

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Dengue virus 2) | BDBM50175982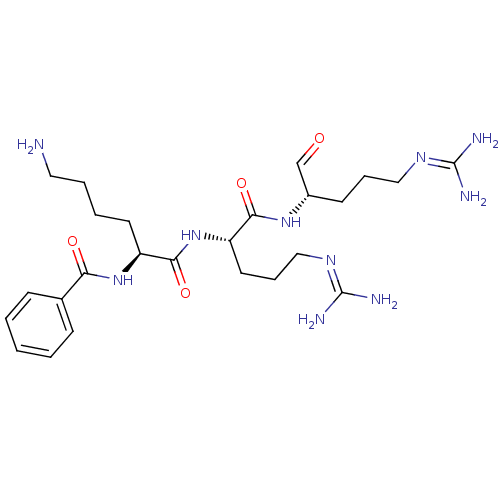 (Bz-Lys-Arg-Arg-H | CHEMBL199510) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175984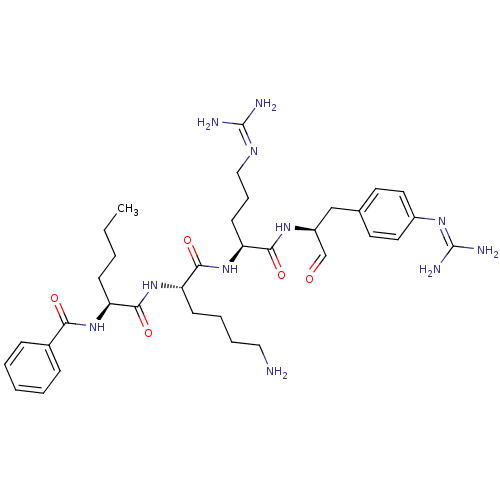 (BZ-Nle-Lys-Arg-(4-guanidinyl)-Phe-H | Bz-Nle-Lys-A...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175976 (Bz-Ala-Lys-Arg-Arg-H | CHEMBL197765) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM33259 (Bz-NKRR-H | Bz-Nle-Lys-Arg-Arg-H | CHEMBL256877) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175988 (Bz-Nle-Lys-Arg-(p-Me)Phe-H | CHEMBL199396) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175987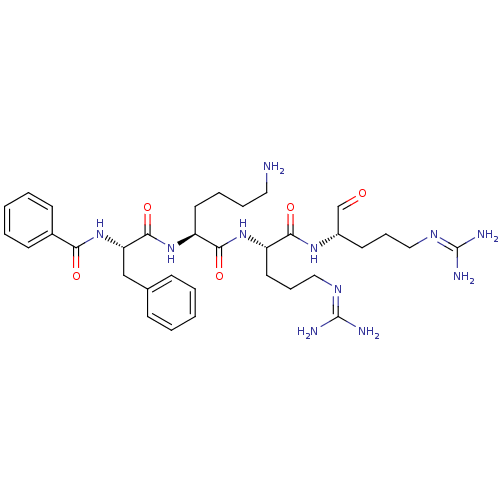 (Bz-Phe-Lys-Arg-Arg-H | CHEMBL199736) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175974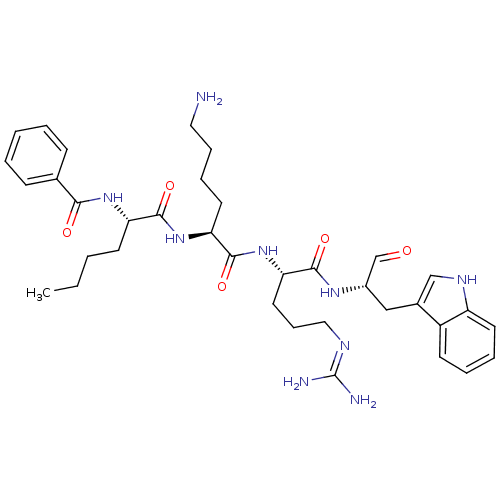 (Bz-Nle-Lys-Arg-Trp-H | CHEMBL433993) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175993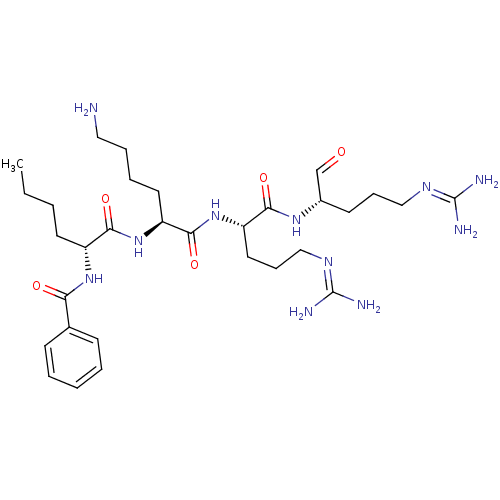 (Bz-D-Nle-Lys-Arg-Arg-H | CHEMBL201775) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175978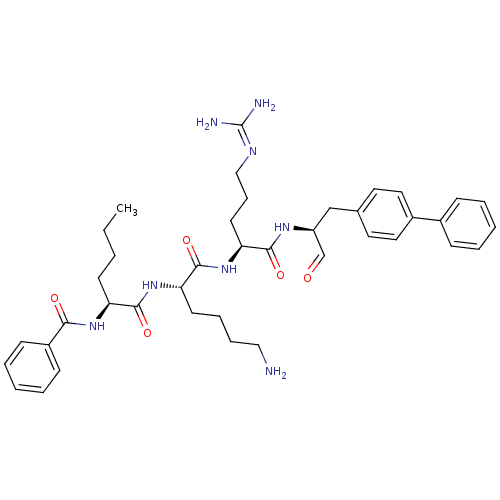 (Bz-Nle-Lys-Arg-(p-Ph)Phe-H | CHEMBL381854) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175981 (Bz-Arg-Arg-H | CHEMBL197766) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175985 (Bz-Nle-Phe-Arg-Arg-H | CHEMBL199582) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175986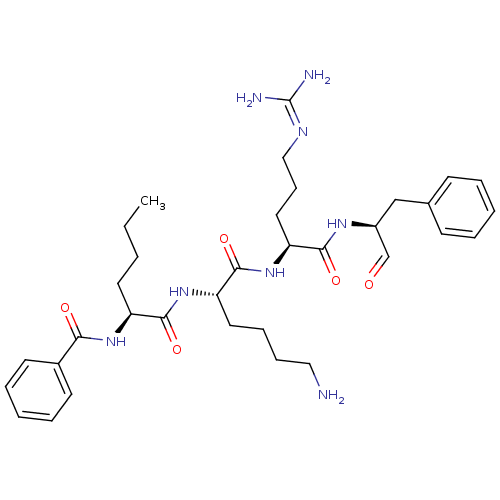 (Bz-Nle-Lys-Arg-Phe-H | CHEMBL199727) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175968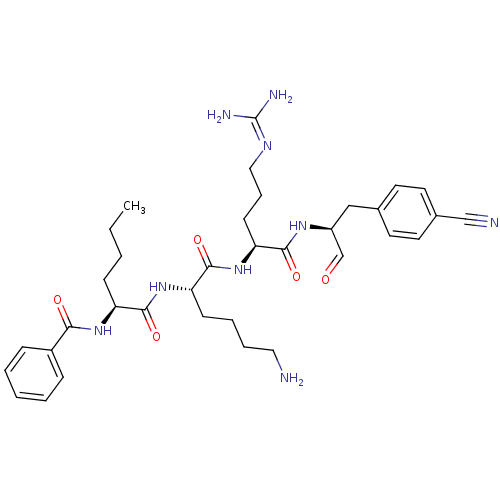 (Bz-Nle-Lys-Arg-(p-CN)Phe-H | CHEMBL200095) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175971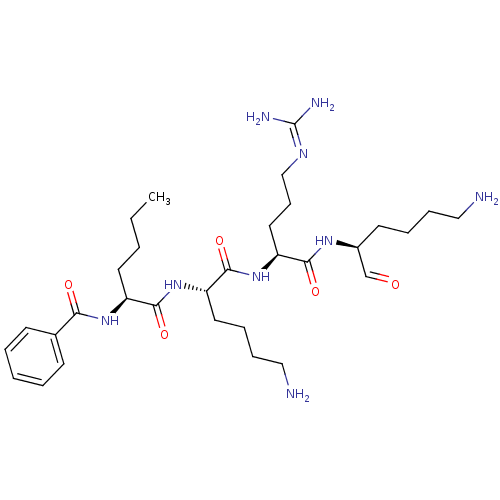 (Bz-Nle-Lys-Arg-Lys-H | CHEMBL377076) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175970 (Bz-Nle-Ala-Arg-Arg-H | CHEMBL369916) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175975 (Bz-Nle-D-Lys-Arg-Arg-H | CHEMBL370138) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175991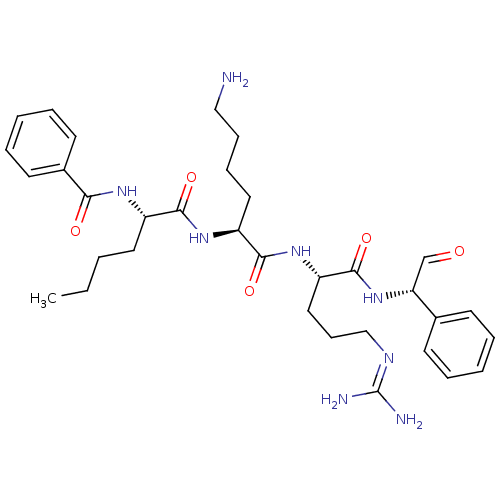 (Bz-Nle-Lys-Arg-Phg-H | CHEMBL200972) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175990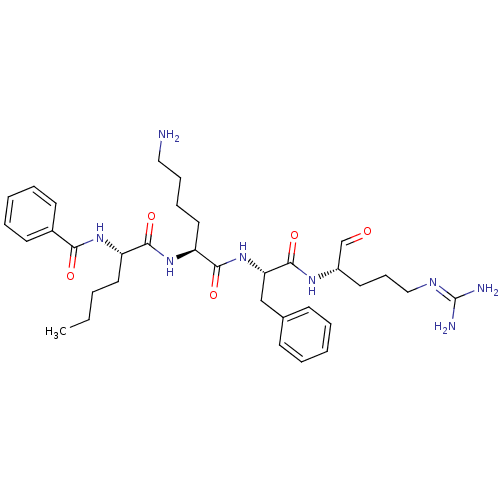 (Bz-Nle-Lys-Phe-Arg-H | CHEMBL435122) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175977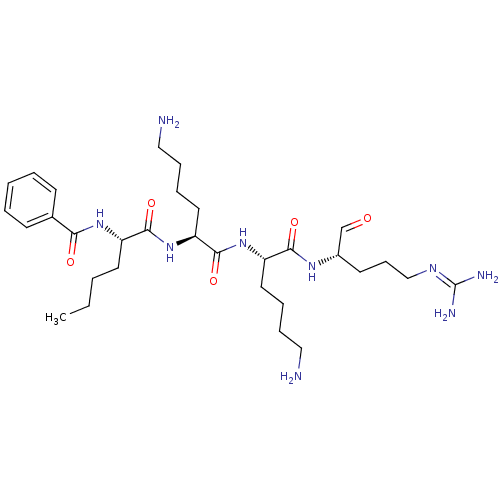 (Bz-Nle-Lys-Lys-Arg-H | CHEMBL201609) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175969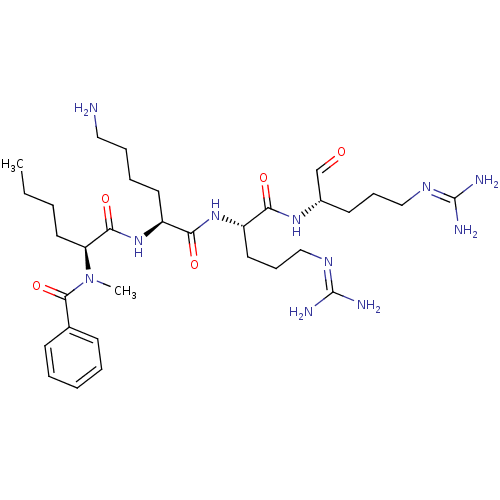 (Bz-N-Me-Nle-Lys-Arg-Arg-H | CHEMBL382143) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175967 (Bz-Nle-Lys-N-Me-Arg-Arg-H | CHEMBL199726) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175980 (Bz-Nle-Lys-Arg-D-Arg-H | CHEMBL199352) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175979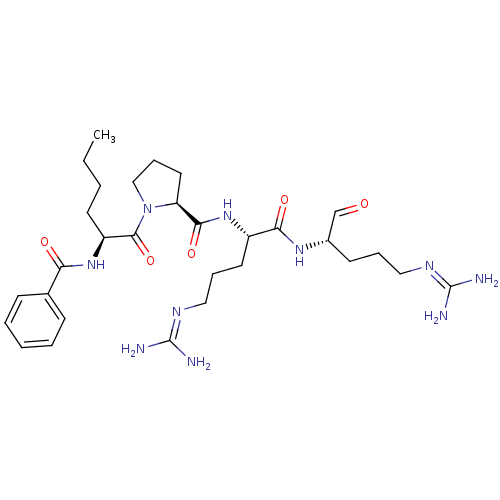 (Bz-Nle-Pro-Arg-Arg-H | CHEMBL382983) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175965 (Bz-Nle-Lys-Pro-Arg-H | CHEMBL199987) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.09E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175973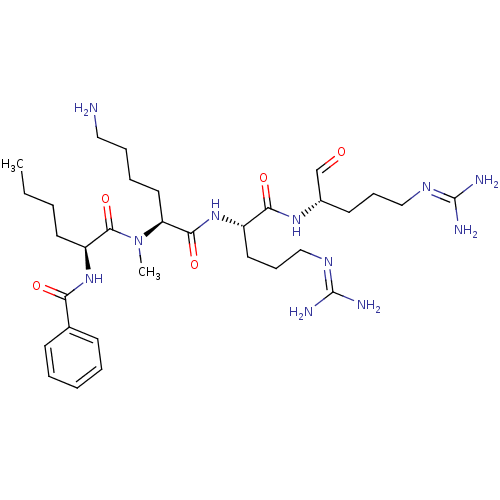 (Bz-Nle-N-Me-Lys-Arg-Arg-H | CHEMBL381639) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.13E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175989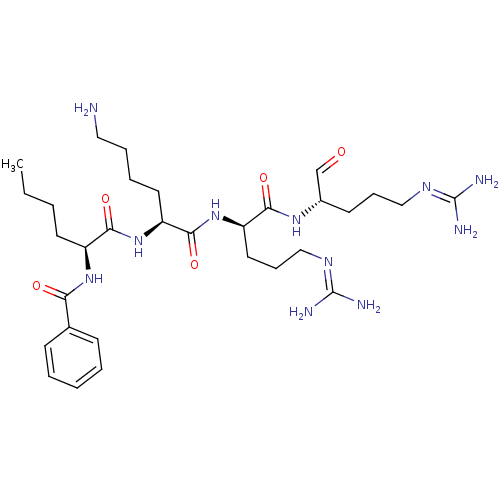 (Bz-Nle-Lys-D-Arg-Arg-H | CHEMBL371447) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175972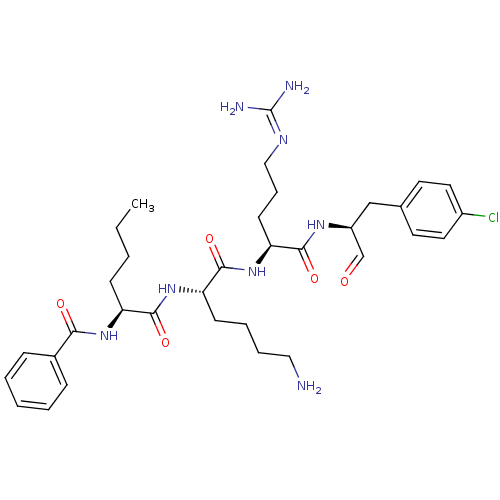 (Bz-Nle-Lys-Arg-(p-Cl)Phe-H | CHEMBL199531) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.38E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175966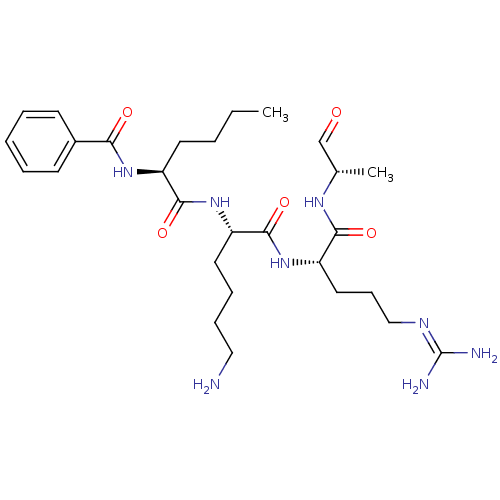 (Bz-Nle-Lys-Arg-Ala-H | CHEMBL199376) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.93E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175992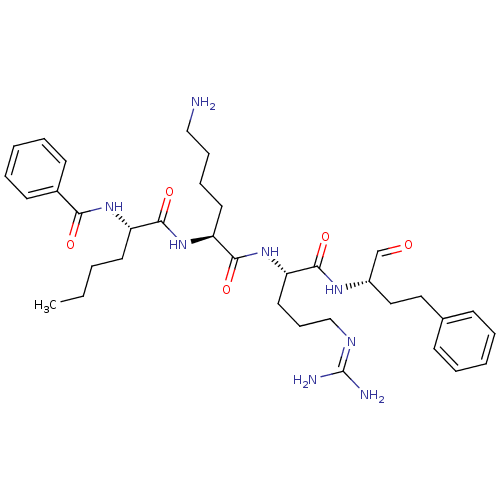 (Bz-Nle-Lys-Arg-homoPhe-H | CHEMBL370338) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175983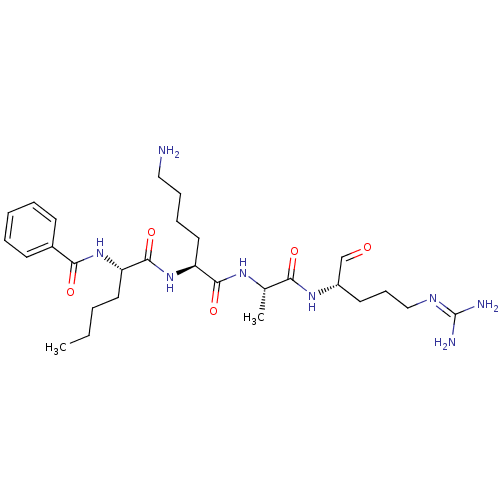 (Bz-Nle-Lys-Ala-Arg-H | CHEMBL372963) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 11 [51-76]/Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM449620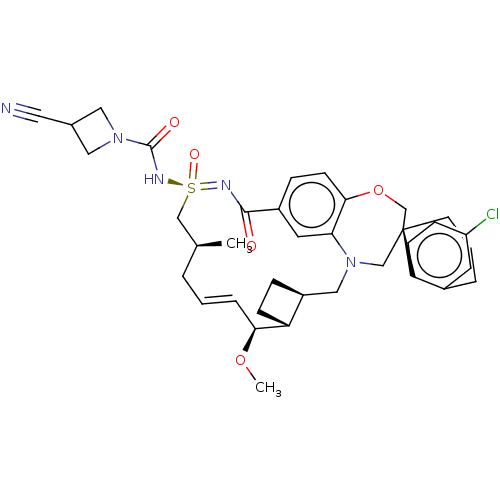 (US10703733, Example 233 | US10988451, Example 233) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The AlphaLISA assay was performed in a 