 Found 140 hits with Last Name = 'koprak' and Initial = 's'
Found 140 hits with Last Name = 'koprak' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50158348

((2S,3R,4E)-2-amino-3-hydroxyoctadec-4-en-1-yl dihy...)Show SMILES CCCCCCCCCCCCC\C=C\[C@@H](O)[C@@H](N)COP(O)(O)=O |r| Show InChI InChI=1S/C18H38NO5P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(20)17(19)16-24-25(21,22)23/h14-15,17-18,20H,2-13,16,19H2,1H3,(H2,21,22,23)/b15-14+/t17-,18+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 3 expressed on CHO cell membranes |
Bioorg Med Chem Lett 14: 3351-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.106
BindingDB Entry DOI: 10.7270/Q2QN667K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50147703
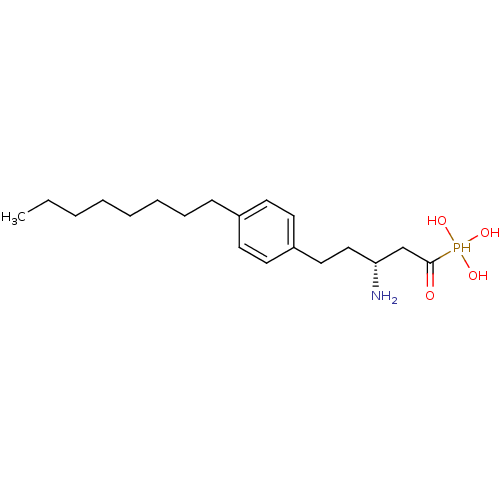
(CHEMBL115554 | CHEMBL115713 | [(1S,3R)-3-Amino-1-h...)Show SMILES CCCCCCCCc1ccc(CC[C@@H](N)CC(=O)P(O)(O)O)cc1 Show InChI InChI=1S/C19H34NO4P/c1-2-3-4-5-6-7-8-16-9-11-17(12-10-16)13-14-18(20)15-19(21)25(22,23)24/h9-12,18,22-25H,2-8,13-15,20H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 1 expressed on CHO cell membranes |
Bioorg Med Chem Lett 14: 3351-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.106
BindingDB Entry DOI: 10.7270/Q2QN667K |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50147713
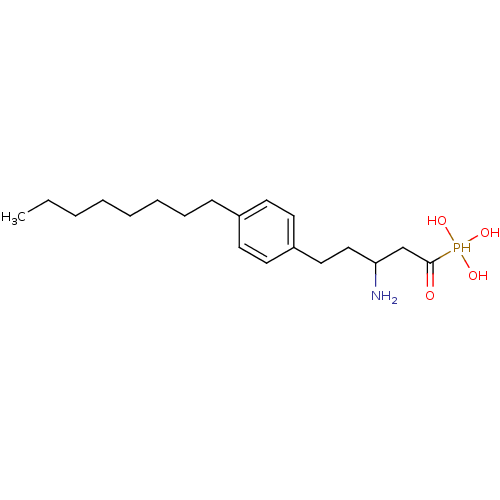
(CHEMBL113344 | [3-Amino-1-hydroxy-5-(4-octyl-pheny...)Show InChI InChI=1S/C19H34NO4P/c1-2-3-4-5-6-7-8-16-9-11-17(12-10-16)13-14-18(20)15-19(21)25(22,23)24/h9-12,18,22-25H,2-8,13-15,20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 1 expressed on CHO cell membranes |
Bioorg Med Chem Lett 14: 3351-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.106
BindingDB Entry DOI: 10.7270/Q2QN667K |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50158348

((2S,3R,4E)-2-amino-3-hydroxyoctadec-4-en-1-yl dihy...)Show SMILES CCCCCCCCCCCCC\C=C\[C@@H](O)[C@@H](N)COP(O)(O)=O |r| Show InChI InChI=1S/C18H38NO5P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(20)17(19)16-24-25(21,22)23/h14-15,17-18,20H,2-13,16,19H2,1H3,(H2,21,22,23)/b15-14+/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 1 expressed on CHO cell membranes |
Bioorg Med Chem Lett 14: 3351-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.106
BindingDB Entry DOI: 10.7270/Q2QN667K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 2
(Homo sapiens (Human)) | BDBM50158348

((2S,3R,4E)-2-amino-3-hydroxyoctadec-4-en-1-yl dihy...)Show SMILES CCCCCCCCCCCCC\C=C\[C@@H](O)[C@@H](N)COP(O)(O)=O |r| Show InChI InChI=1S/C18H38NO5P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(20)17(19)16-24-25(21,22)23/h14-15,17-18,20H,2-13,16,19H2,1H3,(H2,21,22,23)/b15-14+/t17-,18+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 5 expressed on CHO cell membranes |
Bioorg Med Chem Lett 14: 3351-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.106
BindingDB Entry DOI: 10.7270/Q2QN667K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM23163

(CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...)Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 1 expressed on CHO cell membranes |
Bioorg Med Chem Lett 14: 3351-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.106
BindingDB Entry DOI: 10.7270/Q2QN667K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50147714
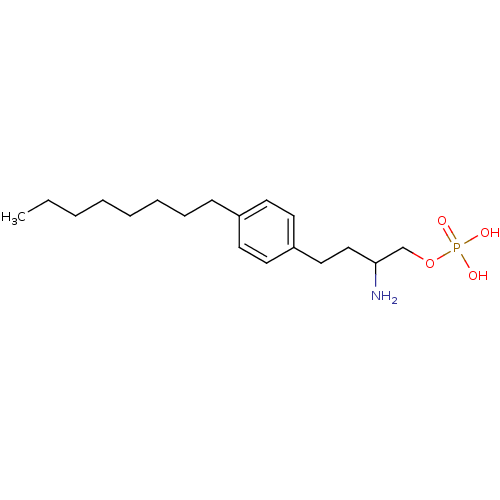
(CHEMBL432067 | Phosphoric acid mono-[2-amino-4-(4-...)Show InChI InChI=1S/C18H32NO4P/c1-2-3-4-5-6-7-8-16-9-11-17(12-10-16)13-14-18(19)15-23-24(20,21)22/h9-12,18H,2-8,13-15,19H2,1H3,(H2,20,21,22) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 3 expressed on CHO cell membranes |
Bioorg Med Chem Lett 14: 3351-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.106
BindingDB Entry DOI: 10.7270/Q2QN667K |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50147714

