 Found 160 hits with Last Name = 'kramer' and Initial = 'a'
Found 160 hits with Last Name = 'kramer' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308167

((3R)-N,N-Dimethyl-1-[3-(1-naphthylsulfonyl)-1H-ind...)Show SMILES CN(C)[C@@H]1CCN(C1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C23H24N4O2S/c1-26(2)18-12-13-27(15-18)17-10-11-21-20(14-17)23(25-24-21)30(28,29)22-9-5-7-16-6-3-4-8-19(16)22/h3-11,14,18H,12-13,15H2,1-2H3,(H,24,25)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308181
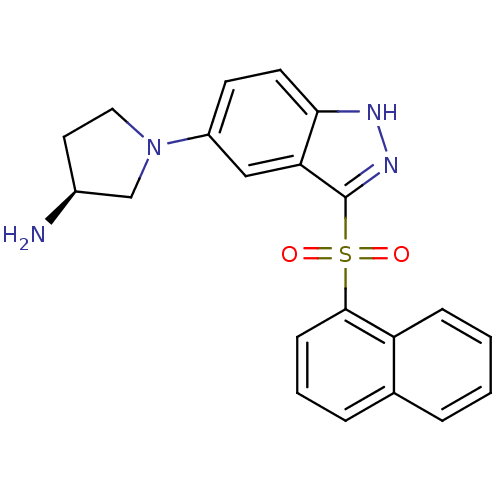
((3S)-1-[3-(1-Naphthylsulfonyl)-1H-indazol-5-yl]pyr...)Show SMILES N[C@H]1CCN(C1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C21H20N4O2S/c22-15-10-11-25(13-15)16-8-9-19-18(12-16)21(24-23-19)28(26,27)20-7-3-5-14-4-1-2-6-17(14)20/h1-9,12,15H,10-11,13,22H2,(H,23,24)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.06 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308183
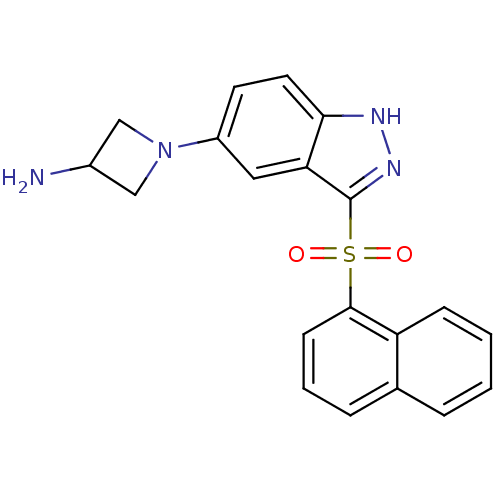
(1-[3-(1-Naphthylsulfonyl)-1H-indazol-5-yl]azetidin...)Show SMILES NC1CN(C1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C20H18N4O2S/c21-14-11-24(12-14)15-8-9-18-17(10-15)20(23-22-18)27(25,26)19-7-3-5-13-4-1-2-6-16(13)19/h1-10,14H,11-12,21H2,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308177

(1-{3-[(4-Methyl-1-naphthyl)sulfonyl]-1H-indazol-5-...)Show SMILES Cc1ccc(c2ccccc12)S(=O)(=O)c1n[nH]c2ccc(cc12)N1CCC(N)CC1 Show InChI InChI=1S/C23H24N4O2S/c1-15-6-9-22(19-5-3-2-4-18(15)19)30(28,29)23-20-14-17(7-8-21(20)25-26-23)27-12-10-16(24)11-13-27/h2-9,14,16H,10-13,24H2,1H3,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308175

(1-[3-(1-Naphthylsulfonyl)-1H-indazol-5-yl]piperidi...)Show SMILES NC1CCN(CC1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C22H22N4O2S/c23-16-10-12-26(13-11-16)17-8-9-20-19(14-17)22(25-24-20)29(27,28)21-7-3-5-15-4-1-2-6-18(15)21/h1-9,14,16H,10-13,23H2,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308182

