 Found 135 hits with Last Name = 'knapp' and Initial = 'ms'
Found 135 hits with Last Name = 'knapp' and Initial = 'ms' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140266
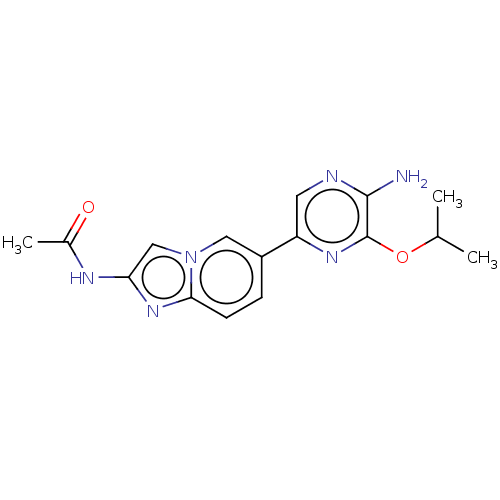
(CHEMBL3753366)Show InChI InChI=1S/C16H18N6O2/c1-9(2)24-16-15(17)18-6-12(20-16)11-4-5-14-21-13(19-10(3)23)8-22(14)7-11/h4-9H,1-3H3,(H2,17,18)(H,19,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439721
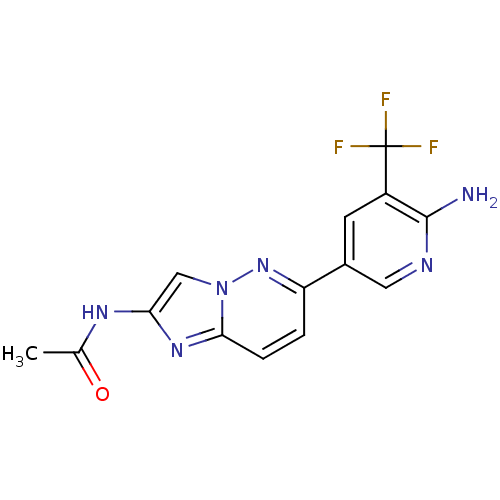
(CHEMBL2418953)Show SMILES CC(=O)Nc1cn2nc(ccc2n1)-c1cnc(N)c(c1)C(F)(F)F Show InChI InChI=1S/C14H11F3N6O/c1-7(24)20-11-6-23-12(21-11)3-2-10(22-23)8-4-9(14(15,16)17)13(18)19-5-8/h2-6H,1H3,(H2,18,19)(H,20,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439721

