-16.02,;18.57,-16.79,;19.91,-16.02,;14.57,-19.09,;14.56,-20.65,;13.21,-21.42,;13.21,-22.96,;11.87,-20.64,;11.88,-19.08,;10.54,-18.32,;9.21,-19.09,;7.87,-18.32,;9.21,-20.64,;10.54,-21.41,;10.54,-22.95,;13.22,-18.3,;19.9,-20.64,;18.58,-21.4,)| Show InChI InChI=1S/C30H18O10/c31-15-4-1-13(2-5-15)24-12-23(38)29-21(36)10-20(35)27(30(29)40-24)17-7-14(3-6-18(17)33)25-11-22(37)28-19(34)8-16(32)9-26(28)39-25/h1-12,31-33,35-37H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of sPLA2-2A in HEK293 cell |
Bioorg Med Chem Lett 16: 2373-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.117
BindingDB Entry DOI: 10.7270/Q20Z72VZ |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50183246

(6-[3-(4-Oxo-4H-chromen-2-yl)-phenyl]-2-phenyl-chro...)Show SMILES O=c1cc(oc2ccccc12)-c1cccc(c1)-c1ccc2oc(cc(=O)c2c1)-c1ccccc1 Show InChI InChI=1S/C30H18O4/c31-25-17-30(33-27-12-5-4-11-23(25)27)22-10-6-9-20(15-22)21-13-14-28-24(16-21)26(32)18-29(34-28)19-7-2-1-3-8-19/h1-18H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yeungnam University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned sPLA2-10 expressed in HEK293 cells |
Bioorg Med Chem 15: 7138-43 (2007)
Article DOI: 10.1016/j.bmc.2007.07.054
BindingDB Entry DOI: 10.7270/Q2WM1D5G |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50183247

(2-{4-[3-(4-oxo-4H-chromen-2-yl)phenyl]phenyl}-4H-c...)Show SMILES O=c1cc(oc2ccccc12)-c1ccc(cc1)-c1cccc(c1)-c1cc(=O)c2ccccc2o1 Show InChI InChI=1S/C30H18O4/c31-25-17-29(33-27-10-3-1-8-23(25)27)20-14-12-19(13-15-20)21-6-5-7-22(16-21)30-18-26(32)24-9-2-4-11-28(24)34-30/h1-18H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yeungnam University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned cPLA2 expressed in HEK293 cells |
Bioorg Med Chem 15: 7138-43 (2007)
Article DOI: 10.1016/j.bmc.2007.07.054
BindingDB Entry DOI: 10.7270/Q2WM1D5G |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50183249

(2,2'-Diphenyl-[6,6']bichromenyl-4,4'-dione | CHEMB...)Show SMILES O=c1cc(oc2ccc(cc12)-c1ccc2oc(cc(=O)c2c1)-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C30H18O4/c31-25-17-29(19-7-3-1-4-8-19)33-27-13-11-21(15-23(25)27)22-12-14-28-24(16-22)26(32)18-30(34-28)20-9-5-2-6-10-20/h1-18H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yeungnam University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned cPLA2 expressed in HEK293 cells |
Bioorg Med Chem 15: 7138-43 (2007)
Article DOI: 10.1016/j.bmc.2007.07.054
BindingDB Entry DOI: 10.7270/Q2WM1D5G |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50254330