((3R)-1-[3-(1-Naphthylsulfonyl)-1H-indazol-5-yl]pyr...)Show SMILES N[C@@H]1CCN(C1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C21H20N4O2S/c22-15-10-11-25(13-15)16-8-9-19-18(12-16)21(24-23-19)28(26,27)20-7-3-5-14-4-1-2-6-17(14)20/h1-9,12,15H,10-11,13,22H2,(H,23,24)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308178
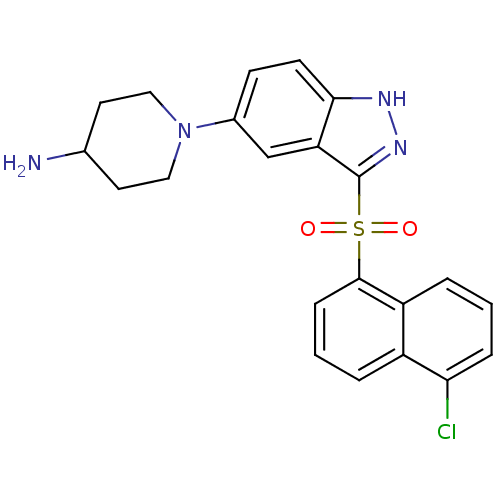
(1-{3-[(5-Chloro-1-naphthyl)sulfonyl]-1H-indazol-5-...)Show SMILES NC1CCN(CC1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2c(Cl)cccc12 Show InChI InChI=1S/C22H21ClN4O2S/c23-19-5-1-4-17-16(19)3-2-6-21(17)30(28,29)22-18-13-15(7-8-20(18)25-26-22)27-11-9-14(24)10-12-27/h1-8,13-14H,9-12,24H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308179

(1-(3-(3-chlorophenylsulfonyl)-1H-indazol-5-yl)pipe...)Show SMILES NC1CCN(CC1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C18H19ClN4O2S/c19-12-2-1-3-15(10-12)26(24,25)18-16-11-14(4-5-17(16)21-22-18)23-8-6-13(20)7-9-23/h1-5,10-11,13H,6-9,20H2,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308180
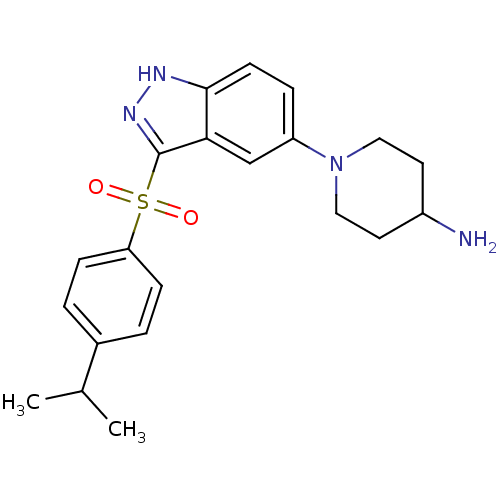
(1-{3-[(4-Isopropylphenyl)sulfonyl]-1H-indazol-5-yl...)Show SMILES CC(C)c1ccc(cc1)S(=O)(=O)c1n[nH]c2ccc(cc12)N1CCC(N)CC1 Show InChI InChI=1S/C21H26N4O2S/c1-14(2)15-3-6-18(7-4-15)28(26,27)21-19-13-17(5-8-20(19)23-24-21)25-11-9-16(22)10-12-25/h3-8,13-14,16H,9-12,22H2,1-2H3,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308170
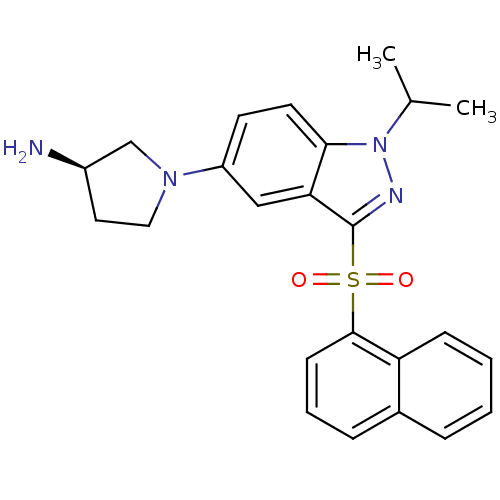
((3R)-1-[1-(1-Methylethyl)-3-(naphthalen-1-ylsulfon...)Show SMILES CC(C)n1nc(c2cc(ccc12)N1CC[C@@H](N)C1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C24H26N4O2S/c1-16(2)28-22-11-10-19(27-13-12-18(25)15-27)14-21(22)24(26-28)31(29,30)23-9-5-7-17-6-3-4-8-20(17)23/h3-11,14,16,18H,12-13,15,25H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308176
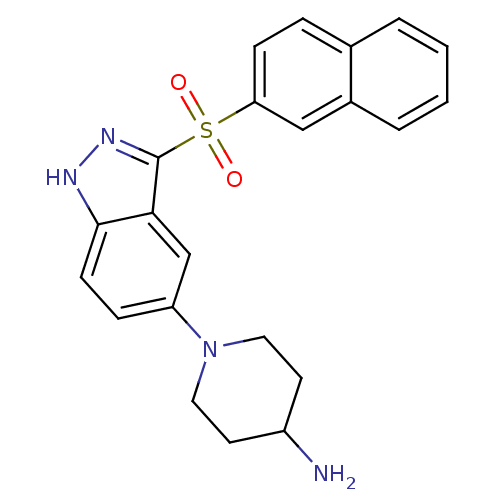
(1-[3-(2-Naphthylsulfonyl)-1H-indazol-5-yl]piperidi...)Show SMILES NC1CCN(CC1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C22H22N4O2S/c23-17-9-11-26(12-10-17)18-6-8-21-20(14-18)22(25-24-21)29(27,28)19-7-5-15-3-1-2-4-16(15)13-19/h1-8,13-14,17H,9-12,23H2,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50308181