(CHEMBL2418953)Show SMILES CC(=O)Nc1cn2nc(ccc2n1)-c1cnc(N)c(c1)C(F)(F)F Show InChI InChI=1S/C14H11F3N6O/c1-7(24)20-11-6-23-12(21-11)3-2-10(22-23)8-4-9(14(15,16)17)13(18)19-5-8/h2-6H,1H3,(H2,18,19)(H,20,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using [gamma33P]ATP as substrate by top counting analysis |
Bioorg Med Chem Lett 23: 4652-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.010
BindingDB Entry DOI: 10.7270/Q2NP25T0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140265
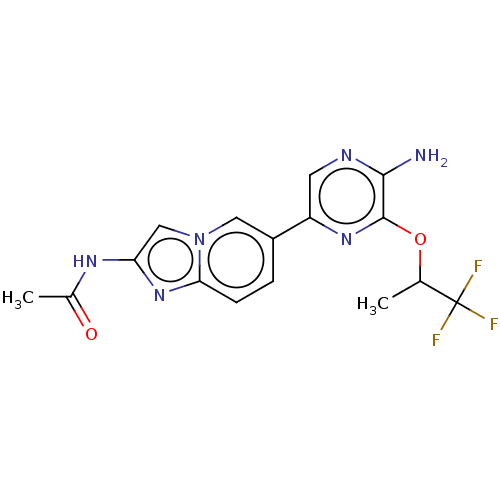
(CHEMBL3753450)Show SMILES CC(Oc1nc(cnc1N)-c1ccc2nc(NC(C)=O)cn2c1)C(F)(F)F Show InChI InChI=1S/C16H15F3N6O2/c1-8(16(17,18)19)27-15-14(20)21-5-11(23-15)10-3-4-13-24-12(22-9(2)26)7-25(13)6-10/h3-8H,1-2H3,(H2,20,21)(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140269
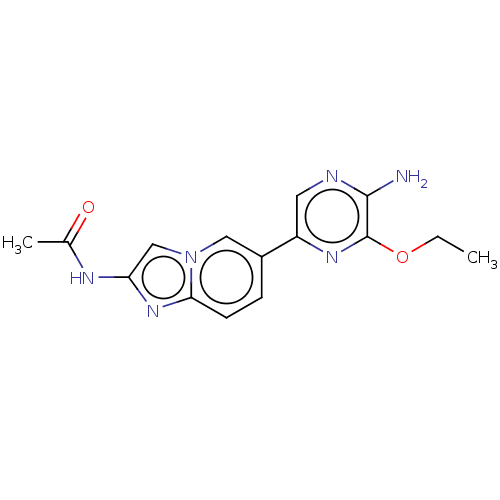
(CHEMBL3752653)Show InChI InChI=1S/C15H16N6O2/c1-3-23-15-14(16)17-6-11(19-15)10-4-5-13-20-12(18-9(2)22)8-21(13)7-10/h4-8H,3H2,1-2H3,(H2,16,17)(H,18,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140267
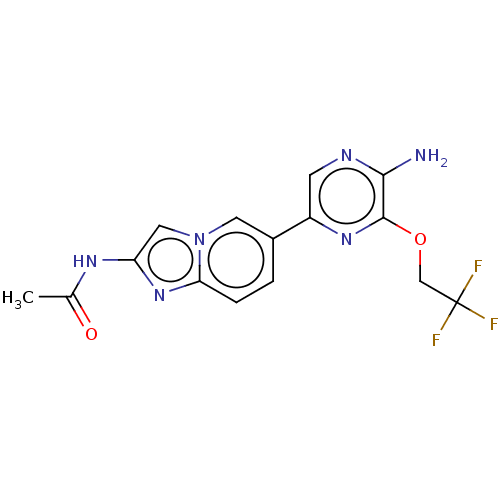
(CHEMBL3752019)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cnc(N)c(OCC(F)(F)F)n1 Show InChI InChI=1S/C15H13F3N6O2/c1-8(25)21-11-6-24-5-9(2-3-12(24)23-11)10-4-20-13(19)14(22-10)26-7-15(16,17)18/h2-6H,7H2,1H3,(H2,19,20)(H,21,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140270
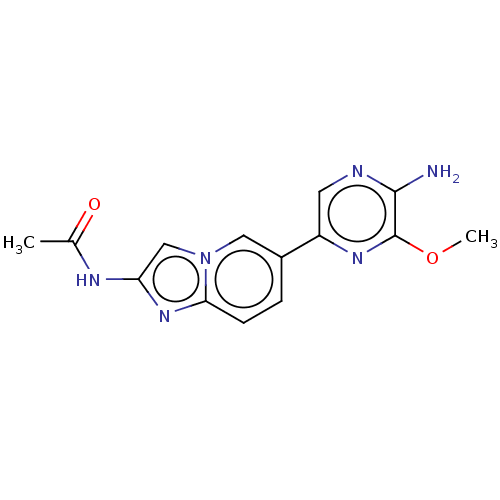
(CHEMBL3752503)Show InChI InChI=1S/C14H14N6O2/c1-8(21)17-11-7-20-6-9(3-4-12(20)19-11)10-5-16-13(15)14(18-10)22-2/h3-7H,1-2H3,(H2,15,16)(H,17,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439711
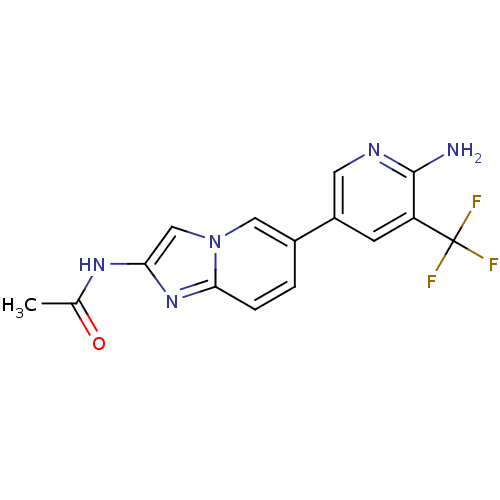
(CHEMBL2418954)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cnc(N)c(c1)C(F)(F)F Show InChI InChI=1S/C15H12F3N5O/c1-8(24)21-12-7-23-6-9(2-3-13(23)22-12)10-4-11(15(16,17)18)14(19)20-5-10/h2-7H,1H3,(H2,19,20)(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1alpha-phosphotidylinositol by luminescence assay |
Bioorg Med Chem Lett 23: 4652-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.010
BindingDB Entry DOI: 10.7270/Q2NP25T0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439711

(CHEMBL2418954)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cnc(N)c(c1)C(F)(F)F Show InChI InChI=1S/C15H12F3N5O/c1-8(24)21-12-7-23-6-9(2-3-13(23)22-12)10-4-11(15(16,17)18)14(19)20-5-10/h2-7H,1H3,(H2,19,20)(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using [gamma33P]ATP as substrate by top counting analysis |
Bioorg Med Chem Lett 23: 4652-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.010
BindingDB Entry DOI: 10.7270/Q2NP25T0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439711

