 Found 341 hits with Last Name = 'kurose' and Initial = 'n'
Found 341 hits with Last Name = 'kurose' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM210894

(US9273043, A123(e))Show SMILES C[C@@H]1CN(CCN1C(=O)Nc1nc2ccc(Cl)cc2s1)c1ncc(cc1F)[C@@H](O)CO |r| Show InChI InChI=1S/C20H21ClFN5O3S/c1-11-9-26(18-14(22)6-12(8-23-18)16(29)10-28)4-5-27(11)20(30)25-19-24-15-3-2-13(21)7-17(15)31-19/h2-3,6-8,11,16,28-29H,4-5,9-10H2,1H3,(H,24,25,30)/t11-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Purdue Pharma L.P.; Shionogi & Co., Ltd.
US Patent
| Assay Description
pH dependent Ca2+ responses in TRPV1/CHO cells cultured in a 96-well plate were determined (see, e.g., FIG. 2 of U.S. Patent Application Publication ... |
US Patent US9273043 (2016)
BindingDB Entry DOI: 10.7270/Q2D7998G |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM210898
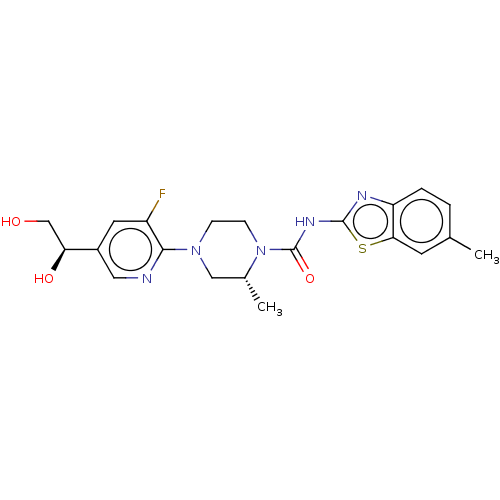
(US9273043, A126(e))Show SMILES C[C@@H]1CN(CCN1C(=O)Nc1nc2ccc(C)cc2s1)c1ncc(cc1F)[C@@H](O)CO |r| Show InChI InChI=1S/C21H24FN5O3S/c1-12-3-4-16-18(7-12)31-20(24-16)25-21(30)27-6-5-26(10-13(27)2)19-15(22)8-14(9-23-19)17(29)11-28/h3-4,7-9,13,17,28-29H,5-6,10-11H2,1-2H3,(H,24,25,30)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Purdue Pharma L.P.; Shionogi & Co., Ltd.
US Patent
| Assay Description
pH dependent Ca2+ responses in TRPV1/CHO cells cultured in a 96-well plate were determined (see, e.g., FIG. 2 of U.S. Patent Application Publication ... |
US Patent US9273043 (2016)
BindingDB Entry DOI: 10.7270/Q2D7998G |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM185339
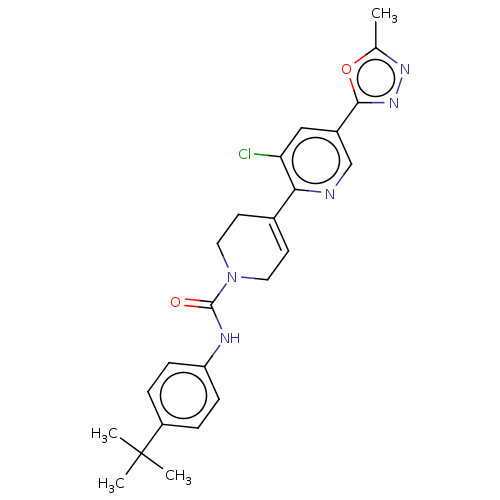
(US9156830, I-7)Show SMILES Cc1nnc(o1)-c1cnc(C2=CCN(CC2)C(=O)Nc2ccc(cc2)C(C)(C)C)c(Cl)c1 |t:11| Show InChI InChI=1S/C24H26ClN5O2/c1-15-28-29-22(32-15)17-13-20(25)21(26-14-17)16-9-11-30(12-10-16)23(31)27-19-7-5-18(6-8-19)24(2,3)4/h5-9,13-14H,10-12H2,1-4H3,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
SHIONOGI & CO., LTD.
US Patent
| Assay Description
Two days prior to performing this assay, cells are seeded on poly-D-lysine-coated 96-well clear-bottom black plates (commercially available from Bect... |
US Patent US9156830 (2015)
BindingDB Entry DOI: 10.7270/Q2SN07R2 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM210896
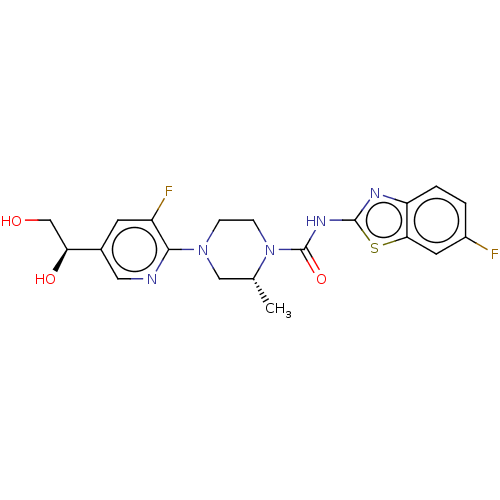
(US9273043, A125(e))Show SMILES C[C@@H]1CN(CCN1C(=O)Nc1nc2ccc(F)cc2s1)c1ncc(cc1F)[C@@H](O)CO |r| Show InChI InChI=1S/C20H21F2N5O3S/c1-11-9-26(18-14(22)6-12(8-23-18)16(29)10-28)4-5-27(11)20(30)25-19-24-15-3-2-13(21)7-17(15)31-19/h2-3,6-8,11,16,28-29H,4-5,9-10H2,1H3,(H,24,25,30)/t11-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Purdue Pharma L.P.; Shionogi & Co., Ltd.
US Patent
| Assay Description
pH dependent Ca2+ responses in TRPV1/CHO cells cultured in a 96-well plate were determined (see, e.g., FIG. 2 of U.S. Patent Application Publication ... |
US Patent US9273043 (2016)
BindingDB Entry DOI: 10.7270/Q2D7998G |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50432632

(CHEMBL2347211)Show SMILES C[C@]1(CCSC(N)=N1)c1cc(NC(=O)c2ccc(Cl)cn2)ccc1F |r,c:6| Show InChI InChI=1S/C17H16ClFN4OS/c1-17(6-7-25-16(20)23-17)12-8-11(3-4-13(12)19)22-15(24)14-5-2-10(18)9-21-14/h2-5,8-9H,6-7H2,1H3,(H2,20,23)(H,22,24)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) assessed as reduction in amyloid beta production by cell based assay |
J Med Chem 61: 5122-5137 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00002
BindingDB Entry DOI: 10.7270/Q27W6FSQ |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM185343