(CHEMBL432067 | Phosphoric acid mono-[2-amino-4-(4-...)Show InChI InChI=1S/C18H32NO4P/c1-2-3-4-5-6-7-8-16-9-11-17(12-10-16)13-14-18(19)15-23-24(20,21)22/h9-12,18H,2-8,13-15,19H2,1H3,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 1 expressed on CHO cell membranes |
Bioorg Med Chem Lett 14: 3351-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.106
BindingDB Entry DOI: 10.7270/Q2QN667K |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 2
(Homo sapiens (Human)) | BDBM23163

(CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...)Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 5 expressed on CHO cell membranes |
Bioorg Med Chem Lett 14: 3351-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.106
BindingDB Entry DOI: 10.7270/Q2QN667K |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50174299
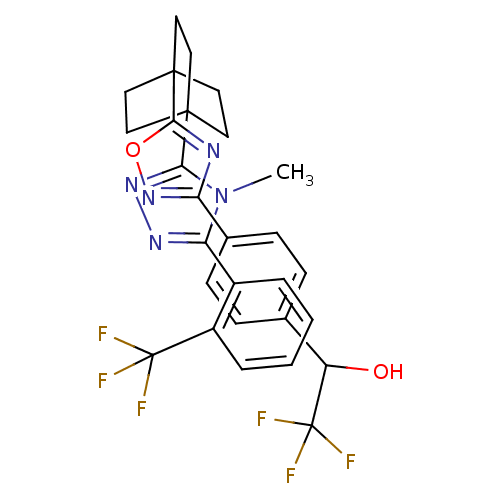
(2,2,2-trifluoro-1-(4-(5-(4-(4-methyl-5-(2-(trifluo...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nc(no1)-c1ccc(cc1)C(O)C(F)(F)F)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C28H25F6N5O2/c1-39-22(18-4-2-3-5-19(18)27(29,30)31)36-37-23(39)25-10-13-26(14-11-25,15-12-25)24-35-21(38-41-24)17-8-6-16(7-9-17)20(40)28(32,33)34/h2-9,20,40H,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50174288
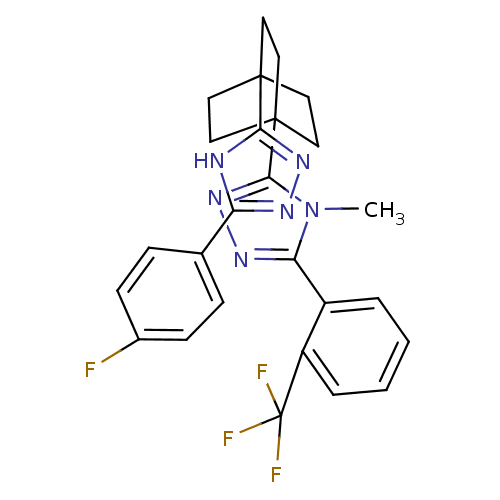
(3-(4-(5-(4-fluorophenyl)-4H-1,2,4-triazol-3-yl)bic...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nnc([nH]1)-c1ccc(F)cc1)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C26H24F4N6/c1-36-21(18-4-2-3-5-19(18)26(28,29)30)33-35-23(36)25-13-10-24(11-14-25,12-15-25)22-31-20(32-34-22)16-6-8-17(27)9-7-16/h2-9H,10-15H2,1H3,(H,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 4
(Homo sapiens (Human)) | BDBM50147714

(CHEMBL432067 | Phosphoric acid mono-[2-amino-4-(4-...)Show InChI InChI=1S/C18H32NO4P/c1-2-3-4-5-6-7-8-16-9-11-17(12-10-16)13-14-18(19)15-23-24(20,21)22/h9-12,18H,2-8,13-15,19H2,1H3,(H2,20,21,22) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 4 expressed on CHO cell membranes |
Bioorg Med Chem Lett 14: 3351-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.106
BindingDB Entry DOI: 10.7270/Q2QN667K |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50174284
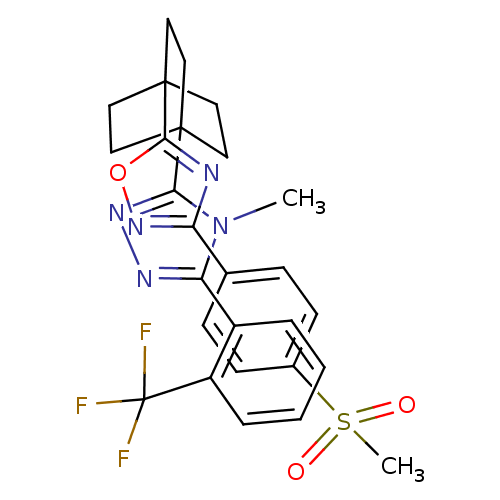
(4-methyl-3-(4-(3-(4-(methylsulfonyl)phenyl)-1,2,4-...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nc(no1)-c1ccc(cc1)S(C)(=O)=O)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C27H26F3N5O3S/c1-35-22(19-5-3-4-6-20(19)27(28,29)30)32-33-23(35)25-11-14-26(15-12-25,16-13-25)24-31-21(34-38-24)17-7-9-18(10-8-17)39(2,36)37/h3-10H,11-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 2
(Homo sapiens (Human)) | BDBM50147714