(CHEMBL2418954)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cnc(N)c(c1)C(F)(F)F Show InChI InChI=1S/C15H12F3N5O/c1-8(24)21-12-7-23-6-9(2-3-13(23)22-12)10-4-11(15(16,17)18)14(19)20-5-10/h2-7H,1H3,(H2,19,20)(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140273
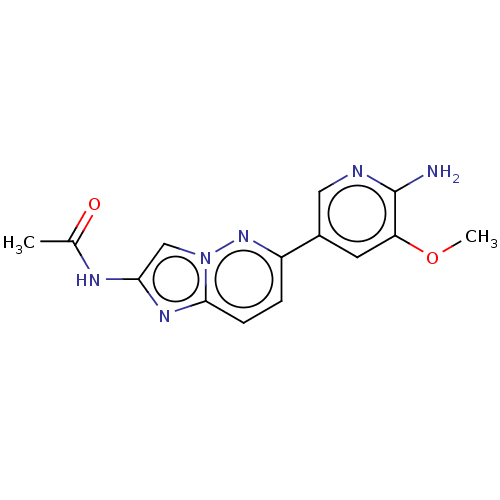
(CHEMBL3752760)Show InChI InChI=1S/C14H14N6O2/c1-8(21)17-12-7-20-13(18-12)4-3-10(19-20)9-5-11(22-2)14(15)16-6-9/h3-7H,1-2H3,(H2,15,16)(H,17,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140271
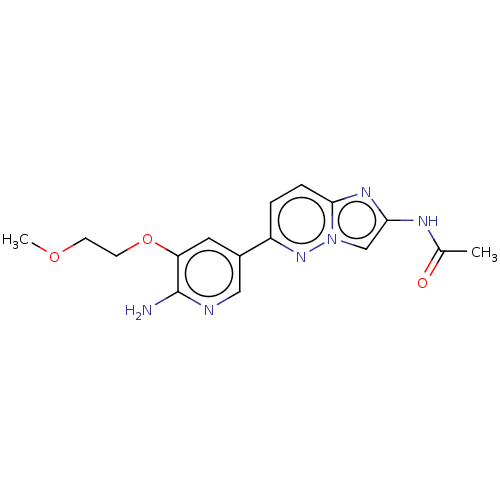
(CHEMBL3751961)Show InChI InChI=1S/C16H18N6O3/c1-10(23)19-14-9-22-15(20-14)4-3-12(21-22)11-7-13(16(17)18-8-11)25-6-5-24-2/h3-4,7-9H,5-6H2,1-2H3,(H2,17,18)(H,19,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50331592

(3-(6-(6-methoxypyridin-3-ylamino)-2-morpholinopyri...)Show SMILES COc1ccc(Nc2cc(nc(n2)N2CCOCC2)-c2cccc(O)c2)cn1 Show InChI InChI=1S/C20H21N5O3/c1-27-19-6-5-15(13-21-19)22-18-12-17(14-3-2-4-16(26)11-14)23-20(24-18)25-7-9-28-10-8-25/h2-6,11-13,26H,7-10H2,1H3,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50140265

(CHEMBL3753450)Show SMILES CC(Oc1nc(cnc1N)-c1ccc2nc(NC(C)=O)cn2c1)C(F)(F)F Show InChI InChI=1S/C16H15F3N6O2/c1-8(16(17,18)19)27-15-14(20)21-5-11(23-15)10-3-4-13-24-12(22-9(2)26)7-25(13)6-10/h3-8H,1-2H3,(H2,20,21)(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439726
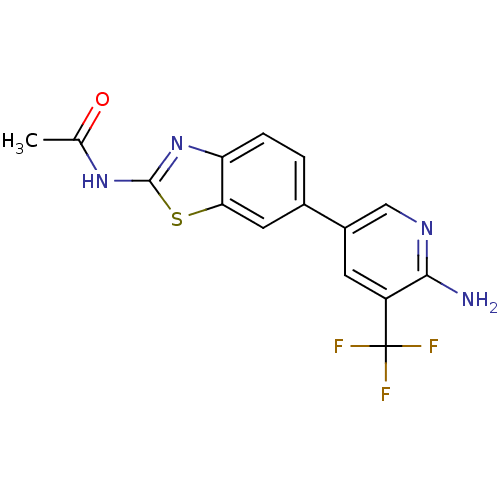
(CHEMBL2418948)Show SMILES CC(=O)Nc1nc2ccc(cc2s1)-c1cnc(N)c(c1)C(F)(F)F Show InChI InChI=1S/C15H11F3N4OS/c1-7(23)21-14-22-11-3-2-8(5-12(11)24-14)9-4-10(15(16,17)18)13(19)20-6-9/h2-6H,1H3,(H2,19,20)(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using [gamma33P]ATP as substrate by top counting analysis |
Bioorg Med Chem Lett 23: 4652-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.010
BindingDB Entry DOI: 10.7270/Q2NP25T0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140268

(CHEMBL3753085)Show InChI InChI=1S/C16H18N6O3/c1-10(23)19-13-9-22-8-11(3-4-14(22)21-13)12-7-18-15(17)16(20-12)25-6-5-24-2/h3-4,7-9H,5-6H2,1-2H3,(H2,17,18)(H,19,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50140265

(CHEMBL3753450)Show SMILES CC(Oc1nc(cnc1N)-c1ccc2nc(NC(C)=O)cn2c1)C(F)(F)F Show InChI InChI=1S/C16H15F3N6O2/c1-8(16(17,18)19)27-15-14(20)21-5-11(23-15)10-3-4-13-24-12(22-9(2)26)7-25(13)6-10/h3-8H,1-2H3,(H2,20,21)(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50439711