384-well Proxiplate in a total volume of 40 μL. The reaction mixture contained 0.0625 nM 6×His-Mcl-1 (171... | US Patent US10703733 (2020) BindingDB Entry DOI: 10.7270/Q2XK8JK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 11 [51-76]/Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM449685 (US10703733, Example 298 | US11643400, Example 298) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q24B357N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM449620 (US10703733, Example 233 | US10988451, Example 233) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The AlphaLISA assay was performed in a 384-well Proxiplate in a total volume of 40 μL. The reaction mixture contained 0.0625 nM 6× His-Mcl-1 (17... | US Patent US10988451 (2021) BindingDB Entry DOI: 10.7270/Q2M048KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 11 [51-76]/Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM449685 (US10703733, Example 298 | US11643400, Example 298) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The AlphaLISA assay was performed in a 384-well Proxiplate in a total volume of 40 μL. The reaction mixture contained 0.0625 nM 6×His-Mcl-1 (171... | US Patent US10703733 (2020) BindingDB Entry DOI: 10.7270/Q2XK8JK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 11 [51-76]/Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM602144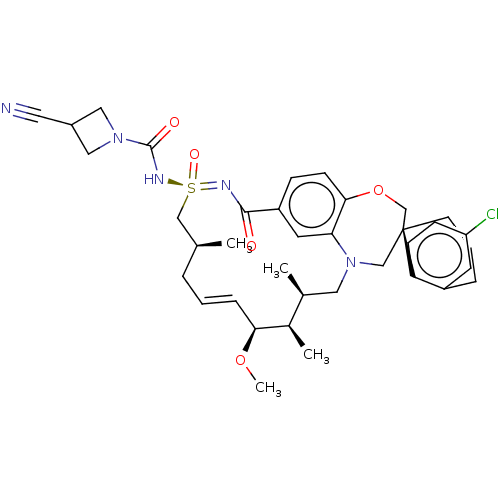 (US11643400, Example 233) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q24B357N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM449686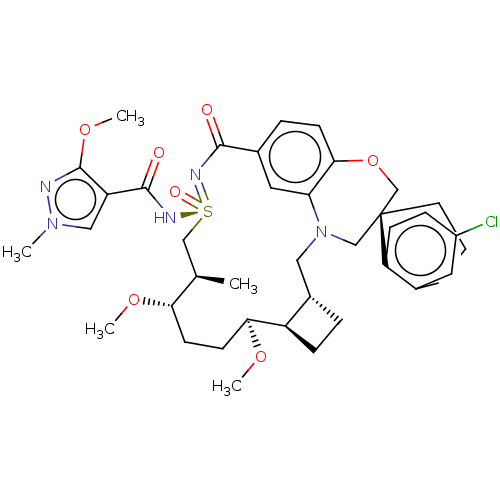 (US10703733, Example 299 | US10988451, Example 298 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The AlphaLISA assay was performed in a 384-well Proxiplate in a total volume of 40 μL. The reaction mixture contained 0.0625 nM 6× His-Mcl-1 (17... | US Patent US10988451 (2021) BindingDB Entry DOI: 10.7270/Q2M048KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM449659 (US10703733, Example 272 | US10988451, Example 272 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The AlphaLISA assay was performed in a 384-well Proxiplate in a total volume of 40 μL. The reaction mixture contained 0.0625 nM 6× His-Mcl-1 (17... | US Patent US10988451 (2021) BindingDB Entry DOI: 10.7270/Q2M048KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM449627 (US10703733, Example 240 | US10988451, Example 240) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The AlphaLISA assay was performed in a 384-well Proxiplate in a total volume of 40 μL. The reaction mixture contained 0.0625 nM 6× His-Mcl-1 (17... | US Patent US10988451 (2021) BindingDB Entry DOI: 10.7270/Q2M048KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 11 [51-76]/Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM449659 (US10703733, Example 272 | US10988451, Example 272 ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The