(2-(2,4-dihydroxyphenyl)-6-(2-(4-hydroxyphenyl)-4-o...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2ccc(Oc3ccc4oc(cc(=O)c4c3)-c3ccc(O)cc3O)cc2o1 Show InChI InChI=1S/C30H18O8/c31-17-3-1-16(2-4-17)28-14-25(34)22-9-6-20(13-29(22)38-28)36-19-7-10-27-23(12-19)26(35)15-30(37-27)21-8-5-18(32)11-24(21)33/h1-15,31-33H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS-mediated NO production in LPS-stimulated mouse RAW264.7 cells by Griess method |
Bioorg Med Chem Lett 19: 74-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.017
BindingDB Entry DOI: 10.7270/Q24B3153 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50254331
(6,7'-oxybis(2-(2,4-dimethoxyphenyl)-4H-chromen-4-o...)Show SMILES COc1ccc(c(OC)c1)-c1cc(=O)c2cc(Oc3ccc4c(c3)oc(cc4=O)-c3ccc(OC)cc3OC)ccc2o1 Show InChI InChI=1S/C34H26O9/c1-37-19-5-10-24(30(14-19)39-3)33-18-28(36)26-13-21(8-12-29(26)42-33)41-22-7-9-23-27(35)17-34(43-32(23)16-22)25-11-6-20(38-2)15-31(25)40-4/h5-18H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS-mediated NO production in LPS-stimulated mouse RAW264.7 cells by Griess method |
Bioorg Med Chem Lett 19: 74-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.017
BindingDB Entry DOI: 10.7270/Q24B3153 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50254329
(2-(2,4-dimethoxyphenyl)-6-(2-(4-methoxyphenyl)-4-o...)Show SMILES COc1ccc(cc1)-c1cc(=O)c2ccc(Oc3ccc4oc(cc(=O)c4c3)-c3ccc(OC)cc3OC)cc2o1 Show InChI InChI=1S/C33H24O8/c1-36-20-6-4-19(5-7-20)30-17-27(34)24-11-9-23(16-32(24)41-30)39-22-10-13-29-26(14-22)28(35)18-33(40-29)25-12-8-21(37-2)15-31(25)38-3/h4-18H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS-mediated NO production in LPS-stimulated mouse RAW264.7 cells by Griess method |
Bioorg Med Chem Lett 19: 74-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.017
BindingDB Entry DOI: 10.7270/Q24B3153 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50254383
(2-(2,4-dimethoxyphenyl)-7-(2-(4-methoxyphenyl)-4-o...)Show SMILES COc1ccc(cc1)-c1cc(=O)c2cc(Oc3ccc4c(c3)oc(cc4=O)-c3ccc(OC)cc3OC)ccc2o1 Show InChI InChI=1S/C33H24O8/c1-36-20-6-4-19(5-7-20)30-17-28(35)26-14-22(10-13-29(26)40-30)39-23-9-11-24-27(34)18-33(41-32(24)16-23)25-12-8-21(37-2)15-31(25)38-3/h4-18H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS-mediated NO production in LPS-stimulated mouse RAW264.7 cells by Griess method |
Bioorg Med Chem Lett 19: 74-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.017
BindingDB Entry DOI: 10.7270/Q24B3153 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50254422
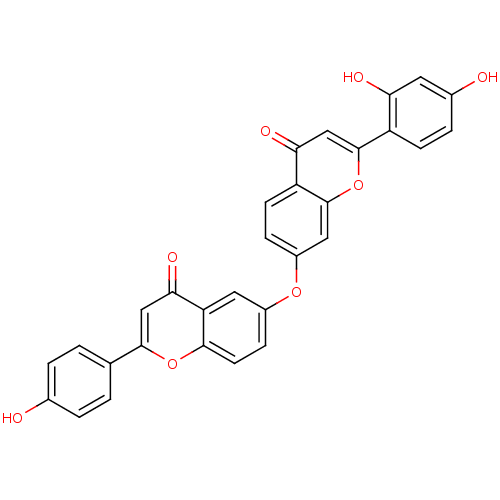
(2-(2,4-dihydroxyphenyl)-7-(2-(4-hydroxyphenyl)-4-o...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2cc(Oc3ccc4c(c3)oc(cc4=O)-c3ccc(O)cc3O)ccc2o1 Show InChI InChI=1S/C30H18O8/c31-17-3-1-16(2-4-17)28-14-26(35)23-12-19(7-10-27(23)37-28)36-20-6-9-22-25(34)15-30(38-29(22)13-20)21-8-5-18(32)11-24(21)33/h1-15,31-33H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of iNOS-mediated NO production in LPS-stimulated mouse RAW264.7 cells by Griess method |
Bioorg Med Chem Lett 19: 74-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.017
BindingDB Entry DOI: 10.7270/Q24B3153 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50254328
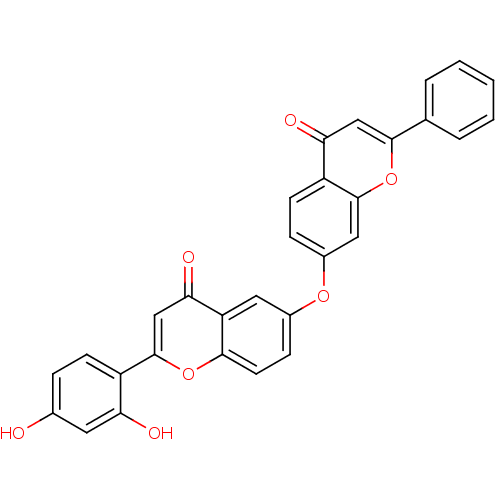
(2-(2,4-dihydroxyphenyl)-6-(4-oxo-2-phenyl-4H-chrom...)Show SMILES Oc1ccc(c(O)c1)-c1cc(=O)c2cc(Oc3ccc4c(c3)oc(cc4=O)-c3ccccc3)ccc2o1 Show InChI InChI=1S/C30H18O7/c31-18-6-9-21(24(32)12-18)30-16-26(34)23-13-19(8-11-27(23)36-30)35-20-7-10-22-25(33)15-28(37-29(22)14-20)17-4-2-1-3-5-17/h1-16,31-32H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of COX2-mediated PGE2 production in LPS-stimulated mouse RAW264.7 cells by ELISA |
Bioorg Med Chem Lett 19: 74-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.017
BindingDB Entry DOI: 10.7270/Q24B3153 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50254329