(CHEMBL2418954)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cnc(N)c(c1)C(F)(F)F Show InChI InChI=1S/C15H12F3N5O/c1-8(24)21-12-7-23-6-9(2-3-13(23)22-12)10-4-11(15(16,17)18)14(19)20-5-10/h2-7H,1H3,(H2,19,20)(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using 1alpha-phosphotidylinositol by luminescence assay |
Bioorg Med Chem Lett 23: 4652-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.010
BindingDB Entry DOI: 10.7270/Q2NP25T0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140272
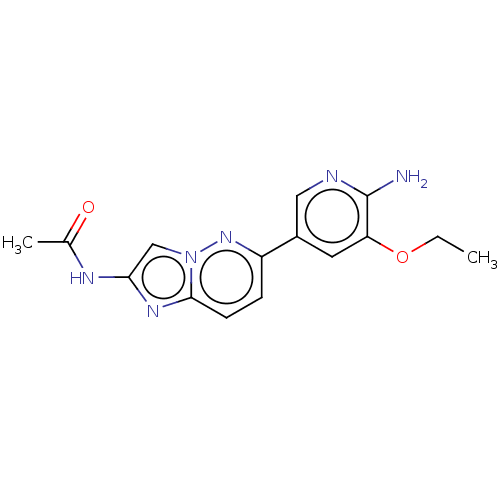
(CHEMBL3754572)Show InChI InChI=1S/C15H16N6O2/c1-3-23-12-6-10(7-17-15(12)16)11-4-5-14-19-13(18-9(2)22)8-21(14)20-11/h4-8H,3H2,1-2H3,(H2,16,17)(H,18,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156294

(CHEMBL3781415)Show InChI InChI=1S/C17H17N7/c18-16-21-10-13(15(23-16)9-11-1-2-11)14-5-8-20-17(24-14)22-12-3-6-19-7-4-12/h3-8,10-11H,1-2,9H2,(H2,18,21,23)(H,19,20,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of VPS34 in human U2OS cells incubated for 2 hrs by GFP-FYVE reporter gene assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140275

(CHEMBL3752775)Show InChI InChI=1S/C15H15N5O2/c1-9(21)18-13-8-20-7-10(3-4-14(20)19-13)11-5-12(22-2)15(16)17-6-11/h3-8H,1-2H3,(H2,16,17)(H,18,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439723
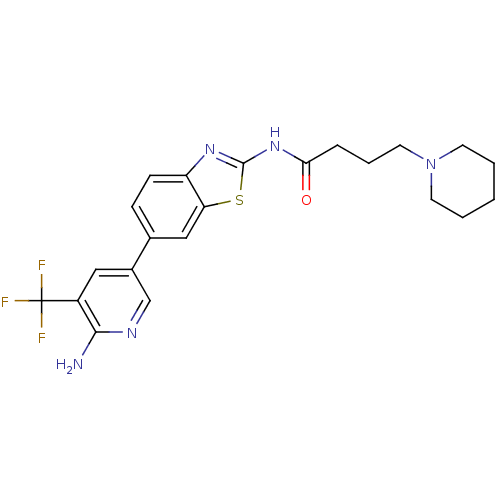
(CHEMBL2418951)Show SMILES Nc1ncc(cc1C(F)(F)F)-c1ccc2nc(NC(=O)CCCN3CCCCC3)sc2c1 Show InChI InChI=1S/C22H24F3N5OS/c23-22(24,25)16-11-15(13-27-20(16)26)14-6-7-17-18(12-14)32-21(28-17)29-19(31)5-4-10-30-8-2-1-3-9-30/h6-7,11-13H,1-5,8-10H2,(H2,26,27)(H,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using [gamma33P]ATP as substrate by top counting analysis |
Bioorg Med Chem Lett 23: 4652-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.010
BindingDB Entry DOI: 10.7270/Q2NP25T0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156302
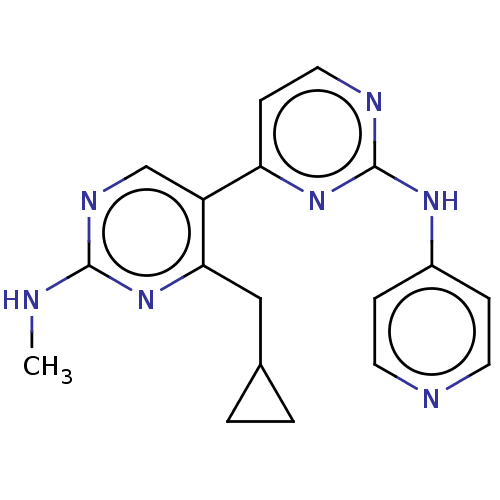
(CHEMBL3780029)Show InChI InChI=1S/C18H19N7/c1-19-17-22-11-14(16(25-17)10-12-2-3-12)15-6-9-21-18(24-15)23-13-4-7-20-8-5-13/h4-9,11-12H,2-3,10H2,1H3,(H,19,22,25)(H,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of VPS34 in human U2OS cells incubated for 2 hrs by GFP-FYVE reporter gene assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156296

(CHEMBL3781466)Show SMILES CC(C)(O)CNc1ncc(c(CC2CC2)n1)-c1ccnc(Nc2ccncc2)n1 Show InChI InChI=1S/C21H25N7O/c1-21(2,29)13-25-19-24-12-16(18(28-19)11-14-3-4-14)17-7-10-23-20(27-17)26-15-5-8-22-9-6-15/h5-10,12,14,29H,3-4,11,13H2,1-2H3,(H,24,25,28)(H,22,23,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140274