(US9156830, I-44)Show SMILES Cc1cc(NC(=O)N2CCC(=CC2)c2ncc(cc2C)-c2nnc(CO)o2)ccc1C(F)(F)F |c:10| Show InChI InChI=1S/C23H22F3N5O3/c1-13-10-17(3-4-18(13)23(24,25)26)28-22(33)31-7-5-15(6-8-31)20-14(2)9-16(11-27-20)21-30-29-19(12-32)34-21/h3-5,9-11,32H,6-8,12H2,1-2H3,(H,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | n/a |
SHIONOGI & CO., LTD.
US Patent
| Assay Description
Two days prior to performing this assay, cells are seeded on poly-D-lysine-coated 96-well clear-bottom black plates (commercially available from Bect... |
US Patent US9156830 (2015)
BindingDB Entry DOI: 10.7270/Q2SN07R2 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM141515
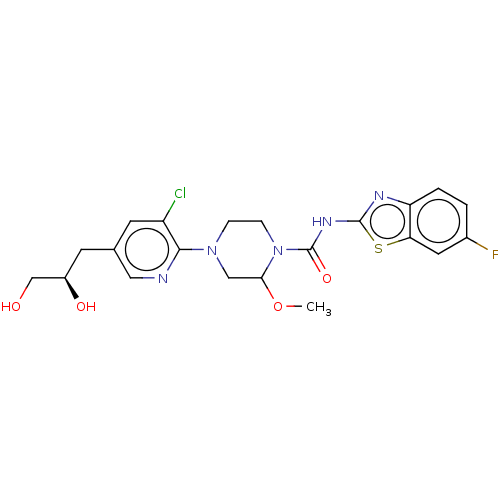
(US8921373, ACU)Show SMILES COC1CN(CCN1C(=O)Nc1nc2ccc(F)cc2s1)c1ncc(C[C@@H](O)CO)cc1Cl |r| Show InChI InChI=1S/C21H23ClFN5O4S/c1-32-18-10-27(19-15(22)7-12(9-24-19)6-14(30)11-29)4-5-28(18)21(31)26-20-25-16-3-2-13(23)8-17(16)33-20/h2-3,7-9,14,18,29-30H,4-6,10-11H2,1H3,(H,25,26,31)/t14-,18?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Shionogi & Co., Ltd.
US Patent
| Assay Description
Two days prior to performing this assay, cells are seeded on poly-D-lysine-coated 96-well clear-bottom black plates (commercially available from Bect... |
US Patent US8921373 (2014)
BindingDB Entry DOI: 10.7270/Q28051BW |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50484379
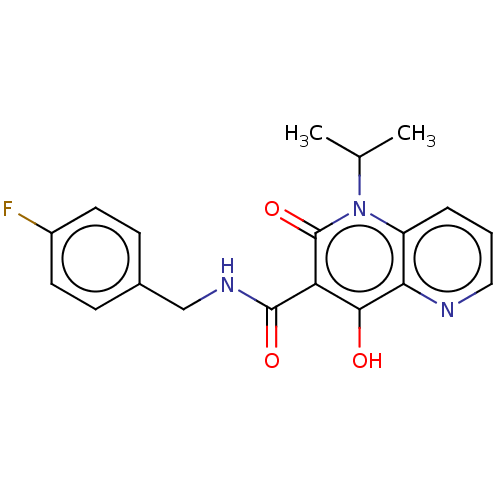
(CHEMBL1917873)Show SMILES CC(C)n1c2cccnc2c(O)c(C(=O)NCc2ccc(F)cc2)c1=O Show InChI InChI=1S/C19H18FN3O3/c1-11(2)23-14-4-3-9-21-16(14)17(24)15(19(23)26)18(25)22-10-12-5-7-13(20)8-6-12/h3-9,11,24H,10H2,1-2H3,(H,22,25) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer by biochemical assay |
Bioorg Med Chem Lett 21: 6461-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.082
BindingDB Entry DOI: 10.7270/Q22Z18CH |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM185349
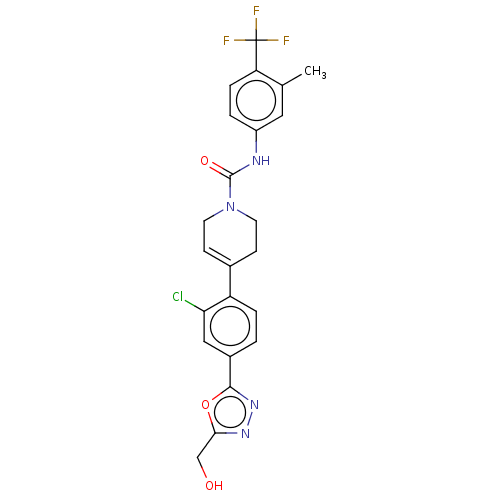
(US9156830, I-76)Show SMILES Cc1cc(NC(=O)N2CCC(=CC2)c2ccc(cc2Cl)-c2nnc(CO)o2)ccc1C(F)(F)F |c:10| Show InChI InChI=1S/C23H20ClF3N4O3/c1-13-10-16(3-5-18(13)23(25,26)27)28-22(33)31-8-6-14(7-9-31)17-4-2-15(11-19(17)24)21-30-29-20(12-32)34-21/h2-6,10-11,32H,7-9,12H2,1H3,(H,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 7.4 | n/a |
SHIONOGI & CO., LTD.
US Patent
| Assay Description
Two days prior to performing this assay, cells are seeded on poly-D-lysine-coated 96-well clear-bottom black plates (commercially available from Bect... |
US Patent US9156830 (2015)
BindingDB Entry DOI: 10.7270/Q2SN07R2 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(Homo sapiens (Human)) | BDBM50580223
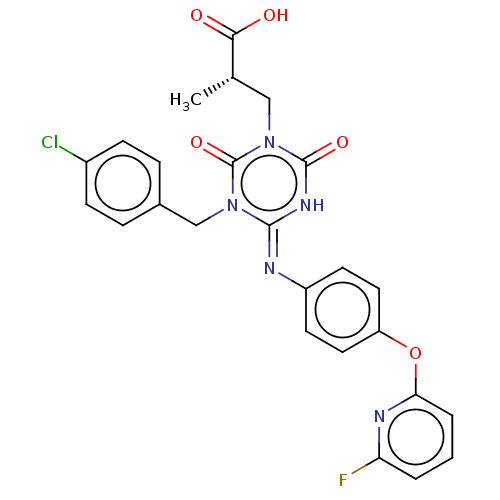
(CHEMBL5079957)Show SMILES C[C@@H](Cn1c(=O)[nH]\c(=N/c2ccc(Oc3cccc(F)n3)cc2)n(Cc2ccc(Cl)cc2)c1=O)C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at P2X3 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128384
BindingDB Entry DOI: 10.7270/Q2S46WTX |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(Homo sapiens (Human)) | BDBM50580222
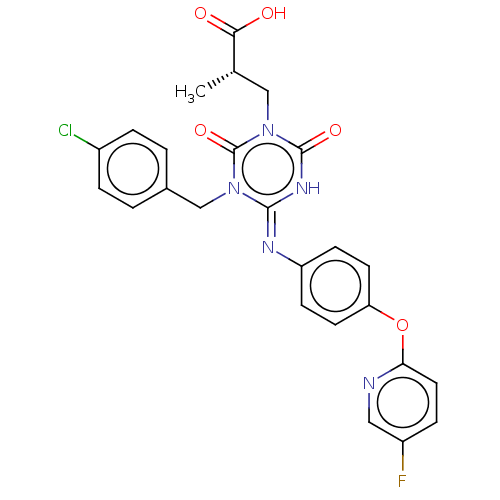
(CHEMBL5076078)Show SMILES C[C@@H](Cn1c(=O)[nH]\c(=N/c2ccc(Oc3ccc(F)cn3)cc2)n(Cc2ccc(Cl)cc2)c1=O)C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at P2X3 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128384
BindingDB Entry DOI: 10.7270/Q2S46WTX |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50457586
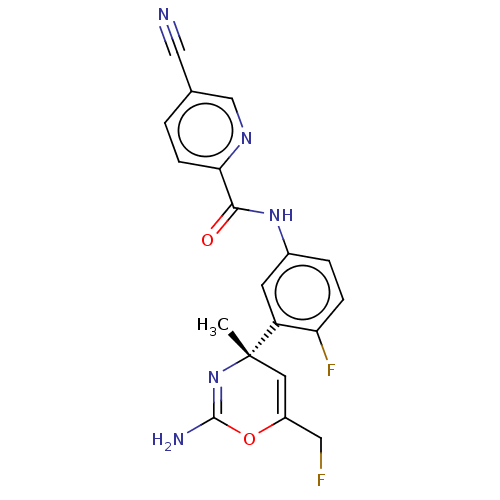
(CHEMBL4213486)Show SMILES C[C@]1(C=C(CF)OC(N)=N1)c1cc(NC(=O)c2ccc(cn2)C#N)ccc1F |r,c:8,t:2| Show InChI InChI=1S/C19H15F2N5O2/c1-19(7-13(8-20)28-18(23)26-19)14-6-12(3-4-15(14)21)25-17(27)16-5-2-11(9-22)10-24-16/h2-7,10H,8H2,1H3,(H2,23,26)(H,25,27)/t19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human SH-SY5Y cells transfected with human wild type beta-APP assessed as reduction in amyloid beta (1 to 40) levels after 24 ... |
J Med Chem 61: 5122-5137 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00002
BindingDB Entry DOI: 10.7270/Q27W6FSQ |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50432609

