 Found 173 hits with Last Name = 'kurup' and Initial = 's'
Found 173 hits with Last Name = 'kurup' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM2483

((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...)Show SMILES FC(F)(F)[C@]1(OC(=O)Nc2ccc(Cl)cc12)C#CC1CC1 |r| Show InChI InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50137113
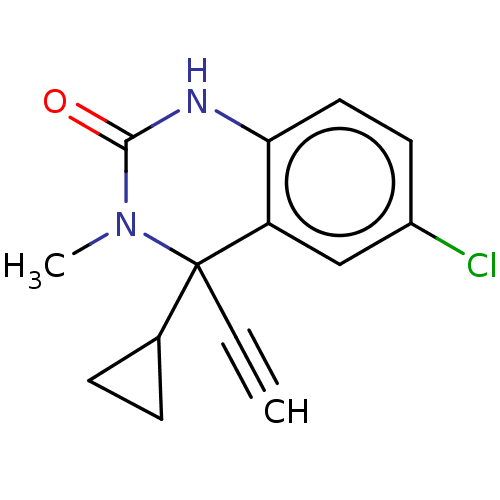
(CHEMBL3753096)Show InChI InChI=1S/C14H13ClN2O/c1-3-14(9-4-5-9)11-8-10(15)6-7-12(11)16-13(18)17(14)2/h1,6-9H,4-5H2,2H3,(H,16,18) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50137112
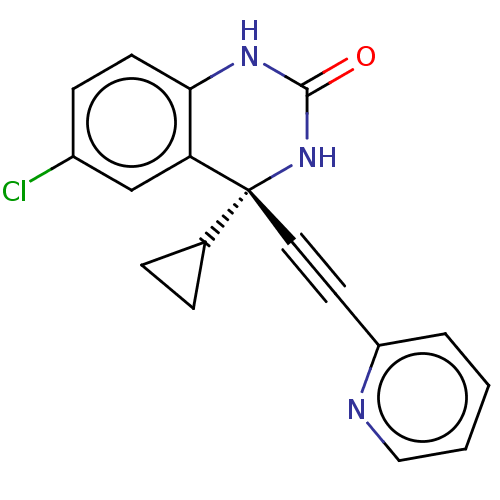
(CHEMBL309896)Show SMILES Clc1ccc2NC(=O)N[C@](C#Cc3ccccn3)(C3CC3)c2c1 Show InChI InChI=1S/C18H14ClN3O/c19-13-6-7-16-15(11-13)18(12-4-5-12,22-17(23)21-16)9-8-14-3-1-2-10-20-14/h1-3,6-7,10-12H,4-5H2,(H2,21,22,23)/t18-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM3107
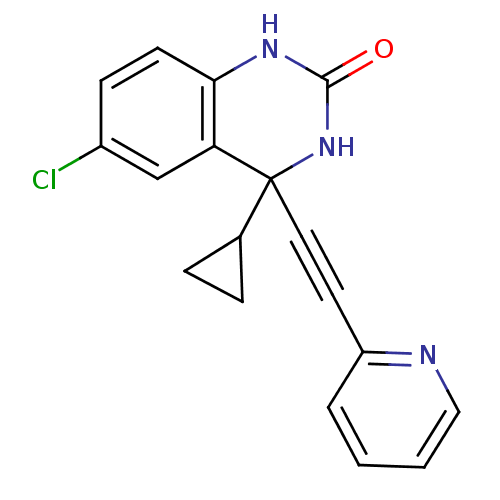
(3,4-Dihydroquinazolinon 4a | 6-Chloro-4-cyclopyrop...)Show InChI InChI=1S/C18H14ClN3O/c19-13-6-7-16-15(11-13)18(12-4-5-12,22-17(23)21-16)9-8-14-3-1-2-10-20-14/h1-3,6-7,10-12H,4-5H2,(H2,21,22,23) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM1517
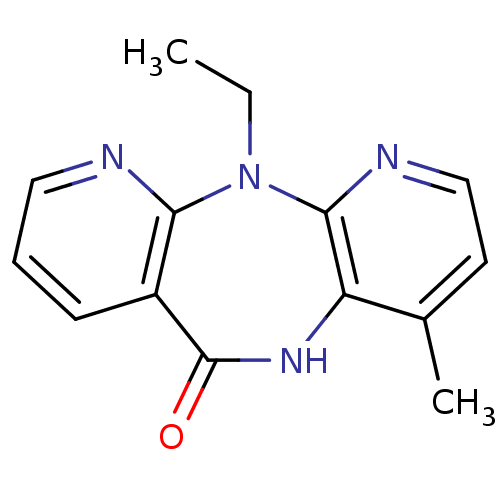
(2-ethyl-7-methyl-2,4,9,15-tetraazatricyclo[9.4.0.0...)Show InChI InChI=1S/C14H14N4O/c1-3-18-12-10(5-4-7-15-12)14(19)17-11-9(2)6-8-16-13(11)18/h4-8H,3H2,1-2H3,(H,17,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase derived from HIV patient plasma by LC/MS/MS analysis |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM2483