(CHEMBL3753665)Show InChI InChI=1S/C16H17N5O2/c1-3-23-13-6-12(7-18-16(13)17)11-4-5-15-20-14(19-10(2)22)9-21(15)8-11/h4-9H,3H2,1-2H3,(H2,17,18)(H,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50331592

(3-(6-(6-methoxypyridin-3-ylamino)-2-morpholinopyri...)Show SMILES COc1ccc(Nc2cc(nc(n2)N2CCOCC2)-c2cccc(O)c2)cn1 Show InChI InChI=1S/C20H21N5O3/c1-27-19-6-5-15(13-21-19)22-18-12-17(14-3-2-4-16(26)11-14)23-20(24-18)25-7-9-28-10-8-25/h2-6,11-13,26H,7-10H2,1H3,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156294

(CHEMBL3781415)Show InChI InChI=1S/C17H17N7/c18-16-21-10-13(15(23-16)9-11-1-2-11)14-5-8-20-17(24-14)22-12-3-6-19-7-4-12/h3-8,10-11H,1-2,9H2,(H2,18,21,23)(H,19,20,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439722
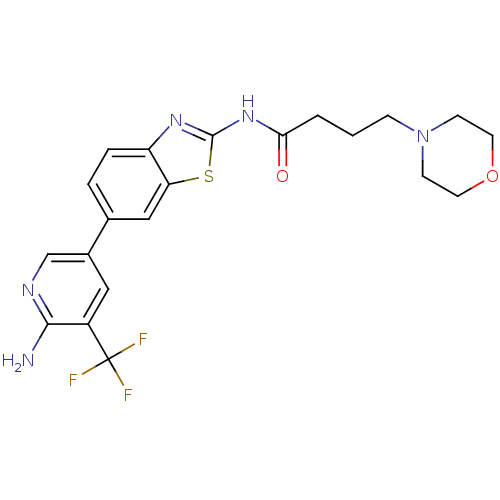
(CHEMBL2418952)Show SMILES Nc1ncc(cc1C(F)(F)F)-c1ccc2nc(NC(=O)CCCN3CCOCC3)sc2c1 Show InChI InChI=1S/C21H22F3N5O2S/c22-21(23,24)15-10-14(12-26-19(15)25)13-3-4-16-17(11-13)32-20(27-16)28-18(30)2-1-5-29-6-8-31-9-7-29/h3-4,10-12H,1-2,5-9H2,(H2,25,26)(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using [gamma33P]ATP as substrate by top counting analysis |
Bioorg Med Chem Lett 23: 4652-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.010
BindingDB Entry DOI: 10.7270/Q2NP25T0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156296

(CHEMBL3781466)Show SMILES CC(C)(O)CNc1ncc(c(CC2CC2)n1)-c1ccnc(Nc2ccncc2)n1 Show InChI InChI=1S/C21H25N7O/c1-21(2,29)13-25-19-24-12-16(18(28-19)11-14-3-4-14)17-7-10-23-20(27-17)26-15-5-8-22-9-6-15/h5-10,12,14,29H,3-4,11,13H2,1-2H3,(H,24,25,28)(H,22,23,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of VPS34 in human U2OS cells incubated for 2 hrs by GFP-FYVE reporter gene assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140276

(CHEMBL3752175)Show InChI InChI=1S/C17H19N5O3/c1-11(23)20-15-10-22-9-12(3-4-16(22)21-15)13-7-14(17(18)19-8-13)25-6-5-24-2/h3-4,7-10H,5-6H2,1-2H3,(H2,18,19)(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50331577

(3-(6-(1H-indazol-5-ylamino)-2-morpholinopyrimidin-...)Show SMILES Oc1cccc(c1)-c1cc(Nc2ccc3[nH]ncc3c2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C21H20N6O2/c28-17-3-1-2-14(11-17)19-12-20(25-21(24-19)27-6-8-29-9-7-27)23-16-4-5-18-15(10-16)13-22-26-18/h1-5,10-13,28H,6-9H2,(H,22,26)(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50331577

(3-(6-(1H-indazol-5-ylamino)-2-morpholinopyrimidin-...)Show SMILES Oc1cccc(c1)-c1cc(Nc2ccc3[nH]ncc3c2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C21H20N6O2/c28-17-3-1-2-14(11-17)19-12-20(25-21(24-19)27-6-8-29-9-7-27)23-16-4-5-18-15(10-16)13-22-26-18/h1-5,10-13,28H,6-9H2,(H,22,26)(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439713
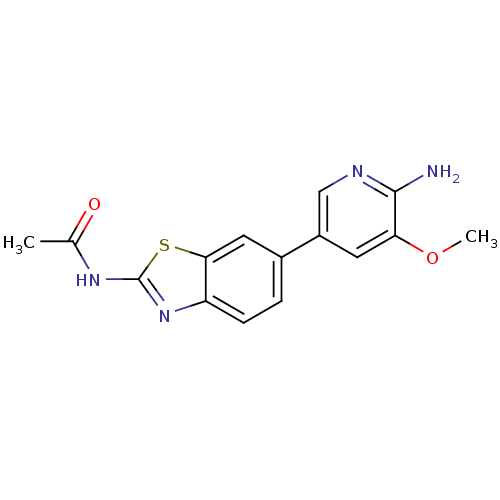
(CHEMBL2418943)Show InChI InChI=1S/C15H14N4O2S/c1-8(20)18-15-19-11-4-3-9(6-13(11)22-15)10-5-12(21-2)14(16)17-7-10/h3-7H,1-2H3,(H2,16,17)(H,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using [gamma33P]ATP as substrate by top counting analysis |
Bioorg Med Chem Lett 23: 4652-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.010
BindingDB Entry DOI: 10.7270/Q2NP25T0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156298