(CHEMBL2347203 | US8999980, I-6)Show SMILES C[C@]1(CCOC(N)=N1)c1cc(NC(=O)c2ccc(cn2)C#N)ccc1F |r,c:6| Show InChI InChI=1S/C18H16FN5O2/c1-18(6-7-26-17(21)24-18)13-8-12(3-4-14(13)19)23-16(25)15-5-2-11(9-20)10-22-15/h2-5,8,10H,6-7H2,1H3,(H2,21,24)(H,23,25)/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human SH-SY5Y cells transfected with human wild type beta-APP assessed as reduction in amyloid beta (1 to 40) levels after 24 ... |
J Med Chem 61: 5122-5137 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00002
BindingDB Entry DOI: 10.7270/Q27W6FSQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
P2X purinoceptor 3
(RAT) | BDBM50580225
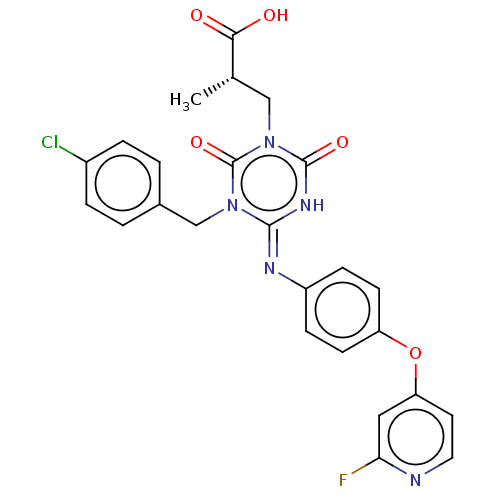
(CHEMBL5084053)Show SMILES C[C@@H](Cn1c(=O)[nH]\c(=N/c2ccc(Oc3ccnc(F)c3)cc2)n(Cc2ccc(Cl)cc2)c1=O)C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at rat P2X3 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128384
BindingDB Entry DOI: 10.7270/Q2S46WTX |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50484397
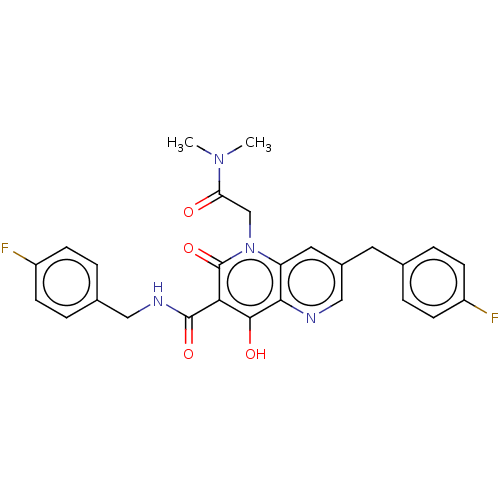
(CHEMBL1914460)Show SMILES CN(C)C(=O)Cn1c2cc(Cc3ccc(F)cc3)cnc2c(O)c(C(=O)NCc2ccc(F)cc2)c1=O Show InChI InChI=1S/C27H24F2N4O4/c1-32(2)22(34)15-33-21-12-18(11-16-3-7-19(28)8-4-16)14-30-24(21)25(35)23(27(33)37)26(36)31-13-17-5-9-20(29)10-6-17/h3-10,12,14,35H,11,13,15H2,1-2H3,(H,31,36) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer by biochemical assay |
Bioorg Med Chem Lett 21: 6461-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.082
BindingDB Entry DOI: 10.7270/Q22Z18CH |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(RAT) | BDBM271626

(US10065941, Compound I-127 | US9732060, Compound I...)Show SMILES C[C@@H](Cn1c(=O)[nH]\c(=N/c2ccc(Oc3ccccn3)cc2)n(Cc2ccc(Cl)cc2)c1=O)C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at rat P2X3 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128384
BindingDB Entry DOI: 10.7270/Q2S46WTX |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(Homo sapiens (Human)) | BDBM271626

(US10065941, Compound I-127 | US9732060, Compound I...)Show SMILES C[C@@H](Cn1c(=O)[nH]\c(=N/c2ccc(Oc3ccccn3)cc2)n(Cc2ccc(Cl)cc2)c1=O)C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at P2X3 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128384
BindingDB Entry DOI: 10.7270/Q2S46WTX |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM153632
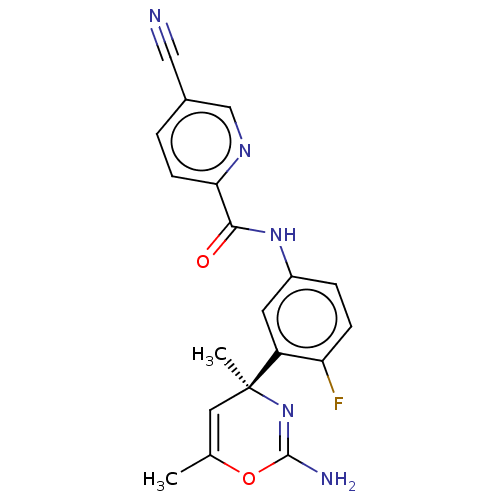
(US8999980, I-54)Show SMILES CC1=C[C@](C)(N=C(N)O1)c1cc(NC(=O)c2ccc(cn2)C#N)ccc1F |r,t:1,5| Show InChI InChI=1S/C19H16FN5O2/c1-11-8-19(2,25-18(22)27-11)14-7-13(4-5-15(14)20)24-17(26)16-6-3-12(9-21)10-23-16/h3-8,10H,1-2H3,(H2,22,25)(H,24,26)/t19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human SH-SY5Y cells transfected with human wild type beta-APP assessed as reduction in amyloid beta (1 to 40) levels after 24 ... |
J Med Chem 61: 5122-5137 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00002
BindingDB Entry DOI: 10.7270/Q27W6FSQ |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(Homo sapiens (Human)) | BDBM271569