((3S)-1-[3-(1-Naphthylsulfonyl)-1H-indazol-5-yl]pyr...)Show SMILES N[C@H]1CCN(C1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C21H20N4O2S/c22-15-10-11-25(13-15)16-8-9-19-18(12-16)21(24-23-19)28(26,27)20-7-3-5-14-4-1-2-6-17(14)20/h1-9,12,15H,10-11,13,22H2,(H,23,24)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2B receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308169

((3R)-1-[1-Methyl-3-(naphthalen-1-ylsulfonyl)-1H-in...)Show SMILES Cn1nc(c2cc(ccc12)N1CC[C@@H](N)C1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C22H22N4O2S/c1-25-20-10-9-17(26-12-11-16(23)14-26)13-19(20)22(24-25)29(27,28)21-8-4-6-15-5-2-3-7-18(15)21/h2-10,13,16H,11-12,14,23H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308168
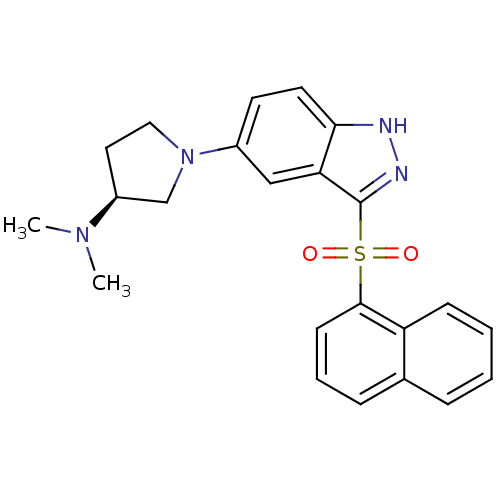
((3S)-N,N-Dimethyl-1-[3-(1-naphthylsulfonyl)-1H-ind...)Show SMILES CN(C)[C@H]1CCN(C1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C23H24N4O2S/c1-26(2)18-12-13-27(15-18)17-10-11-21-20(14-17)23(25-24-21)30(28,29)22-9-5-7-16-6-3-4-8-19(16)22/h3-11,14,18H,12-13,15H2,1-2H3,(H,24,25)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308171

((3R)-1-[1-(2-Methylpropyl)-3-(naphthalen-1-ylsulfo...)Show SMILES CC(C)Cn1nc(c2cc(ccc12)N1CC[C@@H](N)C1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C25H28N4O2S/c1-17(2)15-29-23-11-10-20(28-13-12-19(26)16-28)14-22(23)25(27-29)32(30,31)24-9-5-7-18-6-3-4-8-21(18)24/h3-11,14,17,19H,12-13,15-16,26H2,1-2H3/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50319673
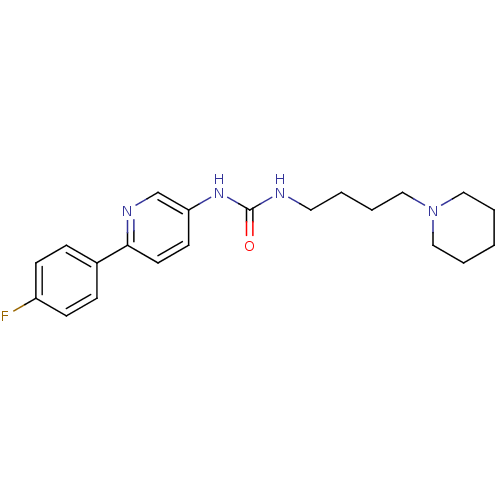
(1-[6-(4-Fluorophenyl)pyridin-3-yl]-3-(4-piperidin-...)Show InChI InChI=1S/C21H27FN4O/c22-18-8-6-17(7-9-18)20-11-10-19(16-24-20)25-21(27)23-12-2-5-15-26-13-3-1-4-14-26/h6-11,16H,1-5,12-15H2,(H2,23,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siena Biotech SpA
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from rat alpha7 nAChR expressed in rat GH4C1 cells |
J Med Chem 53: 4379-89 (2010)
Article DOI: 10.1021/jm901692q
BindingDB Entry DOI: 10.7270/Q2S46S4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308173