(CHEMBL3781926)Show SMILES CC(C)(C)Nc1ncc(c(CC2CC2)n1)-c1ccnc(Nc2ccncc2)n1 Show InChI InChI=1S/C21H25N7/c1-21(2,3)28-20-24-13-16(18(27-20)12-14-4-5-14)17-8-11-23-19(26-17)25-15-6-9-22-10-7-15/h6-11,13-14H,4-5,12H2,1-3H3,(H,24,27,28)(H,22,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439714
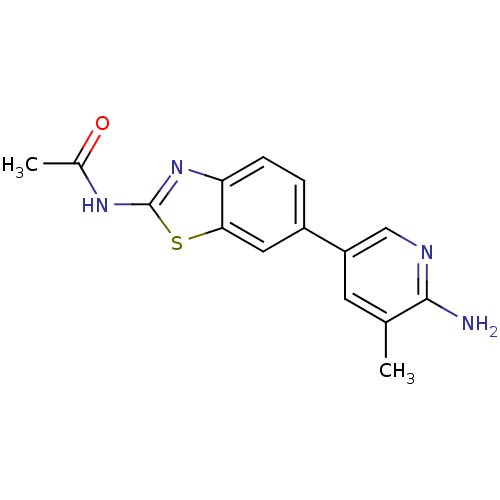
(CHEMBL2418944)Show InChI InChI=1S/C15H14N4OS/c1-8-5-11(7-17-14(8)16)10-3-4-12-13(6-10)21-15(19-12)18-9(2)20/h3-7H,1-2H3,(H2,16,17)(H,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using [gamma33P]ATP as substrate by top counting analysis |
Bioorg Med Chem Lett 23: 4652-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.010
BindingDB Entry DOI: 10.7270/Q2NP25T0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156302

(CHEMBL3780029)Show InChI InChI=1S/C18H19N7/c1-19-17-22-11-14(16(25-17)10-12-2-3-12)15-6-9-21-18(24-15)23-13-4-7-20-8-5-13/h4-9,11-12H,2-3,10H2,1H3,(H,19,22,25)(H,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50140265

(CHEMBL3753450)Show SMILES CC(Oc1nc(cnc1N)-c1ccc2nc(NC(C)=O)cn2c1)C(F)(F)F Show InChI InChI=1S/C16H15F3N6O2/c1-8(16(17,18)19)27-15-14(20)21-5-11(23-15)10-3-4-13-24-12(22-9(2)26)7-25(13)6-10/h3-8H,1-2H3,(H2,20,21)(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50331593

(3-(2-morpholino-6-(pyridin-3-yloxy)pyrimidin-4-yl)...)Show InChI InChI=1S/C19H18N4O3/c24-15-4-1-3-14(11-15)17-12-18(26-16-5-2-6-20-13-16)22-19(21-17)23-7-9-25-10-8-23/h1-6,11-13,24H,7-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439727
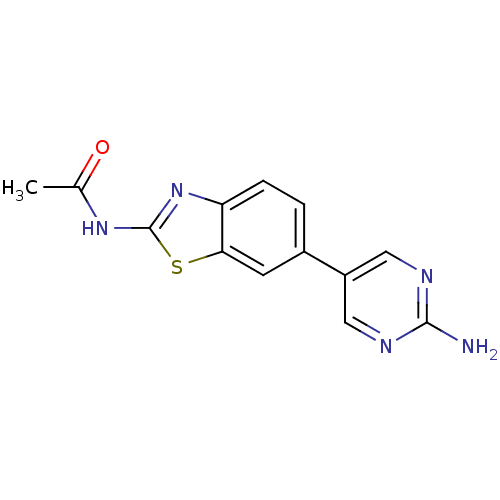
(CHEMBL2418947)Show InChI InChI=1S/C13H11N5OS/c1-7(19)17-13-18-10-3-2-8(4-11(10)20-13)9-5-15-12(14)16-6-9/h2-6H,1H3,(H2,14,15,16)(H,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using [gamma33P]ATP as substrate by top counting analysis |
Bioorg Med Chem Lett 23: 4652-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.010
BindingDB Entry DOI: 10.7270/Q2NP25T0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50331591
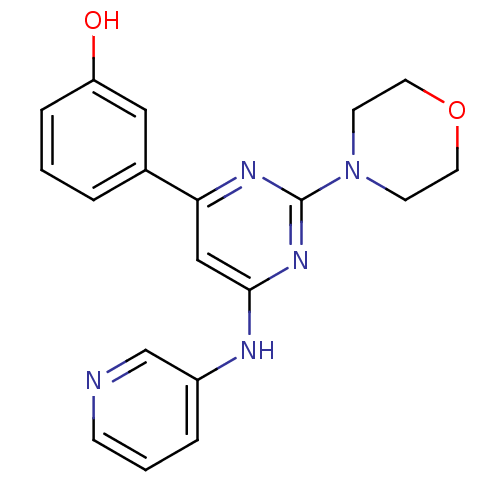
(3-(2-morpholino-6-(pyridin-3-ylamino)pyrimidin-4-y...)Show InChI InChI=1S/C19H19N5O2/c25-16-5-1-3-14(11-16)17-12-18(21-15-4-2-6-20-13-15)23-19(22-17)24-7-9-26-10-8-24/h1-6,11-13,25H,7-10H2,(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6895-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.021
BindingDB Entry DOI: 10.7270/Q2QZ2B6C |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439728