(US10065941, Compound I-070 | US9732060, Compound I...)Show SMILES C[C@@H](Cn1c(=O)[nH]\c(=N/c2ccc(Oc3cc(F)ccn3)cc2)n(Cc2ccc(Cl)cc2)c1=O)C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at P2X3 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128384
BindingDB Entry DOI: 10.7270/Q2S46WTX |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM141532
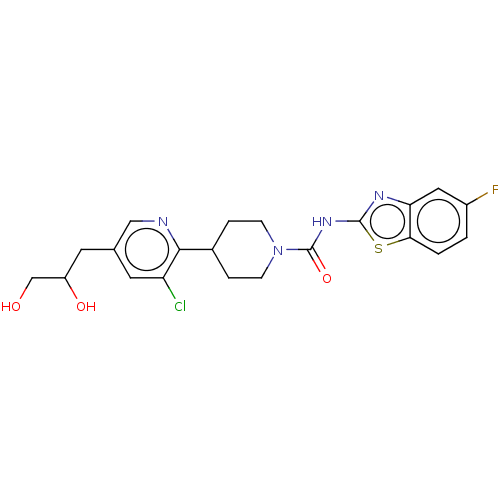
(US8921373, AAD)Show SMILES OCC(O)Cc1cnc(C2CCN(CC2)C(=O)Nc2nc3cc(F)ccc3s2)c(Cl)c1 Show InChI InChI=1S/C21H22ClFN4O3S/c22-16-8-12(7-15(29)11-28)10-24-19(16)13-3-5-27(6-4-13)21(30)26-20-25-17-9-14(23)1-2-18(17)31-20/h1-2,8-10,13,15,28-29H,3-7,11H2,(H,25,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Shionogi & Co., Ltd.
US Patent
| Assay Description
Two days prior to performing this assay, cells are seeded on poly-D-lysine-coated 96-well clear-bottom black plates (commercially available from Bect... |
US Patent US8921373 (2014)
BindingDB Entry DOI: 10.7270/Q28051BW |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(Homo sapiens (Human)) | BDBM271893

(US10065941, Compound R-208 | US9732060, Compound R...)Show SMILES C[C@@H](Cn1c(=O)[nH]\c(=N/c2ccc(Oc3ccncc3)cc2)n(Cc2ccc(Cl)cc2)c1=O)C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at P2X3 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128384
BindingDB Entry DOI: 10.7270/Q2S46WTX |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(Homo sapiens (Human)) | BDBM50580225

(CHEMBL5084053)Show SMILES C[C@@H](Cn1c(=O)[nH]\c(=N/c2ccc(Oc3ccnc(F)c3)cc2)n(Cc2ccc(Cl)cc2)c1=O)C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at P2X3 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128384
BindingDB Entry DOI: 10.7270/Q2S46WTX |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(Homo sapiens (Human)) | BDBM50580221
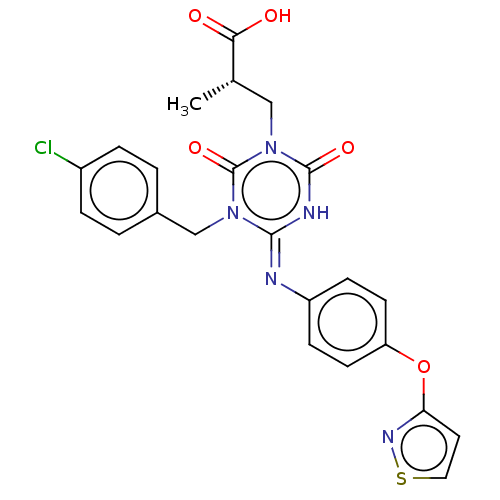
(CHEMBL5073093)Show SMILES C[C@@H](Cn1c(=O)[nH]\c(=N/c2ccc(Oc3ccsn3)cc2)n(Cc2ccc(Cl)cc2)c1=O)C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at P2X3 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128384
BindingDB Entry DOI: 10.7270/Q2S46WTX |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(Homo sapiens (Human)) | BDBM50580224
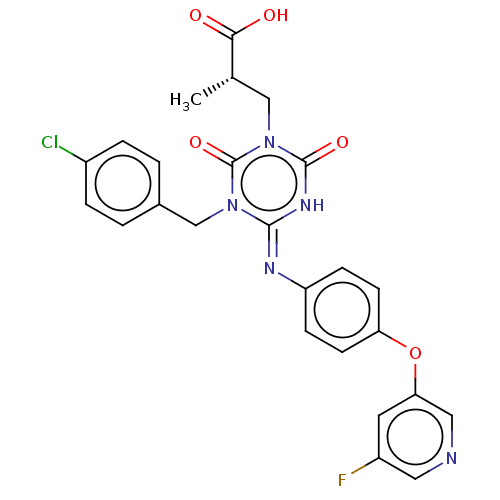
(CHEMBL5090841)Show SMILES C[C@@H](Cn1c(=O)[nH]\c(=N/c2ccc(Oc3cncc(F)c3)cc2)n(Cc2ccc(Cl)cc2)c1=O)C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at P2X3 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128384
BindingDB Entry DOI: 10.7270/Q2S46WTX |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(Homo sapiens (Human)) | BDBM265772

(US9718790, I-0616)Show SMILES CC(C)Oc1ccc(cc1Cl)\N=c1/[nH]c(=O)n(C[C@@H](O)CO)c(=O)n1Cc1ccc(Cl)cc1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at P2X3 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128384
BindingDB Entry DOI: 10.7270/Q2S46WTX |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(Homo sapiens (Human)) | BDBM271685