(CHEMBL432067 | Phosphoric acid mono-[2-amino-4-(4-...)Show InChI InChI=1S/C18H32NO4P/c1-2-3-4-5-6-7-8-16-9-11-17(12-10-16)13-14-18(19)15-23-24(20,21)22/h9-12,18H,2-8,13-15,19H2,1H3,(H2,20,21,22) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 5 expressed on CHO cell membranes |
Bioorg Med Chem Lett 14: 3351-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.106
BindingDB Entry DOI: 10.7270/Q2QN667K |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50174298
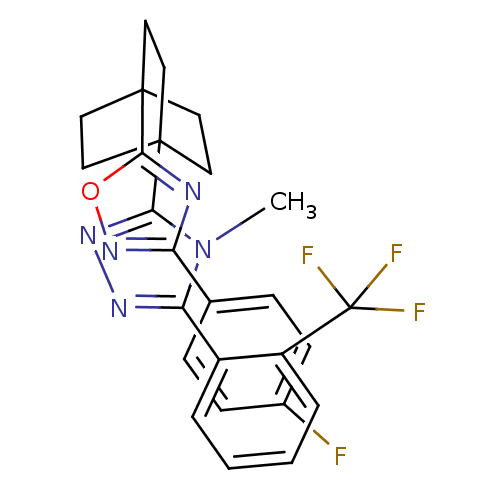
(3-(4-(3-(4-fluorophenyl)-1,2,4-oxadiazol-5-yl)bicy...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nc(no1)-c1ccc(F)cc1)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C26H23F4N5O/c1-35-21(18-4-2-3-5-19(18)26(28,29)30)32-33-22(35)24-10-13-25(14-11-24,15-12-24)23-31-20(34-36-23)16-6-8-17(27)9-7-16/h2-9H,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50174295
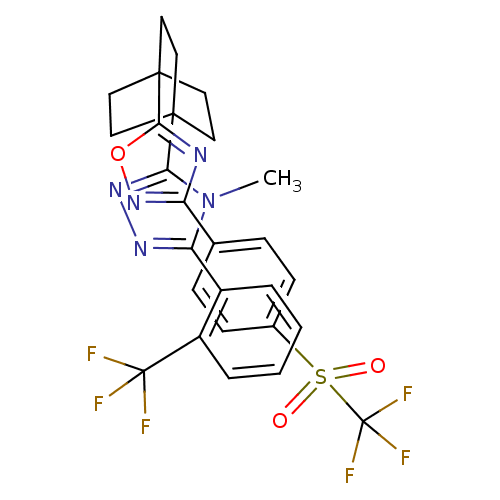
(4-methyl-3-(2-(trifluoromethyl)phenyl)-5-(4-(3-(4-...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nc(no1)-c1ccc(cc1)S(=O)(=O)C(F)(F)F)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C27H23F6N5O3S/c1-38-21(18-4-2-3-5-19(18)26(28,29)30)35-36-22(38)24-10-13-25(14-11-24,15-12-24)23-34-20(37-41-23)16-6-8-17(9-7-16)42(39,40)27(31,32)33/h2-9H,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50174283
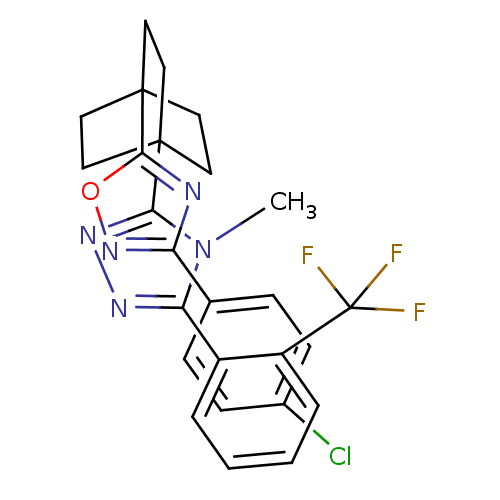
(3-(4-(3-(4-chlorophenyl)-1,2,4-oxadiazol-5-yl)bicy...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nc(no1)-c1ccc(Cl)cc1)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C26H23ClF3N5O/c1-35-21(18-4-2-3-5-19(18)26(28,29)30)32-33-22(35)24-10-13-25(14-11-24,15-12-24)23-31-20(34-36-23)16-6-8-17(27)9-7-16/h2-9H,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50174281
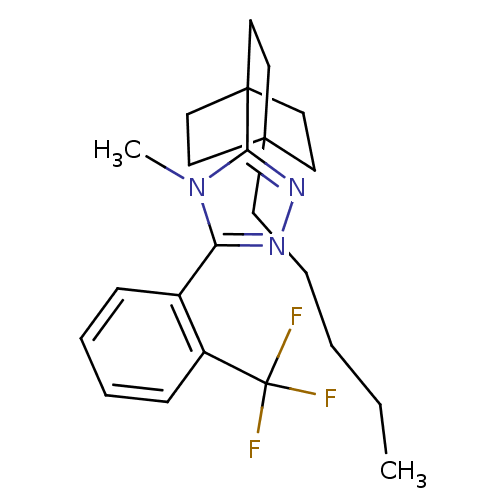
(4-methyl-3-(4-pentylbicyclo[2.2.2]octan-1-yl)-5-(2...)Show SMILES CCCCCC12CCC(CC1)(CC2)c1nnc(-c2ccccc2C(F)(F)F)n1C Show InChI InChI=1S/C23H30F3N3/c1-3-4-7-10-21-11-14-22(15-12-21,16-13-21)20-28-27-19(29(20)2)17-8-5-6-9-18(17)23(24,25)26/h5-6,8-9H,3-4,7,10-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |
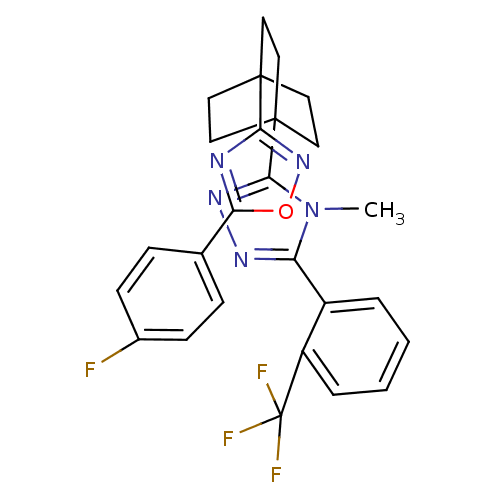
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50174296
(3-(4-(5-(4-fluorophenyl)-1,2,4-oxadiazol-3-yl)bicy...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1noc(n1)-c1ccc(F)cc1)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C26H23F4N5O/c1-35-20(18-4-2-3-5-19(18)26(28,29)30)32-33-23(35)25-13-10-24(11-14-25,12-15-25)22-31-21(36-34-22)16-6-8-17(27)9-7-16/h2-9H,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50174298