(CHEMBL2418946)Show InChI InChI=1S/C14H12N4OS/c1-8(19)17-14-18-11-4-2-9(6-12(11)20-14)10-3-5-13(15)16-7-10/h2-7H,1H3,(H2,15,16)(H,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using [gamma33P]ATP as substrate by top counting analysis |
Bioorg Med Chem Lett 23: 4652-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.010
BindingDB Entry DOI: 10.7270/Q2NP25T0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156295
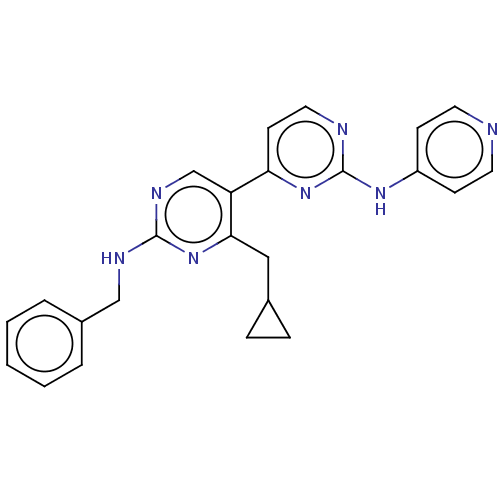
(CHEMBL3781615)Show SMILES C(Nc1ncc(c(CC2CC2)n1)-c1ccnc(Nc2ccncc2)n1)c1ccccc1 Show InChI InChI=1S/C24H23N7/c1-2-4-18(5-3-1)15-27-23-28-16-20(22(31-23)14-17-6-7-17)21-10-13-26-24(30-21)29-19-8-11-25-12-9-19/h1-5,8-13,16-17H,6-7,14-15H2,(H,27,28,31)(H,25,26,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156297
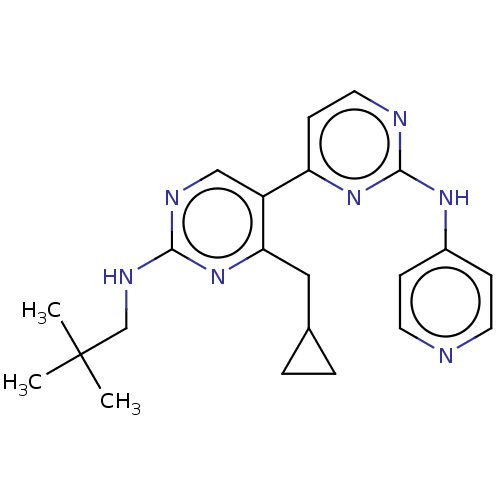
(CHEMBL3780339)Show SMILES CC(C)(C)CNc1ncc(c(CC2CC2)n1)-c1ccnc(Nc2ccncc2)n1 Show InChI InChI=1S/C22H27N7/c1-22(2,3)14-26-20-25-13-17(19(29-20)12-15-4-5-15)18-8-11-24-21(28-18)27-16-6-9-23-10-7-16/h6-11,13,15H,4-5,12,14H2,1-3H3,(H,25,26,29)(H,23,24,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156300

(CHEMBL3781634)Show InChI InChI=1S/C18H18N6O/c1-25-18-21-11-14(16(24-18)10-12-2-3-12)15-6-9-20-17(23-15)22-13-4-7-19-8-5-13/h4-9,11-12H,2-3,10H2,1H3,(H,19,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50439711