(US10065941, Compound R-019 | US9732060, Compound R...)Show SMILES C[C@@H](Cn1c(=O)[nH]\c(=N/c2ccc(Oc3cnccn3)cc2)n(Cc2ccc(Cl)cc2)c1=O)C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at P2X3 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128384
BindingDB Entry DOI: 10.7270/Q2S46WTX |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50457587
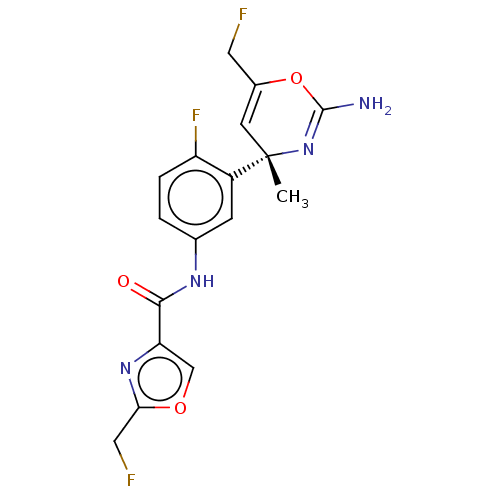
(CHEMBL4207430)Show SMILES C[C@]1(C=C(CF)OC(N)=N1)c1cc(NC(=O)c2coc(CF)n2)ccc1F |r,c:8,t:2| Show InChI InChI=1S/C17H15F3N4O3/c1-17(5-10(6-18)27-16(21)24-17)11-4-9(2-3-12(11)20)22-15(25)13-8-26-14(7-19)23-13/h2-5,8H,6-7H2,1H3,(H2,21,24)(H,22,25)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human SH-SY5Y cells transfected with human wild type beta-APP assessed as reduction in amyloid beta (1 to 40) levels after 24 ... |
J Med Chem 61: 5122-5137 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00002
BindingDB Entry DOI: 10.7270/Q27W6FSQ |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM210893

(US9273043, A122(e))Show SMILES C[C@@H]1CN(CCN1C(=O)Nc1nc2ccccc2s1)c1ncc(cc1F)[C@@H](O)CO |r| Show InChI InChI=1S/C20H22FN5O3S/c1-12-10-25(18-14(21)8-13(9-22-18)16(28)11-27)6-7-26(12)20(29)24-19-23-15-4-2-3-5-17(15)30-19/h2-5,8-9,12,16,27-28H,6-7,10-11H2,1H3,(H,23,24,29)/t12-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Purdue Pharma L.P.; Shionogi & Co., Ltd.
US Patent
| Assay Description
pH dependent Ca2+ responses in TRPV1/CHO cells cultured in a 96-well plate were determined (see, e.g., FIG. 2 of U.S. Patent Application Publication ... |
US Patent US9273043 (2016)
BindingDB Entry DOI: 10.7270/Q2D7998G |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50484393
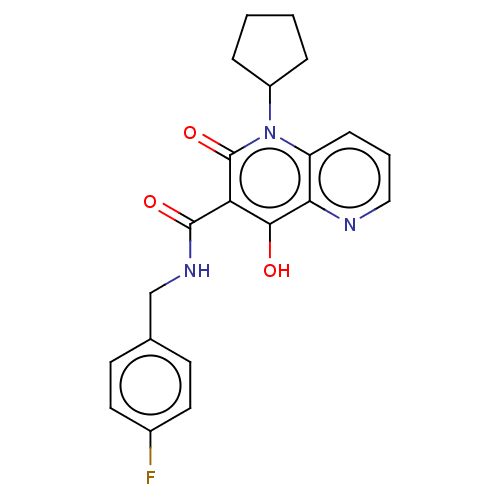
(CHEMBL1917874)Show SMILES Oc1c(C(=O)NCc2ccc(F)cc2)c(=O)n(C2CCCC2)c2cccnc12 Show InChI InChI=1S/C21H20FN3O3/c22-14-9-7-13(8-10-14)12-24-20(27)17-19(26)18-16(6-3-11-23-18)25(21(17)28)15-4-1-2-5-15/h3,6-11,15,26H,1-2,4-5,12H2,(H,24,27) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer by biochemical assay |
Bioorg Med Chem Lett 21: 6461-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.082
BindingDB Entry DOI: 10.7270/Q22Z18CH |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50484392
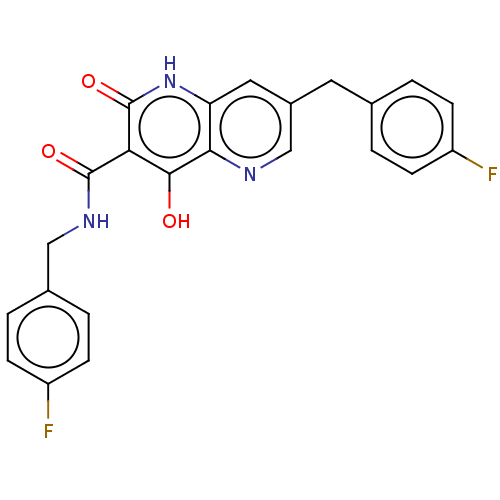
(CHEMBL1917876)Show SMILES Oc1c(C(=O)NCc2ccc(F)cc2)c(=O)[nH]c2cc(Cc3ccc(F)cc3)cnc12 Show InChI InChI=1S/C23H17F2N3O3/c24-16-5-1-13(2-6-16)9-15-10-18-20(26-12-15)21(29)19(23(31)28-18)22(30)27-11-14-3-7-17(25)8-4-14/h1-8,10,12H,9,11H2,(H,27,30)(H2,28,29,31) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer by biochemical assay |
Bioorg Med Chem Lett 21: 6461-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.082
BindingDB Entry DOI: 10.7270/Q22Z18CH |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50484383
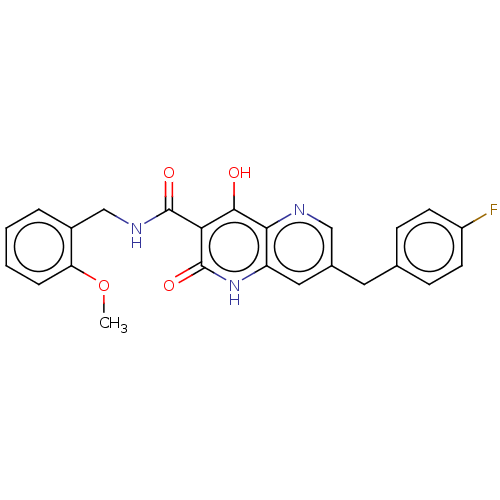
(CHEMBL1914553)Show SMILES COc1ccccc1CNC(=O)c1c(O)c2ncc(Cc3ccc(F)cc3)cc2[nH]c1=O Show InChI InChI=1S/C24H20FN3O4/c1-32-19-5-3-2-4-16(19)13-27-23(30)20-22(29)21-18(28-24(20)31)11-15(12-26-21)10-14-6-8-17(25)9-7-14/h2-9,11-12H,10,13H2,1H3,(H,27,30)(H2,28,29,31) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer by biochemical assay |
Bioorg Med Chem Lett 21: 6461-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.082
BindingDB Entry DOI: 10.7270/Q22Z18CH |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(RAT) | BDBM50563052