(2-(2,4-dimethoxyphenyl)-6-(2-(4-methoxyphenyl)-4-o...)Show SMILES COc1ccc(cc1)-c1cc(=O)c2ccc(Oc3ccc4oc(cc(=O)c4c3)-c3ccc(OC)cc3OC)cc2o1 Show InChI InChI=1S/C33H24O8/c1-36-20-6-4-19(5-7-20)30-17-27(34)24-11-9-23(16-32(24)41-30)39-22-10-13-29-26(14-22)28(35)18-33(40-29)25-12-8-21(37-2)15-31(25)38-3/h4-18H,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of COX2-mediated PGE2 production in LPS-stimulated mouse RAW264.7 cells by ELISA |
Bioorg Med Chem Lett 19: 74-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.017
BindingDB Entry DOI: 10.7270/Q24B3153 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50254330

(2-(2,4-dihydroxyphenyl)-6-(2-(4-hydroxyphenyl)-4-o...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2ccc(Oc3ccc4oc(cc(=O)c4c3)-c3ccc(O)cc3O)cc2o1 Show InChI InChI=1S/C30H18O8/c31-17-3-1-16(2-4-17)28-14-25(34)22-9-6-20(13-29(22)38-28)36-19-7-10-27-23(12-19)26(35)15-30(37-27)21-8-5-18(32)11-24(21)33/h1-15,31-33H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of COX2-mediated PGE2 production in LPS-stimulated mouse RAW264.7 cells by ELISA |
Bioorg Med Chem Lett 19: 74-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.017
BindingDB Entry DOI: 10.7270/Q24B3153 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50254331

(6,7'-oxybis(2-(2,4-dimethoxyphenyl)-4H-chromen-4-o...)Show SMILES COc1ccc(c(OC)c1)-c1cc(=O)c2cc(Oc3ccc4c(c3)oc(cc4=O)-c3ccc(OC)cc3OC)ccc2o1 Show InChI InChI=1S/C34H26O9/c1-37-19-5-10-24(30(14-19)39-3)33-18-28(36)26-13-21(8-12-29(26)42-33)41-22-7-9-23-27(35)17-34(43-32(23)16-22)25-11-6-20(38-2)15-31(25)40-4/h5-18H,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of COX2-mediated PGE2 production in LPS-stimulated mouse RAW264.7 cells by ELISA |
Bioorg Med Chem Lett 19: 74-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.017
BindingDB Entry DOI: 10.7270/Q24B3153 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50254382

(6,7'-oxybis(2-(4-hydroxyphenyl)-4H-chromen-4-one) ...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2cc(Oc3ccc4c(c3)oc(cc4=O)-c3ccc(O)cc3)ccc2o1 Show InChI InChI=1S/C30H18O7/c31-19-5-1-17(2-6-19)28-16-26(34)24-13-21(10-12-27(24)36-28)35-22-9-11-23-25(33)15-29(37-30(23)14-22)18-3-7-20(32)8-4-18/h1-16,31-32H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of COX2-mediated PGE2 production in LPS-stimulated mouse RAW264.7 cells by ELISA |
Bioorg Med Chem Lett 19: 74-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.017
BindingDB Entry DOI: 10.7270/Q24B3153 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50254383

(2-(2,4-dimethoxyphenyl)-7-(2-(4-methoxyphenyl)-4-o...)Show SMILES COc1ccc(cc1)-c1cc(=O)c2cc(Oc3ccc4c(c3)oc(cc4=O)-c3ccc(OC)cc3OC)ccc2o1 Show InChI InChI=1S/C33H24O8/c1-36-20-6-4-19(5-7-20)30-17-28(35)26-14-22(10-13-29(26)40-30)39-23-9-11-24-27(34)18-33(41-32(24)16-23)25-12-8-21(37-2)15-31(25)38-3/h4-18H,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of COX2-mediated PGE2 production in LPS-stimulated mouse RAW264.7 cells by ELISA |
Bioorg Med Chem Lett 19: 74-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.017
BindingDB Entry DOI: 10.7270/Q24B3153 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50254422