((3R)-1-[2-(1-Methylethyl)-3-(naphthalen-1-ylsulfon...)Show SMILES CC(C)n1nc2ccc(cc2c1S(=O)(=O)c1cccc2ccccc12)N1CC[C@@H](N)C1 |r| Show InChI InChI=1S/C24H26N4O2S/c1-16(2)28-24(31(29,30)23-9-5-7-17-6-3-4-8-20(17)23)21-14-19(10-11-22(21)26-28)27-13-12-18(25)15-27/h3-11,14,16,18H,12-13,15,25H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 48.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308172
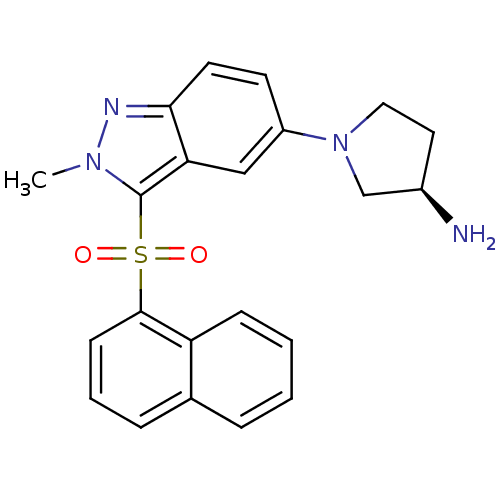
((3R)-1-[2-Methyl-3-(naphthalen-1-ylsulfonyl)-2H-in...)Show SMILES Cn1nc2ccc(cc2c1S(=O)(=O)c1cccc2ccccc12)N1CC[C@@H](N)C1 |r| Show InChI InChI=1S/C22H22N4O2S/c1-25-22(29(27,28)21-8-4-6-15-5-2-3-7-18(15)21)19-13-17(9-10-20(19)24-25)26-12-11-16(23)14-26/h2-10,13,16H,11-12,14,23H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 54.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50308174
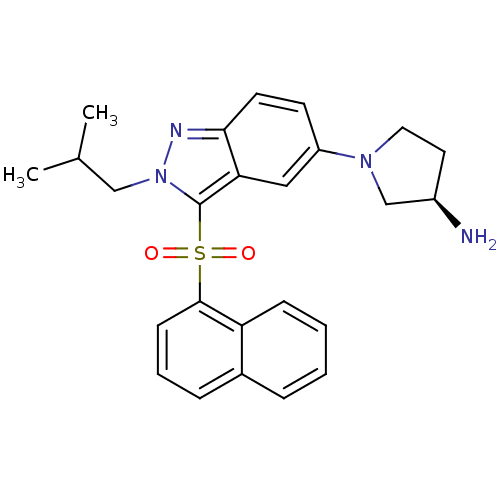
((3R)-1-[2-(2-Methylpropyl)-3-(naphthalen-1-ylsulfo...)Show SMILES CC(C)Cn1nc2ccc(cc2c1S(=O)(=O)c1cccc2ccccc12)N1CC[C@@H](N)C1 |r| Show InChI InChI=1S/C25H28N4O2S/c1-17(2)15-29-25(32(30,31)24-9-5-7-18-6-3-4-8-21(18)24)22-14-20(10-11-23(22)27-29)28-13-12-19(26)16-28/h3-11,14,17,19H,12-13,15-16,26H2,1-2H3/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 75.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50308178

(1-{3-[(5-Chloro-1-naphthyl)sulfonyl]-1H-indazol-5-...)Show SMILES NC1CCN(CC1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2c(Cl)cccc12 Show InChI InChI=1S/C22H21ClN4O2S/c23-19-5-1-4-17-16(19)3-2-6-21(17)30(28,29)22-18-13-15(7-8-20(18)25-26-22)27-11-9-14(24)10-12-27/h1-8,13-14H,9-12,24H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2B receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50308183

(1-[3-(1-Naphthylsulfonyl)-1H-indazol-5-yl]azetidin...)Show SMILES NC1CN(C1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C20H18N4O2S/c21-14-11-24(12-14)15-8-9-18-17(10-15)20(23-22-18)27(25,26)19-7-3-5-13-4-1-2-6-16(13)19/h1-10,14H,11-12,21H2,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 97.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2B receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50308175

(1-[3-(1-Naphthylsulfonyl)-1H-indazol-5-yl]piperidi...)Show SMILES NC1CCN(CC1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C22H22N4O2S/c23-16-10-12-26(13-11-16)17-8-9-20-19(14-17)22(25-24-20)29(27,28)21-7-3-5-15-4-1-2-6-18(15)21/h1-9,14,16H,10-13,23H2,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2B receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50308169