(CHEMBL2418954)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cnc(N)c(c1)C(F)(F)F Show InChI InChI=1S/C15H12F3N5O/c1-8(24)21-12-7-23-6-9(2-3-13(23)22-12)10-4-11(15(16,17)18)14(19)20-5-10/h2-7H,1H3,(H2,19,20)(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using 1alpha-phosphotidylinositol by luminescence assay |
Bioorg Med Chem Lett 23: 4652-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.010
BindingDB Entry DOI: 10.7270/Q2NP25T0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156293
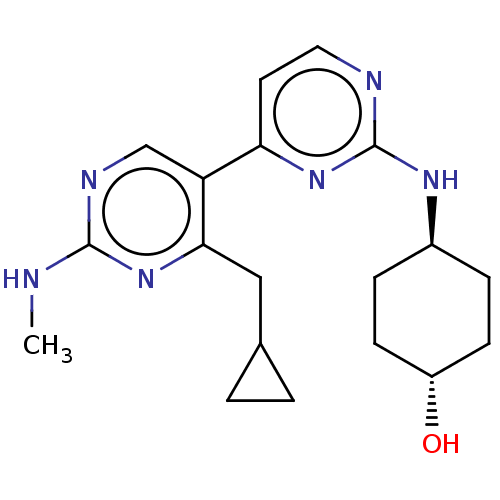
(CHEMBL3781916)Show SMILES CNc1ncc(c(CC2CC2)n1)-c1ccnc(N[C@H]2CC[C@H](O)CC2)n1 |r,wU:21.23,wD:18.19,(-9.3,9.74,;-9.31,8.51,;-7.98,7.73,;-6.64,8.49,;-5.31,7.71,;-5.32,6.17,;-6.66,5.41,;-6.67,3.87,;-8.01,3.11,;-8.71,1.83,;-9.47,3.17,;-7.99,6.19,;-3.99,5.4,;-2.65,6.16,;-1.32,5.39,;-1.33,3.85,;-2.66,3.08,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;2.4,-1.39,;1.33,.77,;,1.54,;-4,3.86,)| Show InChI InChI=1S/C19H26N6O/c1-20-18-22-11-15(17(25-18)10-12-2-3-12)16-8-9-21-19(24-16)23-13-4-6-14(26)7-5-13/h8-9,11-14,26H,2-7,10H2,1H3,(H,20,22,25)(H,21,23,24)/t13-,14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439729
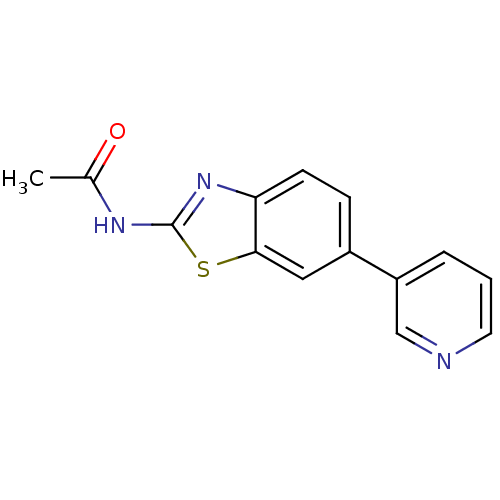
(CHEMBL2418961)Show InChI InChI=1S/C14H11N3OS/c1-9(18)16-14-17-12-5-4-10(7-13(12)19-14)11-3-2-6-15-8-11/h2-8H,1H3,(H,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using [gamma33P]ATP as substrate by top counting analysis |
Bioorg Med Chem Lett 23: 4652-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.010
BindingDB Entry DOI: 10.7270/Q2NP25T0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439720

(CHEMBL2418955)Show SMILES CC(=O)Nc1cn2cc(ncc2n1)-c1cnc(N)c(c1)C(F)(F)F Show InChI InChI=1S/C14H11F3N6O/c1-7(24)21-11-6-23-5-10(19-4-12(23)22-11)8-2-9(14(15,16)17)13(18)20-3-8/h2-6H,1H3,(H2,18,20)(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using [gamma33P]ATP as substrate by top counting analysis |
Bioorg Med Chem Lett 23: 4652-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.010
BindingDB Entry DOI: 10.7270/Q2NP25T0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156337
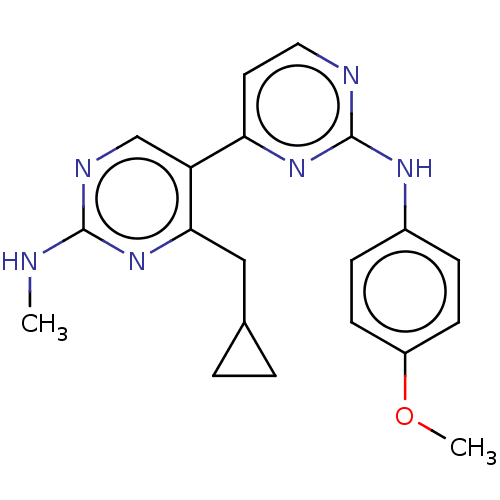
(CHEMBL3781236)Show SMILES CNc1ncc(c(CC2CC2)n1)-c1ccnc(Nc2ccc(OC)cc2)n1 Show InChI InChI=1S/C20H22N6O/c1-21-19-23-12-16(18(26-19)11-13-3-4-13)17-9-10-22-20(25-17)24-14-5-7-15(27-2)8-6-14/h5-10,12-13H,3-4,11H2,1-2H3,(H,21,23,26)(H,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156338

(CHEMBL3782016)Show SMILES CNc1ncc(c(CC2CC2)n1)-c1ccnc(Nc2cnc3ccccc3c2)n1 Show InChI InChI=1S/C22H21N7/c1-23-21-26-13-17(20(29-21)10-14-6-7-14)19-8-9-24-22(28-19)27-16-11-15-4-2-3-5-18(15)25-12-16/h2-5,8-9,11-14H,6-7,10H2,1H3,(H,23,26,29)(H,24,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data