(2-(2,4-dihydroxyphenyl)-7-(2-(4-hydroxyphenyl)-4-o...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2cc(Oc3ccc4c(c3)oc(cc4=O)-c3ccc(O)cc3O)ccc2o1 Show InChI InChI=1S/C30H18O8/c31-17-3-1-16(2-4-17)28-14-26(35)23-12-19(7-10-27(23)37-28)36-20-6-9-22-25(34)15-30(38-29(22)13-20)21-8-5-18(32)11-24(21)33/h1-15,31-33H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of COX2-mediated PGE2 production in LPS-stimulated mouse RAW264.7 cells by ELISA |
Bioorg Med Chem Lett 19: 74-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.017
BindingDB Entry DOI: 10.7270/Q24B3153 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50254423
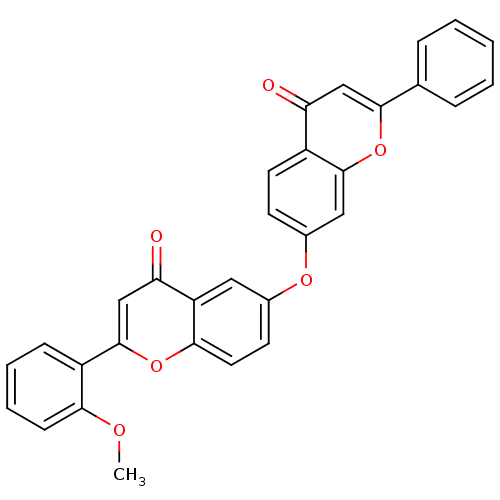
(2-(2-methoxyphenyl)-6-(4-oxo-2-phenyl-4H-chromen-7...)Show SMILES COc1ccccc1-c1cc(=O)c2cc(Oc3ccc4c(c3)oc(cc4=O)-c3ccccc3)ccc2o1 Show InChI InChI=1S/C31H20O6/c1-34-27-10-6-5-9-23(27)31-18-26(33)24-15-20(12-14-28(24)36-31)35-21-11-13-22-25(32)17-29(37-30(22)16-21)19-7-3-2-4-8-19/h2-18H,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of COX2-mediated PGE2 production in LPS-stimulated mouse RAW264.7 cells by ELISA |
Bioorg Med Chem Lett 19: 74-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.017
BindingDB Entry DOI: 10.7270/Q24B3153 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50254424
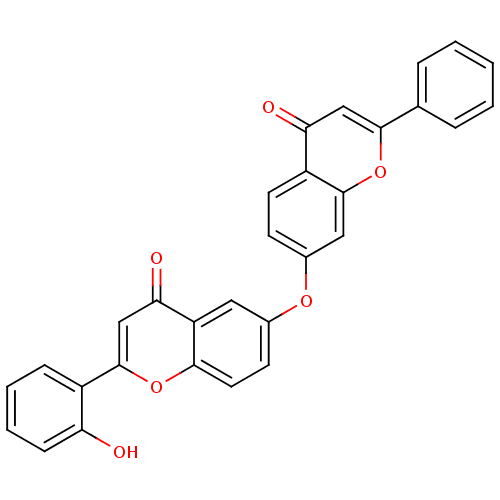
(2-(2-hydroxyphenyl)-6-(4-oxo-2-phenyl-4H-chromen-7...)Show SMILES Oc1ccccc1-c1cc(=O)c2cc(Oc3ccc4c(c3)oc(cc4=O)-c3ccccc3)ccc2o1 Show InChI InChI=1S/C30H18O6/c31-24-9-5-4-8-21(24)30-17-26(33)23-14-19(11-13-27(23)35-30)34-20-10-12-22-25(32)16-28(36-29(22)15-20)18-6-2-1-3-7-18/h1-17,31H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kangwon National University
Curated by ChEMBL
| Assay Description
Inhibition of COX2-mediated PGE2 production in LPS-stimulated mouse RAW264.7 cells by ELISA |
Bioorg Med Chem Lett 19: 74-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.017
BindingDB Entry DOI: 10.7270/Q24B3153 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data