((3R)-1-[1-Methyl-3-(naphthalen-1-ylsulfonyl)-1H-in...)Show SMILES Cn1nc(c2cc(ccc12)N1CC[C@@H](N)C1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C22H22N4O2S/c1-25-20-10-9-17(26-12-11-16(23)14-26)13-19(20)22(24-25)29(27,28)21-8-4-6-15-5-2-3-7-18(15)21/h2-10,13,16H,11-12,14,23H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 185 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2B receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50308170

((3R)-1-[1-(1-Methylethyl)-3-(naphthalen-1-ylsulfon...)Show SMILES CC(C)n1nc(c2cc(ccc12)N1CC[C@@H](N)C1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C24H26N4O2S/c1-16(2)28-22-11-10-19(27-13-12-18(25)15-27)14-21(22)24(26-28)31(29,30)23-9-5-7-17-6-3-4-8-20(17)23/h3-11,14,16,18H,12-13,15,25H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2B receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50308174

((3R)-1-[2-(2-Methylpropyl)-3-(naphthalen-1-ylsulfo...)Show SMILES CC(C)Cn1nc2ccc(cc2c1S(=O)(=O)c1cccc2ccccc12)N1CC[C@@H](N)C1 |r| Show InChI InChI=1S/C25H28N4O2S/c1-17(2)15-29-25(32(30,31)24-9-5-7-18-6-3-4-8-21(18)24)22-14-20(10-11-23(22)27-29)28-13-12-19(26)16-28/h3-11,14,17,19H,12-13,15-16,26H2,1-2H3/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2B receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50308182

((3R)-1-[3-(1-Naphthylsulfonyl)-1H-indazol-5-yl]pyr...)Show SMILES N[C@@H]1CCN(C1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C21H20N4O2S/c22-15-10-11-25(13-15)16-8-9-19-18(12-16)21(24-23-19)28(26,27)20-7-3-5-14-4-1-2-6-17(14)20/h1-9,12,15H,10-11,13,22H2,(H,23,24)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 274 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2B receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50308167

((3R)-N,N-Dimethyl-1-[3-(1-naphthylsulfonyl)-1H-ind...)Show SMILES CN(C)[C@@H]1CCN(C1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C23H24N4O2S/c1-26(2)18-12-13-27(15-18)17-10-11-21-20(14-17)23(25-24-21)30(28,29)22-9-5-7-16-6-3-4-8-19(16)22/h3-11,14,18H,12-13,15H2,1-2H3,(H,24,25)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 423 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2B receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50308179

(1-(3-(3-chlorophenylsulfonyl)-1H-indazol-5-yl)pipe...)Show SMILES NC1CCN(CC1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C18H19ClN4O2S/c19-12-2-1-3-15(10-12)26(24,25)18-16-11-14(4-5-17(16)21-22-18)23-8-6-13(20)7-9-23/h1-5,10-11,13H,6-9,20H2,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 442 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2B receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50308172

((3R)-1-[2-Methyl-3-(naphthalen-1-ylsulfonyl)-2H-in...)Show SMILES Cn1nc2ccc(cc2c1S(=O)(=O)c1cccc2ccccc12)N1CC[C@@H](N)C1 |r| Show InChI InChI=1S/C22H22N4O2S/c1-25-22(29(27,28)21-8-4-6-15-5-2-3-7-18(15)21)19-13-17(9-10-20(19)24-25)26-12-11-16(23)14-26/h2-10,13,16H,11-12,14,23H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 455 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2B receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50308177

(1-{3-[(4-Methyl-1-naphthyl)sulfonyl]-1H-indazol-5-...)Show SMILES Cc1ccc(c2ccccc12)S(=O)(=O)c1n[nH]c2ccc(cc12)N1CCC(N)CC1 Show InChI InChI=1S/C23H24N4O2S/c1-15-6-9-22(19-5-3-2-4-18(15)19)30(28,29)23-20-14-17(7-8-21(20)25-26-23)27-12-10-16(24)11-13-27/h2-9,14,16H,10-13,24H2,1H3,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 493 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2B receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50308168

((3S)-N,N-Dimethyl-1-[3-(1-naphthylsulfonyl)-1H-ind...)Show SMILES CN(C)[C@H]1CCN(C1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C23H24N4O2S/c1-26(2)18-12-13-27(15-18)17-10-11-21-20(14-17)23(25-24-21)30(28,29)22-9-5-7-16-6-3-4-8-19(16)22/h3-11,14,18H,12-13,15H2,1-2H3,(H,24,25)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 583 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2B receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50308176

(1-[3-(2-Naphthylsulfonyl)-1H-indazol-5-yl]piperidi...)Show SMILES NC1CCN(CC1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C22H22N4O2S/c23-17-9-11-26(12-10-17)18-6-8-21-20(14-18)22(25-24-21)29(27,28)19-7-5-15-3-1-2-4-16(15)13-19/h1-8,13-14,17H,9-12,23H2,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2B receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50308182