(3-(4-(3-(4-fluorophenyl)-1,2,4-oxadiazol-5-yl)bicy...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nc(no1)-c1ccc(F)cc1)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C26H23F4N5O/c1-35-21(18-4-2-3-5-19(18)26(28,29)30)32-33-22(35)24-10-13-25(14-11-24,15-12-24)23-31-20(34-36-23)16-6-8-17(27)9-7-16/h2-9H,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50174290
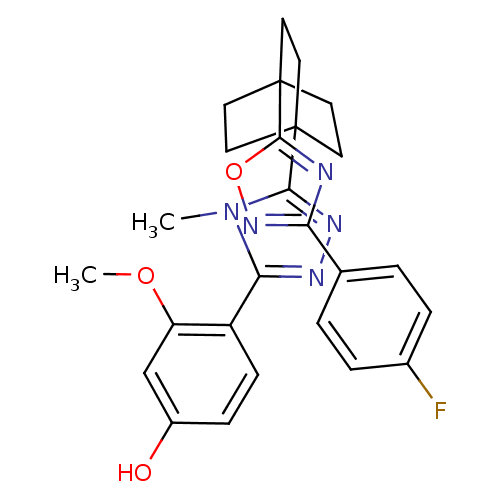
(4-(5-(4-(3-(4-fluorophenyl)-1,2,4-oxadiazol-5-yl)b...)Show SMILES COc1cc(O)ccc1-c1nnc(n1C)C12CCC(CC1)(CC2)c1nc(no1)-c1ccc(F)cc1 Show InChI InChI=1S/C26H26FN5O3/c1-32-22(19-8-7-18(33)15-20(19)34-2)29-30-23(32)25-9-12-26(13-10-25,14-11-25)24-28-21(31-35-24)16-3-5-17(27)6-4-16/h3-8,15,33H,9-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50174282
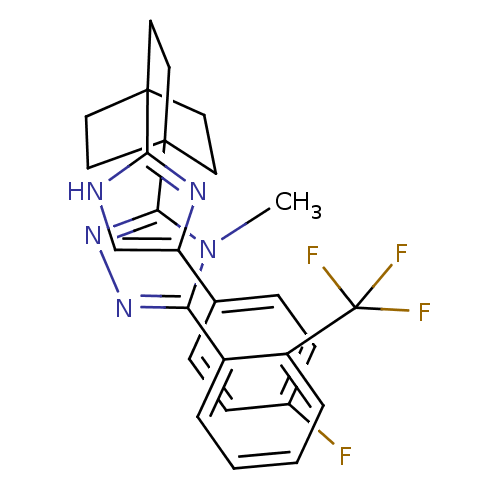
(3-(4-(4-(4-fluorophenyl)-1H-imidazol-2-yl)bicyclo[...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nc(c[nH]1)-c1ccc(F)cc1)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C27H25F4N5/c1-36-22(19-4-2-3-5-20(19)27(29,30)31)34-35-24(36)26-13-10-25(11-14-26,12-15-26)23-32-16-21(33-23)17-6-8-18(28)9-7-17/h2-9,16H,10-15H2,1H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50174297
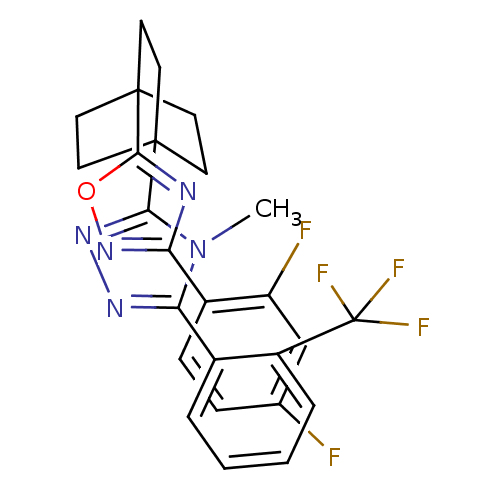
(3-(4-(3-(2,4-difluorophenyl)-1,2,4-oxadiazol-5-yl)...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nc(no1)-c1ccc(F)cc1F)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C26H22F5N5O/c1-36-21(16-4-2-3-5-18(16)26(29,30)31)33-34-22(36)24-8-11-25(12-9-24,13-10-24)23-32-20(35-37-23)17-7-6-15(27)14-19(17)28/h2-7,14H,8-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50174297

(3-(4-(3-(2,4-difluorophenyl)-1,2,4-oxadiazol-5-yl)...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nc(no1)-c1ccc(F)cc1F)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C26H22F5N5O/c1-36-21(16-4-2-3-5-18(16)26(29,30)31)33-34-22(36)24-8-11-25(12-9-24,13-10-24)23-32-20(35-37-23)17-7-6-15(27)14-19(17)28/h2-7,14H,8-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |
Protein phosphatase 3 catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50079775
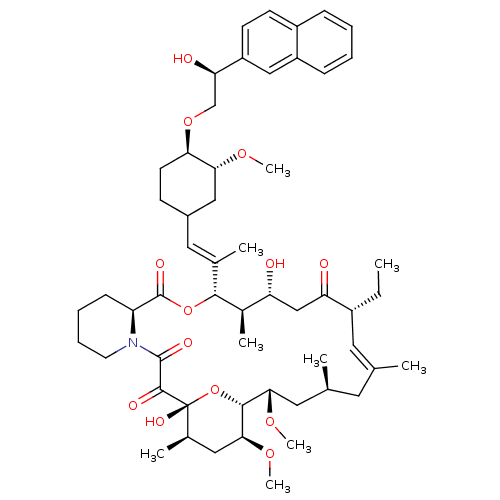
(17-Ethyl-1,14-dihydroxy-12-{2-[4-(2-hydroxy-2-naph...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@H](O)CC1=O)C(\C)=C\C1CC[C@@H](OC[C@@H](O)c2ccc3ccccc3c2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C55H79NO13/c1-10-38-24-32(2)23-33(3)25-48(65-8)51-49(66-9)27-35(5)55(63,69-51)52(60)53(61)56-22-14-13-17-42(56)54(62)68-50(36(6)43(57)30-44(38)58)34(4)26-37-18-21-46(47(28-37)64-7)67-31-45(59)41-20-19-39-15-11-12-16-40(39)29-41/h11-12,15-16,19-20,24,26,29,33,35-38,42-43,45-51,57,59,63H,10,13-14,17-18,21-23,25,27-28,30-31H2,1-9H3/b32-24+,34-26+/t33-,35+,36+,37?,38+,42-,43+,45+,46+,47+,48-,49-,50+,51+,55+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Calcineurin (CaN phosphatase) |
Bioorg Med Chem Lett 9: 2089-94 (1999)
BindingDB Entry DOI: 10.7270/Q2XG9QBD |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50174281