AlphaLISA assay was performed in a 384-well Proxiplate in a total volume of 40 μL. The reaction mixture contained 0.0625 nM 6×His-Mcl-1 (171... | US Patent US10703733 (2020) BindingDB Entry DOI: 10.7270/Q2XK8JK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 11 [51-76]/Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM449627 (US10703733, Example 240 | US10988451, Example 240) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The AlphaLISA assay was performed in a 384-well Proxiplate in a total volume of 40 μL. The reaction mixture contained 0.0625 nM 6×His-Mcl-1 (171... | US Patent US10703733 (2020) BindingDB Entry DOI: 10.7270/Q2XK8JK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 11 [51-76]/Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM602151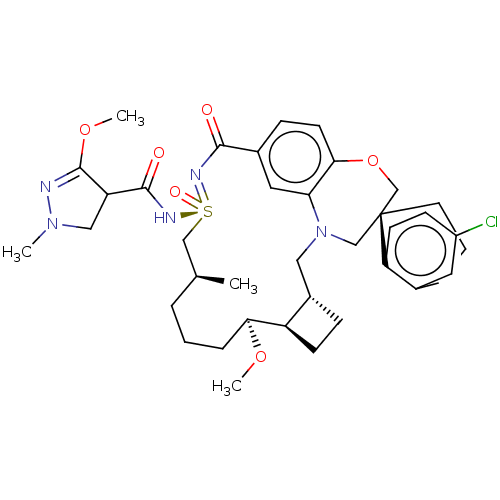 (US11643400, Example 240) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q24B357N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 11 [51-76]/Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM449659 (US10703733, Example 272 | US10988451, Example 272 ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q24B357N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 11 [51-76]/Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM449730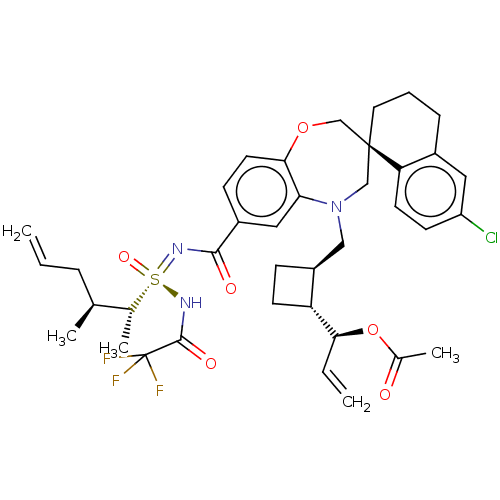 (US10703733, Example 343 | US10988451, Example 343 ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q24B357N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM449730 (US10703733, Example 343 | US10988451, Example 343 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The AlphaLISA assay was performed in a 384-well Proxiplate in a total volume of 40 μL. The reaction mixture contained 0.0625 nM 6× His-Mcl-1 (17... | US Patent US10988451 (2021) BindingDB Entry DOI: 10.7270/Q2M048KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 11 [51-76]/Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM449730 (US10703733, Example 343 | US10988451, Example 343 ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The AlphaLISA assay was performed in a 384-well Proxiplate in a total volume of 40 μL. The reaction mixture contained 0.0625 nM 6×His-Mcl-1 (171... | US Patent US10703733 (2020) BindingDB Entry DOI: 10.7270/Q2XK8JK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 11 [51-76]/Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM449679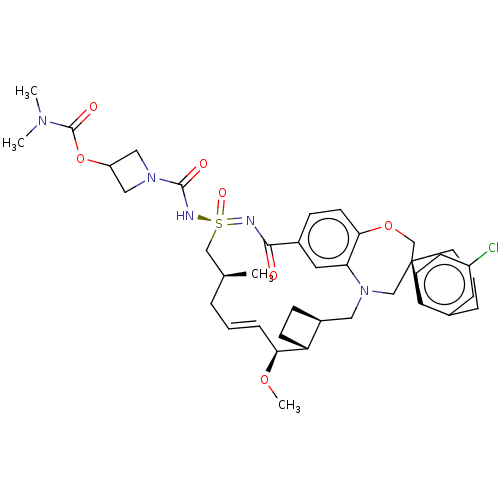 (US10703733, Example 292 | US11643400, Example 292) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The AlphaLISA assay was performed in a 384-well Proxiplate in a total volume of 40 μL. The reaction mixture contained 0.0625 nM 6×His-Mcl-1 (171... | US Patent US10703733 (2020) BindingDB Entry DOI: 10.7270/Q2XK8JK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 11 [51-76]/Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM449679 (US10703733, Example 292 | US11643400, Example 292) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q24B357N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM493822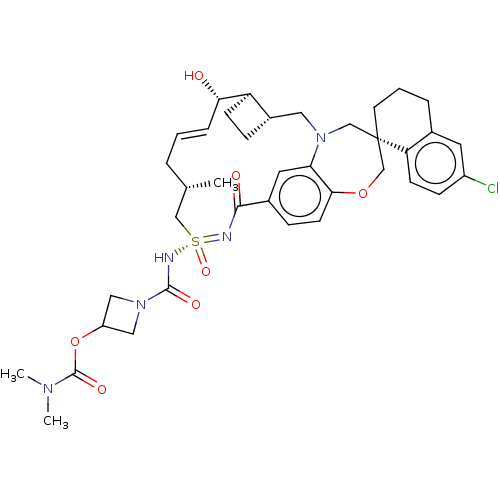 (US10988451, Example 292) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The AlphaLISA assay was performed in a 384-well Proxiplate in a total volume of 40 μL. The reaction mixture contained 0.0625 nM 6× His-Mcl-1 (17... | US Patent US10988451 (2021) BindingDB Entry DOI: 10.7270/Q2M048KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 11 [51-76]/Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM449559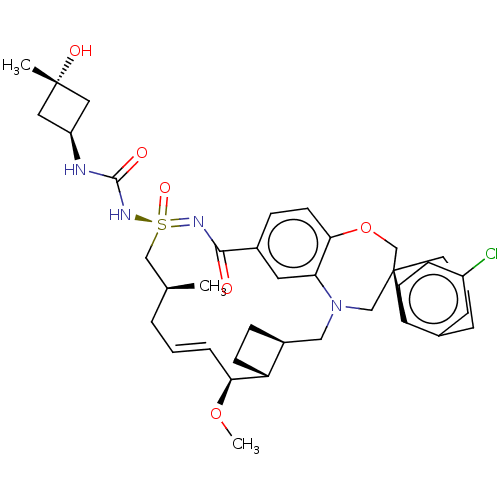 (US10703733, Example 172 | US10988451, Example 172 ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The AlphaLISA assay was performed in a 384-well Proxiplate in a total volume of 40 μL. The reaction mixture contained 0.0625 nM 6×His-Mcl-1 (171... | US Patent US10703733 (2020) BindingDB Entry DOI: 10.7270/Q2XK8JK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 [171-327] (Homo sapiens (Human)) | BDBM449559 (US10703733, Example 172 | US10988451, Example 172 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The AlphaLISA assay was performed in a 384-well Proxiplate in a total volume of 40 μL. The reaction mixture contained 0.0625 nM 6× His-Mcl-1 (17... | US Patent US10988451 (2021) BindingDB Entry DOI: 10.7270/Q2M048KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 8656 total ) | Next | Last >> |