((3R)-1-[3-(1-Naphthylsulfonyl)-1H-indazol-5-yl]pyr...)Show SMILES N[C@@H]1CCN(C1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C21H20N4O2S/c22-15-10-11-25(13-15)16-8-9-19-18(12-16)21(24-23-19)28(26,27)20-7-3-5-14-4-1-2-6-17(14)20/h1-9,12,15H,10-11,13,22H2,(H,23,24)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT1D receptor |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50308182

((3R)-1-[3-(1-Naphthylsulfonyl)-1H-indazol-5-yl]pyr...)Show SMILES N[C@@H]1CCN(C1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C21H20N4O2S/c22-15-10-11-25(13-15)16-8-9-19-18(12-16)21(24-23-19)28(26,27)20-7-3-5-14-4-1-2-6-17(14)20/h1-9,12,15H,10-11,13,22H2,(H,23,24)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D2 receptor |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50308173

((3R)-1-[2-(1-Methylethyl)-3-(naphthalen-1-ylsulfon...)Show SMILES CC(C)n1nc2ccc(cc2c1S(=O)(=O)c1cccc2ccccc12)N1CC[C@@H](N)C1 |r| Show InChI InChI=1S/C24H26N4O2S/c1-16(2)28-24(31(29,30)23-9-5-7-17-6-3-4-8-20(17)23)21-14-19(10-11-22(21)26-28)27-13-12-18(25)15-27/h3-11,14,16,18H,12-13,15,25H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2B receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50308171

((3R)-1-[1-(2-Methylpropyl)-3-(naphthalen-1-ylsulfo...)Show SMILES CC(C)Cn1nc(c2cc(ccc12)N1CC[C@@H](N)C1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C25H28N4O2S/c1-17(2)15-29-23-11-10-20(28-13-12-19(26)16-28)14-22(23)25(27-29)32(30,31)24-9-5-7-18-6-3-4-8-21(18)24/h3-11,14,17,19H,12-13,15-16,26H2,1-2H3/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2B receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50308182

((3R)-1-[3-(1-Naphthylsulfonyl)-1H-indazol-5-yl]pyr...)Show SMILES N[C@@H]1CCN(C1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C21H20N4O2S/c22-15-10-11-25(13-15)16-8-9-19-18(12-16)21(24-23-19)28(26,27)20-7-3-5-14-4-1-2-6-17(14)20/h1-9,12,15H,10-11,13,22H2,(H,23,24)/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT1A receptor |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50319673

(1-[6-(4-Fluorophenyl)pyridin-3-yl]-3-(4-piperidin-...)Show InChI InChI=1S/C21H27FN4O/c22-18-8-6-17(7-9-18)20-11-10-19(16-24-20)25-21(27)23-12-2-5-15-26-13-3-1-4-14-26/h6-11,16H,1-5,12-15H2,(H2,23,25,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Siena Biotech SpA
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT4 receptor |
J Med Chem 53: 4379-89 (2010)
Article DOI: 10.1021/jm901692q
BindingDB Entry DOI: 10.7270/Q2S46S4Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50308182

((3R)-1-[3-(1-Naphthylsulfonyl)-1H-indazol-5-yl]pyr...)Show SMILES N[C@@H]1CCN(C1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C21H20N4O2S/c22-15-10-11-25(13-15)16-8-9-19-18(12-16)21(24-23-19)28(26,27)20-7-3-5-14-4-1-2-6-17(14)20/h1-9,12,15H,10-11,13,22H2,(H,23,24)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT1B receptor |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50308180

(1-{3-[(4-Isopropylphenyl)sulfonyl]-1H-indazol-5-yl...)Show SMILES CC(C)c1ccc(cc1)S(=O)(=O)c1n[nH]c2ccc(cc12)N1CCC(N)CC1 Show InChI InChI=1S/C21H26N4O2S/c1-14(2)15-3-6-18(7-4-15)28(26,27)21-19-13-17(5-8-20(19)23-24-21)25-11-9-16(22)10-12-25/h3-8,13-14,16H,9-12,22H2,1-2H3,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT2B receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50308182