(4-methyl-3-(4-pentylbicyclo[2.2.2]octan-1-yl)-5-(2...)Show SMILES CCCCCC12CCC(CC1)(CC2)c1nnc(-c2ccccc2C(F)(F)F)n1C Show InChI InChI=1S/C23H30F3N3/c1-3-4-7-10-21-11-14-22(15-12-21,16-13-21)20-28-27-19(29(20)2)17-8-5-6-9-18(17)23(24,25)26/h5-6,8-9H,3-4,7,10-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50174285
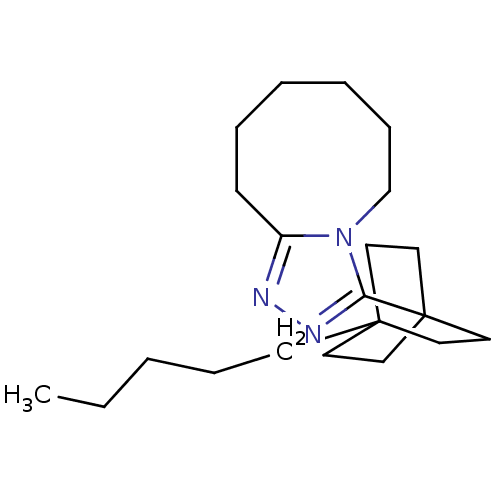
(3-(4-pentylbicyclo[2.2.2]octan-1-yl)-5,6,7,8,9,10-...)Show InChI InChI=1S/C21H35N3/c1-2-3-7-10-20-11-14-21(15-12-20,16-13-20)19-23-22-18-9-6-4-5-8-17-24(18)19/h2-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50174293
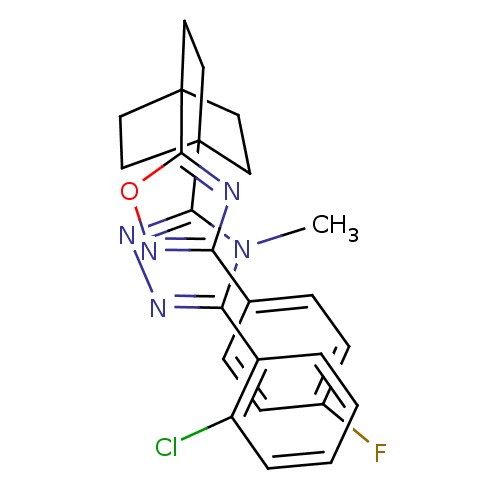
(3-(2-chlorophenyl)-5-(4-(3-(4-fluorophenyl)-1,2,4-...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nc(no1)-c1ccc(F)cc1)-c1ccccc1Cl Show InChI InChI=1S/C25H23ClFN5O/c1-32-21(18-4-2-3-5-19(18)26)29-30-22(32)24-10-13-25(14-11-24,15-12-24)23-28-20(31-33-23)16-6-8-17(27)9-7-16/h2-9H,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |
Protein phosphatase 3 catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50079784
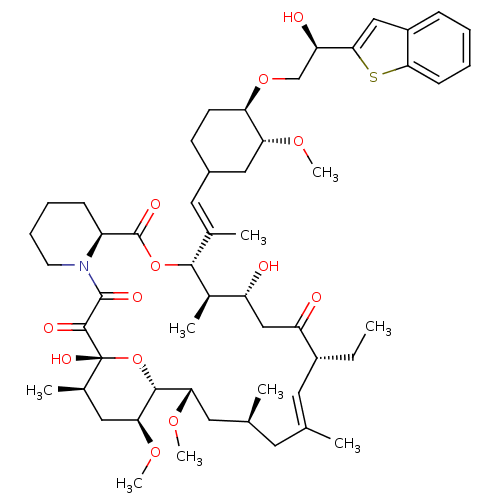
(12-{2-[4-(2-Benzo[b]thiophen-2-yl-2-hydroxy-ethoxy...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@H](O)CC1=O)C(\C)=C\C1CC[C@@H](OC[C@@H](O)c2cc3ccccc3s2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C53H77NO13S/c1-10-36-22-30(2)21-31(3)23-44(63-8)49-45(64-9)25-33(5)53(61,67-49)50(58)51(59)54-20-14-13-16-38(54)52(60)66-48(34(6)39(55)28-40(36)56)32(4)24-35-18-19-42(43(26-35)62-7)65-29-41(57)47-27-37-15-11-12-17-46(37)68-47/h11-12,15,17,22,24,27,31,33-36,38-39,41-45,48-49,55,57,61H,10,13-14,16,18-21,23,25-26,28-29H2,1-9H3/b30-22+,32-24+/t31-,33+,34+,35?,36+,38-,39+,41+,42+,43+,44-,45-,48+,49+,53+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Calcineurin (CaN phosphatase) |
Bioorg Med Chem Lett 9: 2089-94 (1999)
BindingDB Entry DOI: 10.7270/Q2XG9QBD |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50174283

(3-(4-(3-(4-chlorophenyl)-1,2,4-oxadiazol-5-yl)bicy...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nc(no1)-c1ccc(Cl)cc1)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C26H23ClF3N5O/c1-35-21(18-4-2-3-5-19(18)26(28,29)30)32-33-22(35)24-10-13-25(14-11-24,15-12-24)23-31-20(34-36-23)16-6-8-17(27)9-7-16/h2-9H,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50174293