(CHEMBL4748113)Show SMILES CC(C)Oc1ccc(Nc2nc(=O)n(CCC(O)=O)c(=O)n2Cc2ccc(C)cc2)cc1Cl | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at rat P2X3 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128384
BindingDB Entry DOI: 10.7270/Q2S46WTX |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(Homo sapiens (Human)) | BDBM50580220
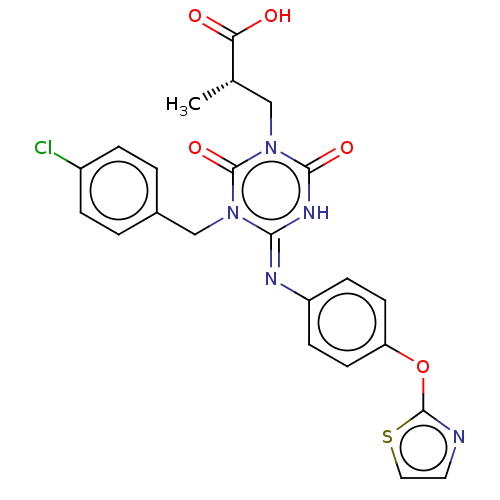
(CHEMBL5086991)Show SMILES C[C@@H](Cn1c(=O)[nH]\c(=N/c2ccc(Oc3nccs3)cc2)n(Cc2ccc(Cl)cc2)c1=O)C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at P2X3 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128384
BindingDB Entry DOI: 10.7270/Q2S46WTX |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50457599
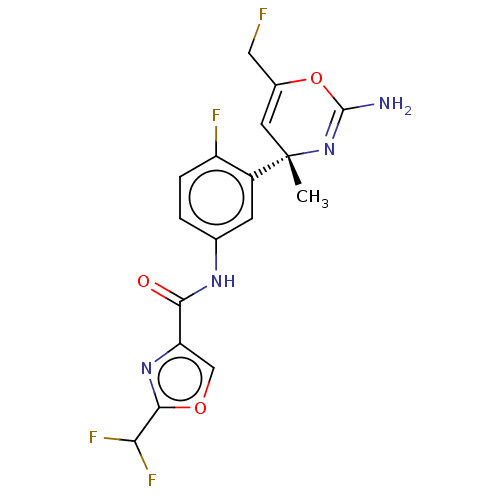
(CHEMBL4212335)Show SMILES C[C@]1(C=C(CF)OC(N)=N1)c1cc(NC(=O)c2coc(n2)C(F)F)ccc1F |r,c:8,t:2| Show InChI InChI=1S/C17H14F4N4O3/c1-17(5-9(6-18)28-16(22)25-17)10-4-8(2-3-11(10)19)23-14(26)12-7-27-15(24-12)13(20)21/h2-5,7,13H,6H2,1H3,(H2,22,25)(H,23,26)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human SH-SY5Y cells transfected with human wild type beta-APP assessed as reduction in amyloid beta (1 to 40) levels after 24 ... |
J Med Chem 61: 5122-5137 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00002
BindingDB Entry DOI: 10.7270/Q27W6FSQ |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50457595
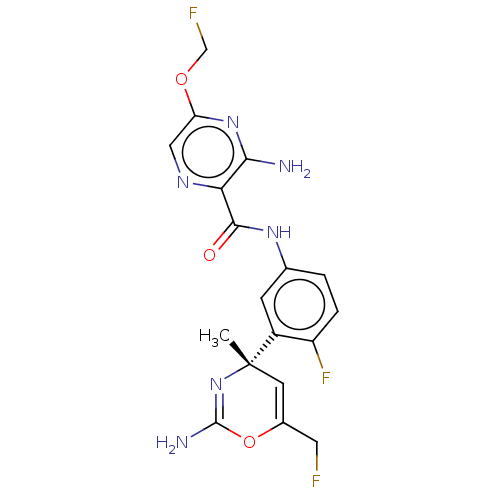
(CHEMBL4211790)Show SMILES C[C@]1(C=C(CF)OC(N)=N1)c1cc(NC(=O)c2ncc(OCF)nc2N)ccc1F |r,c:8,t:2| Show InChI InChI=1S/C18H17F3N6O3/c1-18(5-10(6-19)30-17(23)27-18)11-4-9(2-3-12(11)21)25-16(28)14-15(22)26-13(7-24-14)29-8-20/h2-5,7H,6,8H2,1H3,(H2,22,26)(H2,23,27)(H,25,28)/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human SH-SY5Y cells transfected with human wild type beta-APP assessed as reduction in amyloid beta (1 to 40) levels after 24 ... |
J Med Chem 61: 5122-5137 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00002
BindingDB Entry DOI: 10.7270/Q27W6FSQ |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(Homo sapiens (Human)) | BDBM183231
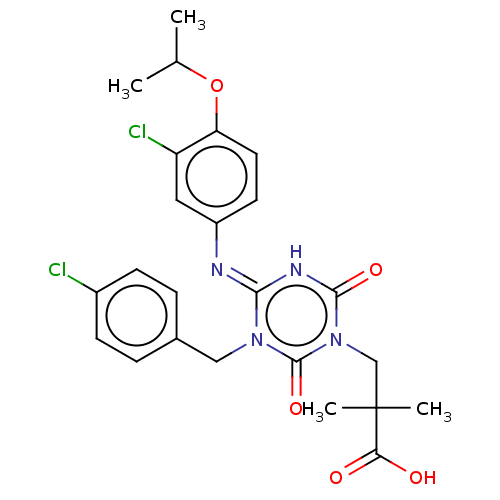
(US9150546, I-401 | US9688643, I-401 | US9718790, I...)Show SMILES CC(C)Oc1ccc(cc1Cl)\N=c1/[nH]c(=O)n(CC(C)(C)C(O)=O)c(=O)n1Cc1ccc(Cl)cc1 Show InChI InChI=1S/C24H26Cl2N4O5/c1-14(2)35-19-10-9-17(11-18(19)26)27-21-28-22(33)30(13-24(3,4)20(31)32)23(34)29(21)12-15-5-7-16(25)8-6-15/h5-11,14H,12-13H2,1-4H3,(H,31,32)(H,27,28,33) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at P2X3 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128384
BindingDB Entry DOI: 10.7270/Q2S46WTX |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50432632