((3R)-1-[3-(1-Naphthylsulfonyl)-1H-indazol-5-yl]pyr...)Show SMILES N[C@@H]1CCN(C1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C21H20N4O2S/c22-15-10-11-25(13-15)16-8-9-19-18(12-16)21(24-23-19)28(26,27)20-7-3-5-14-4-1-2-6-17(14)20/h1-9,12,15H,10-11,13,22H2,(H,23,24)/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity to alpha2A adrenergic receptor |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type 1
(Homo sapiens (Human)) | BDBM50545573
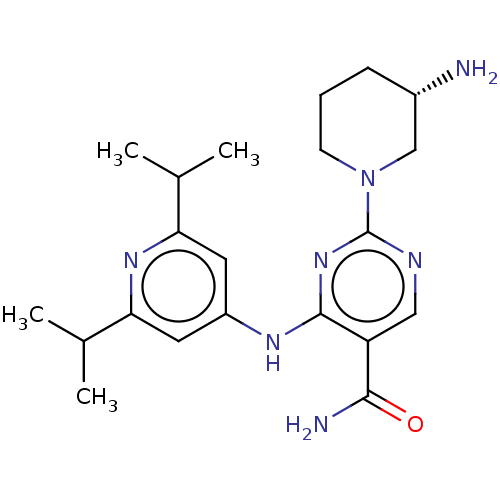
(CHEMBL4635883 | US11530193, Example 54)Show SMILES CC(C)c1cc(Nc2nc(ncc2C(N)=O)N2CCC[C@H](N)C2)cc(n1)C(C)C |r| Show InChI InChI=1S/C21H31N7O/c1-12(2)17-8-15(9-18(26-17)13(3)4)25-20-16(19(23)29)10-24-21(27-20)28-7-5-6-14(22)11-28/h8-10,12-14H,5-7,11,22H2,1-4H3,(H2,23,29)(H,24,25,26,27)/t14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of human CAMK1A using KKALRRQETVDAL as substrate in presence of [gamma-33P]-ATP by hotspot kinase assay |
J Med Chem 63: 6784-6801 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01803
BindingDB Entry DOI: 10.7270/Q2QF8XG3 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type 1G
(Homo sapiens (Human)) | BDBM50545573

(CHEMBL4635883 | US11530193, Example 54)Show SMILES CC(C)c1cc(Nc2nc(ncc2C(N)=O)N2CCC[C@H](N)C2)cc(n1)C(C)C |r| Show InChI InChI=1S/C21H31N7O/c1-12(2)17-8-15(9-18(26-17)13(3)4)25-20-16(19(23)29)10-24-21(27-20)28-7-5-6-14(22)11-28/h8-10,12-14H,5-7,11,22H2,1-4H3,(H2,23,29)(H,24,25,26,27)/t14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of human CAMK1G using KKALRRQETVDAL as substrate in presence of [gamma-33P]-ATP by hotspot assay |
J Med Chem 63: 6784-6801 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01803
BindingDB Entry DOI: 10.7270/Q2QF8XG3 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type 1
(Homo sapiens (Human)) | BDBM50545576
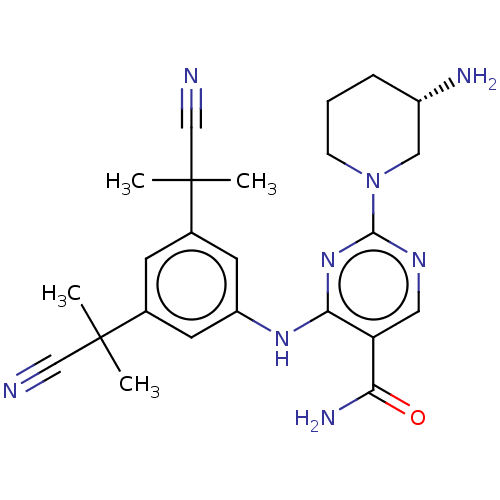
(CHEMBL4640712 | US11530193, Example 50)Show SMILES CC(C)(C#N)c1cc(Nc2nc(ncc2C(N)=O)N2CCC[C@H](N)C2)cc(c1)C(C)(C)C#N |r| Show InChI InChI=1S/C24H30N8O/c1-23(2,13-25)15-8-16(24(3,4)14-26)10-18(9-15)30-21-19(20(28)33)11-29-22(31-21)32-7-5-6-17(27)12-32/h8-11,17H,5-7,12,27H2,1-4H3,(H2,28,33)(H,29,30,31)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of human CAMK1A using KKALRRQETVDAL as substrate in presence of [gamma-33P]-ATP by hotspot kinase assay |
J Med Chem 63: 6784-6801 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01803
BindingDB Entry DOI: 10.7270/Q2QF8XG3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM78940

(METHIOTHEPIN | MLS000859918 | Methiothepin mesylat...)Show InChI InChI=1S/C20H24N2S2/c1-21-9-11-22(12-10-21)18-13-15-5-3-4-6-19(15)24-20-8-7-16(23-2)14-17(18)20/h3-8,14,18H,9-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting |
J Med Chem 53: 2521-7 (2010)
Article DOI: 10.1021/jm901674f
BindingDB Entry DOI: 10.7270/Q22B8Z43 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type 1B
(Homo sapiens (Human)) | BDBM50545573