(3-(2-chlorophenyl)-5-(4-(3-(4-fluorophenyl)-1,2,4-...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nc(no1)-c1ccc(F)cc1)-c1ccccc1Cl Show InChI InChI=1S/C25H23ClFN5O/c1-32-21(18-4-2-3-5-19(18)26)29-30-22(32)24-10-13-25(14-11-24,15-12-24)23-28-20(31-33-23)16-6-8-17(27)9-7-16/h2-9H,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50174278
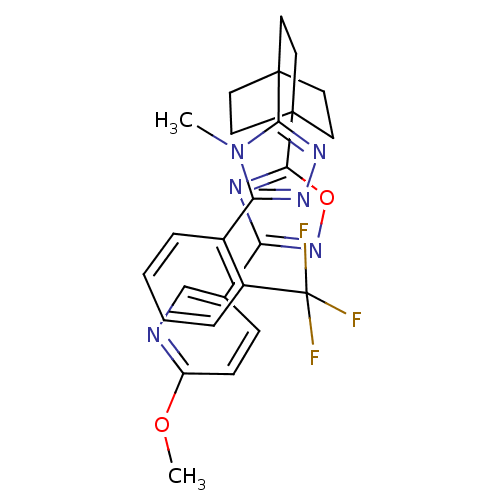
(2-methoxy-5-(5-(4-(4-methyl-5-(2-(trifluoromethyl)...)Show SMILES COc1ccc(cn1)-c1noc(n1)C12CCC(CC1)(CC2)c1nnc(-c2ccccc2C(F)(F)F)n1C Show InChI InChI=1S/C26H25F3N6O2/c1-35-21(17-5-3-4-6-18(17)26(27,28)29)32-33-22(35)24-9-12-25(13-10-24,14-11-24)23-31-20(34-37-23)16-7-8-19(36-2)30-15-16/h3-8,15H,9-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50174301
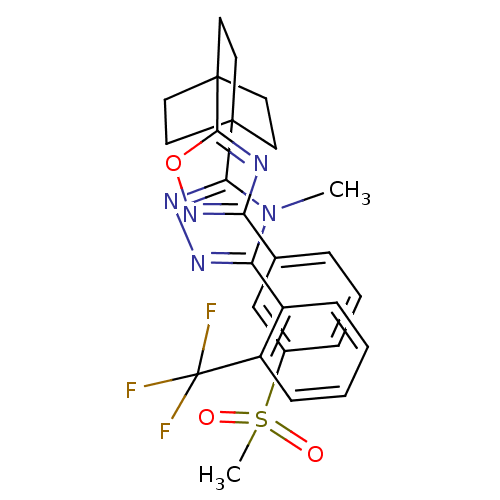
(4-methyl-3-(4-(3-(3-(methylsulfonyl)phenyl)-1,2,4-...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nc(no1)-c1cccc(c1)S(C)(=O)=O)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C27H26F3N5O3S/c1-35-22(19-8-3-4-9-20(19)27(28,29)30)32-33-23(35)25-10-13-26(14-11-25,15-12-25)24-31-21(34-38-24)17-6-5-7-18(16-17)39(2,36)37/h3-9,16H,10-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |
Protein phosphatase 3 catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50079783
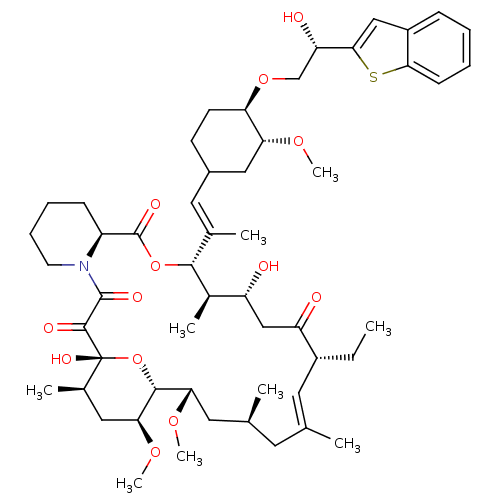
(12-{2-[4-(2-Benzo[b]thiophen-2-yl-2-hydroxy-ethoxy...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@H](O)CC1=O)C(\C)=C\C1CC[C@@H](OC[C@H](O)c2cc3ccccc3s2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C53H77NO13S/c1-10-36-22-30(2)21-31(3)23-44(63-8)49-45(64-9)25-33(5)53(61,67-49)50(58)51(59)54-20-14-13-16-38(54)52(60)66-48(34(6)39(55)28-40(36)56)32(4)24-35-18-19-42(43(26-35)62-7)65-29-41(57)47-27-37-15-11-12-17-46(37)68-47/h11-12,15,17,22,24,27,31,33-36,38-39,41-45,48-49,55,57,61H,10,13-14,16,18-21,23,25-26,28-29H2,1-9H3/b30-22+,32-24+/t31-,33+,34+,35?,36+,38-,39+,41-,42+,43+,44-,45-,48+,49+,53+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Calcineurin (CaN phosphatase) |
Bioorg Med Chem Lett 9: 2089-94 (1999)
BindingDB Entry DOI: 10.7270/Q2XG9QBD |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50174278

(2-methoxy-5-(5-(4-(4-methyl-5-(2-(trifluoromethyl)...)Show SMILES COc1ccc(cn1)-c1noc(n1)C12CCC(CC1)(CC2)c1nnc(-c2ccccc2C(F)(F)F)n1C Show InChI InChI=1S/C26H25F3N6O2/c1-35-21(17-5-3-4-6-18(17)26(27,28)29)32-33-22(35)24-9-12-25(13-10-24,14-11-24)23-31-20(34-37-23)16-7-8-19(36-2)30-15-16/h3-8,15H,9-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 2
(Homo sapiens (Human)) | BDBM50147703

(CHEMBL115554 | CHEMBL115713 | [(1S,3R)-3-Amino-1-h...)Show SMILES CCCCCCCCc1ccc(CC[C@@H](N)CC(=O)P(O)(O)O)cc1 Show InChI InChI=1S/C19H34NO4P/c1-2-3-4-5-6-7-8-16-9-11-17(12-10-16)13-14-18(20)15-19(21)25(22,23)24/h9-12,18,22-25H,2-8,13-15,20H2,1H3/t18-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 5 expressed on CHO cell membranes |
Bioorg Med Chem Lett 14: 3351-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.106
BindingDB Entry DOI: 10.7270/Q2QN667K |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50174288

(3-(4-(5-(4-fluorophenyl)-4H-1,2,4-triazol-3-yl)bic...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nnc([nH]1)-c1ccc(F)cc1)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C26H24F4N6/c1-36-21(18-4-2-3-5-19(18)26(28,29)30)33-35-23(36)25-13-10-24(11-14-25,12-15-25)22-31-20(32-34-22)16-6-8-17(27)9-7-16/h2-9H,10-15H2,1H3,(H,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50147703