(CHEMBL2347211)Show SMILES C[C@]1(CCSC(N)=N1)c1cc(NC(=O)c2ccc(Cl)cn2)ccc1F |r,c:6| Show InChI InChI=1S/C17H16ClFN4OS/c1-17(6-7-25-16(20)23-17)12-8-11(3-4-13(12)19)22-15(24)14-5-2-10(18)9-21-14/h2-5,8-9H,6-7H2,1H3,(H2,20,23)(H,22,24)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) |
J Med Chem 61: 5122-5137 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00002
BindingDB Entry DOI: 10.7270/Q27W6FSQ |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(Homo sapiens (Human)) | BDBM183205
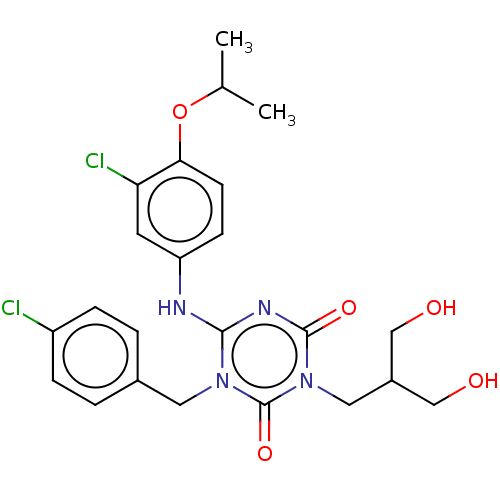
(US9150546, I-369 | US9688643, I-369 | US9718790, I...)Show SMILES CC(C)Oc1ccc(Nc2nc(=O)n(CC(CO)CO)c(=O)n2Cc2ccc(Cl)cc2)cc1Cl Show InChI InChI=1S/C23H26Cl2N4O5/c1-14(2)34-20-8-7-18(9-19(20)25)26-21-27-22(32)29(11-16(12-30)13-31)23(33)28(21)10-15-3-5-17(24)6-4-15/h3-9,14,16,30-31H,10-13H2,1-2H3,(H,26,27,32) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at P2X3 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128384
BindingDB Entry DOI: 10.7270/Q2S46WTX |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(Homo sapiens (Human)) | BDBM183198
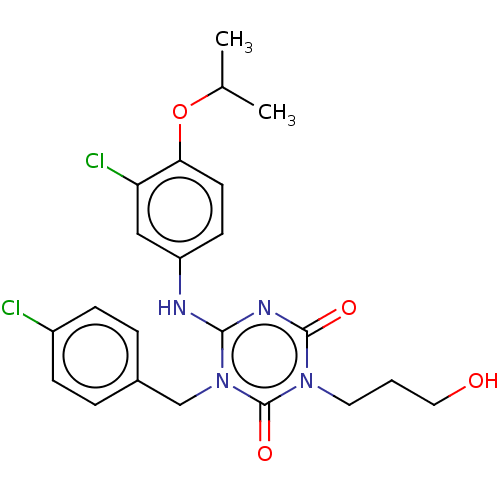
(US9150546, I-362 | US9688643, I-362 | US9718790, I...)Show SMILES CC(C)Oc1ccc(Nc2nc(=O)n(CCCO)c(=O)n2Cc2ccc(Cl)cc2)cc1Cl Show InChI InChI=1S/C22H24Cl2N4O4/c1-14(2)32-19-9-8-17(12-18(19)24)25-20-26-21(30)27(10-3-11-29)22(31)28(20)13-15-4-6-16(23)7-5-15/h4-9,12,14,29H,3,10-11,13H2,1-2H3,(H,25,26,30) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at P2X3 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128384
BindingDB Entry DOI: 10.7270/Q2S46WTX |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50484394
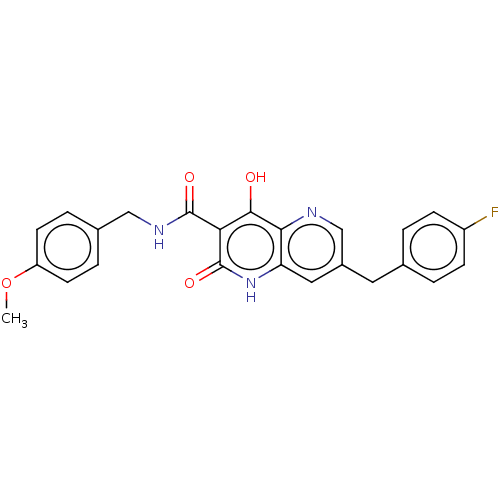
(CHEMBL1914551)Show SMILES COc1ccc(CNC(=O)c2c(O)c3ncc(Cc4ccc(F)cc4)cc3[nH]c2=O)cc1 Show InChI InChI=1S/C24H20FN3O4/c1-32-18-8-4-15(5-9-18)12-27-23(30)20-22(29)21-19(28-24(20)31)11-16(13-26-21)10-14-2-6-17(25)7-3-14/h2-9,11,13H,10,12H2,1H3,(H,27,30)(H2,28,29,31) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer by biochemical assay |
Bioorg Med Chem Lett 21: 6461-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.082
BindingDB Entry DOI: 10.7270/Q22Z18CH |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(Homo sapiens (Human)) | BDBM183344

(US9150546, I-518 | US9688643, I-518 | US9718790, I...)Show SMILES CC(C)Oc1ccc(cc1Cl)\N=c1/[nH]c(=O)n(C[C@H](C)C(O)=O)c(=O)n1Cc1ccc(C)cc1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at P2X3 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128384
BindingDB Entry DOI: 10.7270/Q2S46WTX |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(RAT) | BDBM50580221

(CHEMBL5073093)Show SMILES C[C@@H](Cn1c(=O)[nH]\c(=N/c2ccc(Oc3ccsn3)cc2)n(Cc2ccc(Cl)cc2)c1=O)C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at rat P2X3 receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128384
BindingDB Entry DOI: 10.7270/Q2S46WTX |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(Homo sapiens (Human)) | BDBM271805

(US10065941, Compound R-120 | US9732060, Compound R...)Show SMILES C[C@@H](Cn1c(=O)[nH]\c(=N/c2ccc(Oc3cccnn3)cc2)n(Cc2ccc(Cl)cc2)c1=O)C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at P2X3 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128384
BindingDB Entry DOI: 10.7270/Q2S46WTX |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 3
(Homo sapiens (Human)) | BDBM183287
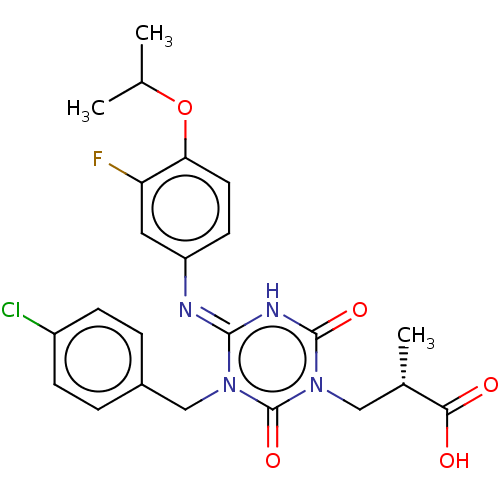
(US9150546, I-456 | US9688643, I-456 | US9718790, I...)Show SMILES CC(C)Oc1ccc(cc1F)\N=c1/[nH]c(=O)n(C[C@H](C)C(O)=O)c(=O)n1Cc1ccc(Cl)cc1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at P2X3 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128384
BindingDB Entry DOI: 10.7270/Q2S46WTX |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50457596
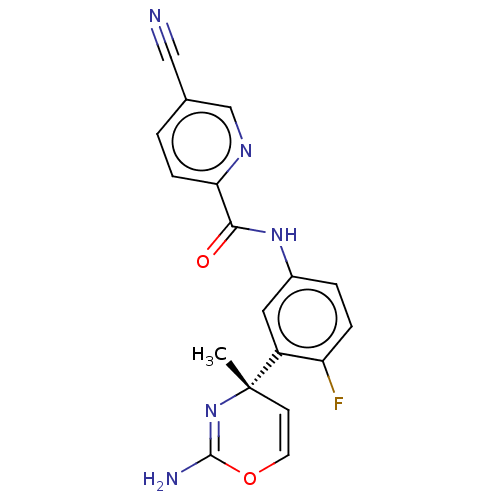
(CHEMBL4205656)Show SMILES C[C@]1(C=COC(N)=N1)c1cc(NC(=O)c2ccc(cn2)C#N)ccc1F |r,c:2,6| Show InChI InChI=1S/C18H14FN5O2/c1-18(6-7-26-17(21)24-18)13-8-12(3-4-14(13)19)23-16(25)15-5-2-11(9-20)10-22-15/h2-8,10H,1H3,(H2,21,24)(H,23,25)/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human SH-SY5Y cells transfected with human wild type beta-APP assessed as reduction in amyloid beta (1 to 40) levels after 24 ... |
J Med Chem 61: 5122-5137 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00002
BindingDB Entry DOI: 10.7270/Q27W6FSQ |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM141534