(CHEMBL4635883 | US11530193, Example 54)Show SMILES CC(C)c1cc(Nc2nc(ncc2C(N)=O)N2CCC[C@H](N)C2)cc(n1)C(C)C |r| Show InChI InChI=1S/C21H31N7O/c1-12(2)17-8-15(9-18(26-17)13(3)4)25-20-16(19(23)29)10-24-21(27-20)28-7-5-6-14(22)11-28/h8-10,12-14H,5-7,11,22H2,1-4H3,(H2,23,29)(H,24,25,26,27)/t14-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of human CAMK1B using KKALRRQETVDAL as substrate in presence of [gamma-33P]-ATP by hotspot kinase assay |
J Med Chem 63: 6784-6801 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01803
BindingDB Entry DOI: 10.7270/Q2QF8XG3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50249319
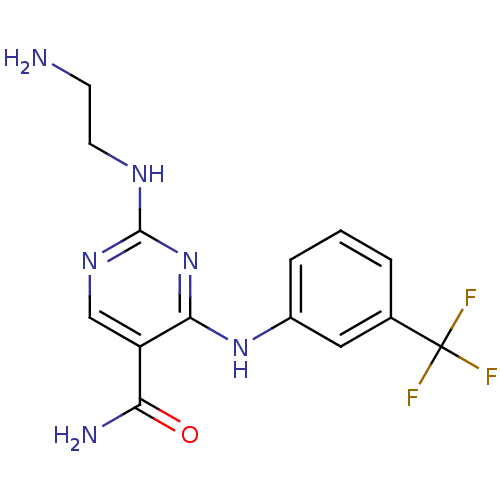
(2-(2-aminoethylamino)-4-(3-(trifluoromethyl)phenyl...)Show InChI InChI=1S/C14H15F3N6O/c15-14(16,17)8-2-1-3-9(6-8)22-12-10(11(19)24)7-21-13(23-12)20-5-4-18/h1-3,6-7H,4-5,18H2,(H2,19,24)(H2,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of human SYK using poly[Glu:Tyr] (4:1) as substrate in presence of [gamma-33P]-ATP by hotspot kinase assay |
J Med Chem 63: 6784-6801 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01803
BindingDB Entry DOI: 10.7270/Q2QF8XG3 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type 1D
(Homo sapiens (Human)) | BDBM50545573

(CHEMBL4635883 | US11530193, Example 54)Show SMILES CC(C)c1cc(Nc2nc(ncc2C(N)=O)N2CCC[C@H](N)C2)cc(n1)C(C)C |r| Show InChI InChI=1S/C21H31N7O/c1-12(2)17-8-15(9-18(26-17)13(3)4)25-20-16(19(23)29)10-24-21(27-20)28-7-5-6-14(22)11-28/h8-10,12-14H,5-7,11,22H2,1-4H3,(H2,23,29)(H,24,25,26,27)/t14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full-length His-tagged CAMK1D expressed in baculovirus expression system using autocamtide-2 as substrate preincubate... |
J Med Chem 63: 6784-6801 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01803
BindingDB Entry DOI: 10.7270/Q2QF8XG3 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type 1D
(Homo sapiens (Human)) | BDBM50545576

(CHEMBL4640712 | US11530193, Example 50)Show SMILES CC(C)(C#N)c1cc(Nc2nc(ncc2C(N)=O)N2CCC[C@H](N)C2)cc(c1)C(C)(C)C#N |r| Show InChI InChI=1S/C24H30N8O/c1-23(2,13-25)15-8-16(24(3,4)14-26)10-18(9-15)30-21-19(20(28)33)11-29-22(31-21)32-7-5-6-17(27)12-32/h8-11,17H,5-7,12,27H2,1-4H3,(H2,28,33)(H,29,30,31)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of HA-tagged CAMK1D (unknown origin) expressed in human MDA-MB-231 cells assessed as reduction in CAMK1D autophosphorylation measured afte... |
J Med Chem 63: 6784-6801 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01803
BindingDB Entry DOI: 10.7270/Q2QF8XG3 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type 1D
(Homo sapiens (Human)) | BDBM50545573

(CHEMBL4635883 | US11530193, Example 54)Show SMILES CC(C)c1cc(Nc2nc(ncc2C(N)=O)N2CCC[C@H](N)C2)cc(n1)C(C)C |r| Show InChI InChI=1S/C21H31N7O/c1-12(2)17-8-15(9-18(26-17)13(3)4)25-20-16(19(23)29)10-24-21(27-20)28-7-5-6-14(22)11-28/h8-10,12-14H,5-7,11,22H2,1-4H3,(H2,23,29)(H,24,25,26,27)/t14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of human CAMK1D using KKALRRQETVDAL as substrate in presence of [gamma-33P]-ATP by hotspot kinase assay |
J Med Chem 63: 6784-6801 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01803
BindingDB Entry DOI: 10.7270/Q2QF8XG3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data