(CHEMBL115554 | CHEMBL115713 | [(1S,3R)-3-Amino-1-h...)Show SMILES CCCCCCCCc1ccc(CC[C@@H](N)CC(=O)P(O)(O)O)cc1 Show InChI InChI=1S/C19H34NO4P/c1-2-3-4-5-6-7-8-16-9-11-17(12-10-16)13-14-18(20)15-19(21)25(22,23)24/h9-12,18,22-25H,2-8,13-15,20H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 1 expressed on CHO cell membranes |
Bioorg Med Chem Lett 14: 3351-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.106
BindingDB Entry DOI: 10.7270/Q2QN667K |
More data for this
Ligand-Target Pair | |
Protein phosphatase 3 catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50079776

(17-Ethyl-1,14-dihydroxy-12-(2-{4-[2-hydroxy-2-(3-t...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@H](O)CC1=O)C(\C)=C\C1CC[C@@H](OC[C@@H](O)c2cccc(c2)C(F)(F)F)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C52H76F3NO13/c1-10-35-21-29(2)20-30(3)22-44(65-8)47-45(66-9)24-32(5)51(63,69-47)48(60)49(61)56-19-12-11-16-38(56)50(62)68-46(33(6)39(57)27-40(35)58)31(4)23-34-17-18-42(43(25-34)64-7)67-28-41(59)36-14-13-15-37(26-36)52(53,54)55/h13-15,21,23,26,30,32-35,38-39,41-47,57,59,63H,10-12,16-20,22,24-25,27-28H2,1-9H3/b29-21+,31-23+/t30-,32+,33+,34?,35+,38-,39+,41+,42+,43+,44-,45-,46+,47+,51+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Calcineurin (CaN phosphatase) |
Bioorg Med Chem Lett 9: 2089-94 (1999)
BindingDB Entry DOI: 10.7270/Q2XG9QBD |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50174284

(4-methyl-3-(4-(3-(4-(methylsulfonyl)phenyl)-1,2,4-...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nc(no1)-c1ccc(cc1)S(C)(=O)=O)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C27H26F3N5O3S/c1-35-22(19-5-3-4-6-20(19)27(28,29)30)32-33-23(35)25-11-14-26(15-12-25,16-13-25)24-31-21(34-38-24)17-7-9-18(10-8-17)39(2,36)37/h3-10H,11-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50174285

(3-(4-pentylbicyclo[2.2.2]octan-1-yl)-5,6,7,8,9,10-...)Show InChI InChI=1S/C21H35N3/c1-2-3-7-10-20-11-14-21(15-12-20,16-13-20)19-23-22-18-9-6-4-5-8-17-24(18)19/h2-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50174292
(3-(4-(5-(4-fluorophenyl)-1,3,4-oxadiazol-2-yl)bicy...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nnc(o1)-c1ccc(F)cc1)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C26H23F4N5O/c1-35-20(18-4-2-3-5-19(18)26(28,29)30)31-33-22(35)24-10-13-25(14-11-24,15-12-24)23-34-32-21(36-23)16-6-8-17(27)9-7-16/h2-9H,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50174300
(3-(2-(difluoromethoxy)phenyl)-5-(4-(3-(4-fluorophe...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nc(no1)-c1ccc(F)cc1)-c1ccccc1OC(F)F Show InChI InChI=1S/C26H24F3N5O2/c1-34-21(18-4-2-3-5-19(18)35-24(28)29)31-32-22(34)25-10-13-26(14-11-25,15-12-25)23-30-20(33-36-23)16-6-8-17(27)9-7-16/h2-9,24H,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50174282

(3-(4-(4-(4-fluorophenyl)-1H-imidazol-2-yl)bicyclo[...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nc(c[nH]1)-c1ccc(F)cc1)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C27H25F4N5/c1-36-22(19-4-2-3-5-20(19)27(29,30)31)34-35-24(36)26-13-10-25(11-14-26,12-15-26)23-32-16-21(33-23)17-6-8-18(28)9-7-17/h2-9,16H,10-15H2,1H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |
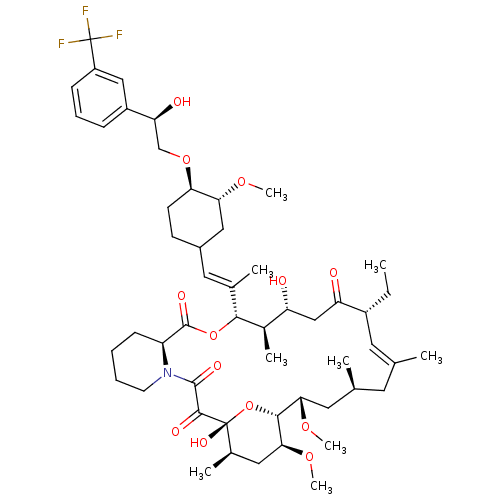
Protein phosphatase 3 catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50079782
(17-Ethyl-1,14-dihydroxy-12-(2-{4-[2-hydroxy-2-(3-t...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@H](O)CC1=O)C(\C)=C\C1CC[C@@H](OC[C@H](O)c2cccc(c2)C(F)(F)F)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C52H76F3NO13/c1-10-35-21-29(2)20-30(3)22-44(65-8)47-45(66-9)24-32(5)51(63,69-47)48(60)49(61)56-19-12-11-16-38(56)50(62)68-46(33(6)39(57)27-40(35)58)31(4)23-34-17-18-42(43(25-34)64-7)67-28-41(59)36-14-13-15-37(26-36)52(53,54)55/h13-15,21,23,26,30,32-35,38-39,41-47,57,59,63H,10-12,16-20,22,24-25,27-28H2,1-9H3/b29-21+,31-23+/t30-,32+,33+,34?,35+,38-,39+,41-,42+,43+,44-,45-,46+,47+,51+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Calcineurin (CaN phosphatase) |
Bioorg Med Chem Lett 9: 2089-94 (1999)
BindingDB Entry DOI: 10.7270/Q2XG9QBD |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM23163

(CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...)Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [33P]-S1P binding to human Sphingosine 1-phosphate receptor 3 expressed on CHO cell membranes |
Bioorg Med Chem Lett 14: 3351-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.106
BindingDB Entry DOI: 10.7270/Q2QN667K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50174292