(US8921373, ADK)Show SMILES C[C@@H]1CC(F)(CCN1C(=O)Nc1nc2cc(Cl)ccc2s1)c1ncc(C[C@H](O)CO)cc1F |r| Show InChI InChI=1S/C22H23ClF2N4O3S/c1-12-9-22(25,19-16(24)7-13(10-26-19)6-15(31)11-30)4-5-29(12)21(32)28-20-27-17-8-14(23)2-3-18(17)33-20/h2-3,7-8,10,12,15,30-31H,4-6,9,11H2,1H3,(H,27,28,32)/t12-,15+,22?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Shionogi & Co., Ltd.
US Patent
| Assay Description
Two days prior to performing this assay, cells are seeded in poly-D-lysine-coated 96-well clear-bottom black plates (50,000 cells/well) in growth med... |
US Patent US8921373 (2014)
BindingDB Entry DOI: 10.7270/Q28051BW |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50484390
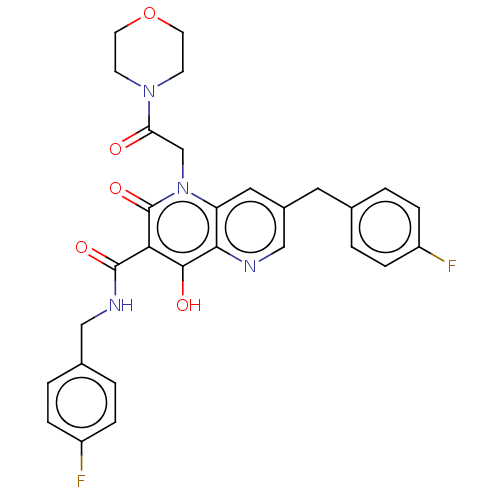
(CHEMBL1914564)Show SMILES Oc1c(C(=O)NCc2ccc(F)cc2)c(=O)n(CC(=O)N2CCOCC2)c2cc(Cc3ccc(F)cc3)cnc12 Show InChI InChI=1S/C29H26F2N4O5/c30-21-5-1-18(2-6-21)13-20-14-23-26(32-16-20)27(37)25(28(38)33-15-19-3-7-22(31)8-4-19)29(39)35(23)17-24(36)34-9-11-40-12-10-34/h1-8,14,16,37H,9-13,15,17H2,(H,33,38) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer by biochemical assay |
Bioorg Med Chem Lett 21: 6461-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.082
BindingDB Entry DOI: 10.7270/Q22Z18CH |
More data for this
Ligand-Target Pair | |
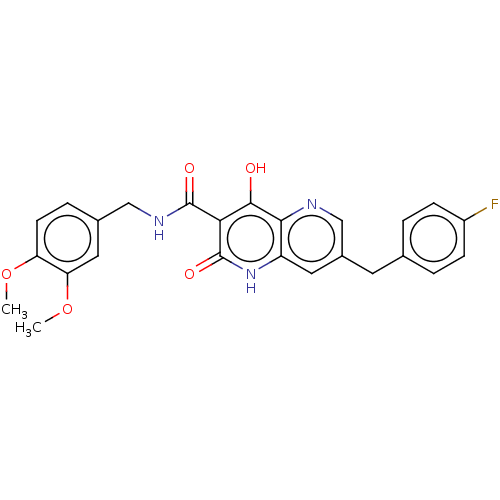
Integrase
(Human immunodeficiency virus 1) | BDBM50484384
(CHEMBL1914554)Show SMILES COc1ccc(CNC(=O)c2c(O)c3ncc(Cc4ccc(F)cc4)cc3[nH]c2=O)cc1OC Show InChI InChI=1S/C25H22FN3O5/c1-33-19-8-5-15(11-20(19)34-2)12-28-24(31)21-23(30)22-18(29-25(21)32)10-16(13-27-22)9-14-3-6-17(26)7-4-14/h3-8,10-11,13H,9,12H2,1-2H3,(H,28,31)(H2,29,30,32) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase strand transfer by biochemical assay |
Bioorg Med Chem Lett 21: 6461-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.082
BindingDB Entry DOI: 10.7270/Q22Z18CH |
More data for this
Ligand-Target Pair | |
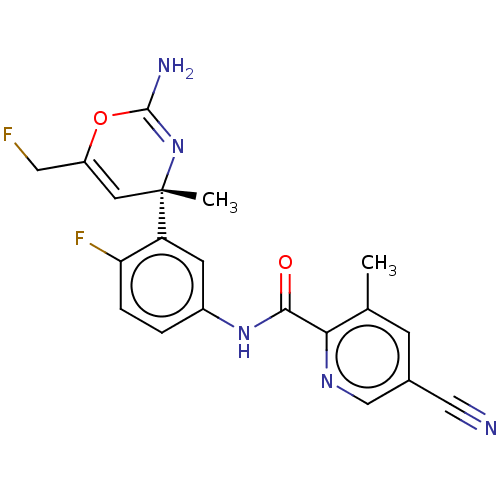
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50457592
(CHEMBL4204742)Show SMILES Cc1cc(cnc1C(=O)Nc1ccc(F)c(c1)[C@]1(C)C=C(CF)OC(N)=N1)C#N |r,c:27,t:21| Show InChI InChI=1S/C20H17F2N5O2/c1-11-5-12(9-23)10-25-17(11)18(28)26-13-3-4-16(22)15(6-13)20(2)7-14(8-21)29-19(24)27-20/h3-7,10H,8H2,1-2H3,(H2,24,27)(H,26,28)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human SH-SY5Y cells transfected with human wild type beta-APP assessed as reduction in amyloid beta (1 to 40) levels after 24 ... |
J Med Chem 61: 5122-5137 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00002
BindingDB Entry DOI: 10.7270/Q27W6FSQ |
More data for this
Ligand-Target Pair | |
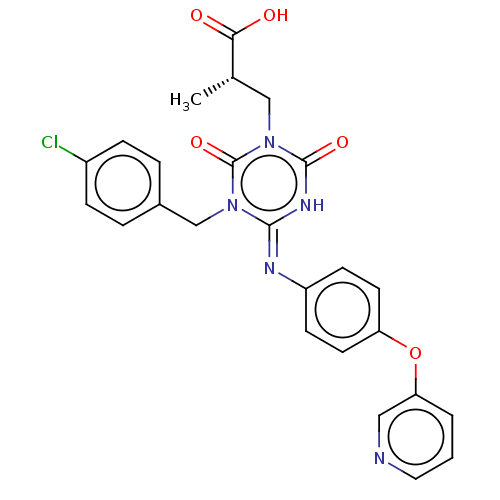
P2X purinoceptor 3
(Homo sapiens (Human)) | BDBM271892
(US10065941, Compound R-207 | US9732060, Compound R...)Show SMILES C[C@@H](Cn1c(=O)[nH]\c(=N/c2ccc(Oc3cccnc3)cc2)n(Cc2ccc(Cl)cc2)c1=O)C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at P2X3 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128384
BindingDB Entry DOI: 10.7270/Q2S46WTX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data