(3-(4-(5-(4-fluorophenyl)-1,3,4-oxadiazol-2-yl)bicy...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nnc(o1)-c1ccc(F)cc1)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C26H23F4N5O/c1-35-20(18-4-2-3-5-19(18)26(28,29)30)31-33-22(35)24-10-13-25(14-11-24,15-12-24)23-34-32-21(36-23)16-6-8-17(27)9-7-16/h2-9H,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human 11beta-HSD1 |
Bioorg Med Chem Lett 15: 5266-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.052
BindingDB Entry DOI: 10.7270/Q2QC031M |
More data for this
Ligand-Target Pair | |
Protein phosphatase 3 catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50079778
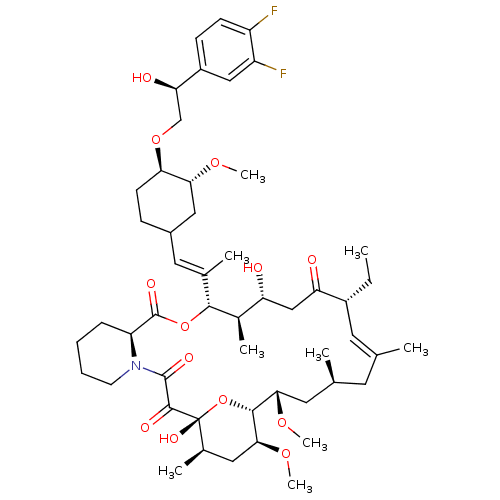
(12-(2-{4-[2-(3,4-Difluoro-phenyl)-2-hydroxy-ethoxy...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@H](O)CC1=O)C(\C)=C\C1CC[C@@H](OC[C@@H](O)c2ccc(F)c(F)c2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C51H75F2NO13/c1-10-34-20-28(2)19-29(3)21-44(63-8)47-45(64-9)23-31(5)51(61,67-47)48(58)49(59)54-18-12-11-13-38(54)50(60)66-46(32(6)39(55)26-40(34)56)30(4)22-33-14-17-42(43(24-33)62-7)65-27-41(57)35-15-16-36(52)37(53)25-35/h15-16,20,22,25,29,31-34,38-39,41-47,55,57,61H,10-14,17-19,21,23-24,26-27H2,1-9H3/b28-20+,30-22+/t29-,31+,32+,33?,34+,38-,39+,41+,42+,43+,44-,45-,46+,47+,51+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Calcineurin (CaN phosphatase) |
Bioorg Med Chem Lett 9: 2089-94 (1999)
BindingDB Entry DOI: 10.7270/Q2XG9QBD |
More data for this
Ligand-Target Pair | |
Protein phosphatase 3 catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50079779
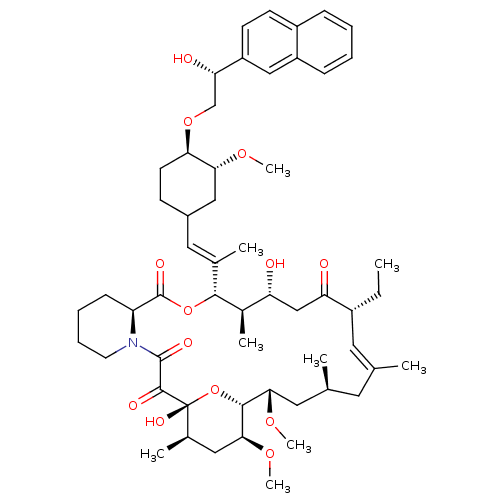
(17-Ethyl-1,14-dihydroxy-12-{2-[4-(2-hydroxy-2-naph...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@H](O)CC1=O)C(\C)=C\C1CC[C@@H](OC[C@H](O)c2ccc3ccccc3c2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C55H79NO13/c1-10-38-24-32(2)23-33(3)25-48(65-8)51-49(66-9)27-35(5)55(63,69-51)52(60)53(61)56-22-14-13-17-42(56)54(62)68-50(36(6)43(57)30-44(38)58)34(4)26-37-18-21-46(47(28-37)64-7)67-31-45(59)41-20-19-39-15-11-12-16-40(39)29-41/h11-12,15-16,19-20,24,26,29,33,35-38,42-43,45-51,57,59,63H,10,13-14,17-18,21-23,25,27-28,30-31H2,1-9H3/b32-24+,34-26+/t33-,35+,36+,37?,38+,42-,43+,45-,46+,47+,48-,49-,50+,51+,55+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Calcineurin (CaN phosphatase) |
Bioorg Med Chem Lett 9: 2089-94 (1999)
BindingDB Entry DOI: 10.7270/Q2XG9QBD |
More data for this
Ligand-Target Pair | |
Protein phosphatase 3 catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50079780
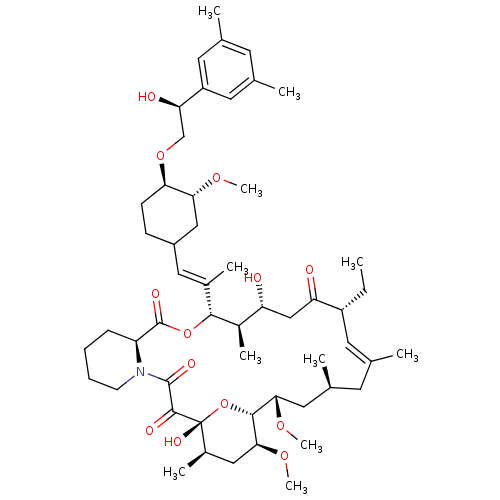
(12-(2-{4-[2-(3,5-Dimethyl-phenyl)-2-hydroxy-ethoxy...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@H](O)CC1=O)C(\C)=C\C1CC[C@@H](OC[C@@H](O)c2cc(C)cc(C)c2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C53H81NO13/c1-12-38-21-30(2)20-33(5)24-46(63-10)49-47(64-11)26-35(7)53(61,67-49)50(58)51(59)54-18-14-13-15-40(54)52(60)66-48(36(8)41(55)28-42(38)56)34(6)25-37-16-17-44(45(27-37)62-9)65-29-43(57)39-22-31(3)19-32(4)23-39/h19,21-23,25,33,35-38,40-41,43-49,55,57,61H,12-18,20,24,26-29H2,1-11H3/b30-21+,34-25+/t33-,35+,36+,37?,38+,40-,41+,43+,44+,45+,46-,47-,48+,49+,53+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Calcineurin (CaN phosphatase) |
Bioorg Med Chem Lett 9: 2089-94 (1999)
BindingDB Entry DOI: 10.7270/Q2XG9QBD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data