((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...)Show SMILES FC(F)(F)[C@]1(OC(=O)Nc2ccc(Cl)cc12)C#CC1CC1 |r| Show InChI InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50524092
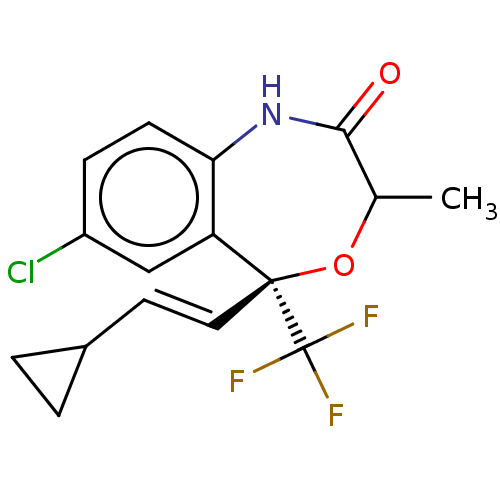
(CHEMBL4436313)Show SMILES CC1O[C@@](\C=C\C2CC2)(c2cc(Cl)ccc2NC1=O)C(F)(F)F |r| Show InChI InChI=1S/C16H15ClF3NO2/c1-9-14(22)21-13-5-4-11(17)8-12(13)15(23-9,16(18,19)20)7-6-10-2-3-10/h4-10H,2-3H2,1H3,(H,21,22)/b7-6+/t9?,15-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50524088
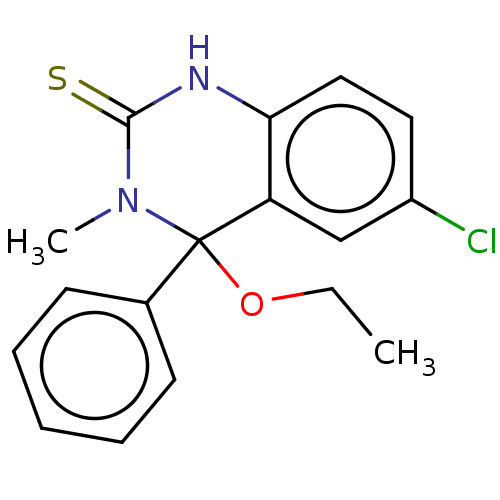
(CHEMBL4483481)Show InChI InChI=1S/C17H17ClN2OS/c1-3-21-17(12-7-5-4-6-8-12)14-11-13(18)9-10-15(14)19-16(22)20(17)2/h4-11H,3H2,1-2H3,(H,19,22) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50524089
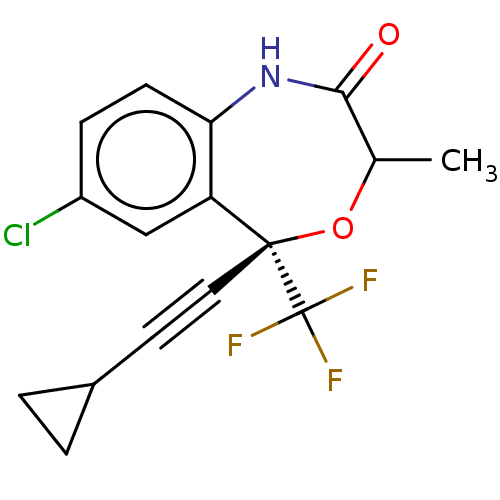
(CHEMBL4529500)Show SMILES CC1O[C@@](C#CC2CC2)(c2cc(Cl)ccc2NC1=O)C(F)(F)F |r| Show InChI InChI=1S/C16H13ClF3NO2/c1-9-14(22)21-13-5-4-11(17)8-12(13)15(23-9,16(18,19)20)7-6-10-2-3-10/h4-5,8-10H,2-3H2,1H3,(H,21,22)/t9?,15-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM1434

(11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...)Show InChI InChI=1S/C15H14N4O/c1-9-6-8-17-14-12(9)18-15(20)11-3-2-7-16-13(11)19(14)10-4-5-10/h2-3,6-8,10H,4-5H2,1H3,(H,18,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase derived from HIV patient plasma by LC/MS/MS analysis |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50318173
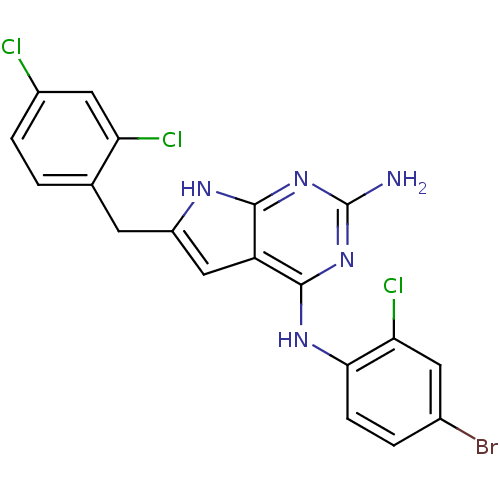
(CHEMBL1095762 | N4-(4-Bromo-2-chlorophenyl)-6-(2,4...)Show SMILES Nc1nc(Nc2ccc(Br)cc2Cl)c2cc(Cc3ccc(Cl)cc3Cl)[nH]c2n1 Show InChI InChI=1S/C19H13BrCl3N5/c20-10-2-4-16(15(23)6-10)26-18-13-8-12(25-17(13)27-19(24)28-18)5-9-1-3-11(21)7-14(9)22/h1-4,6-8H,5H2,(H4,24,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 phosphorylation in human U251 cells after 10 mins by FLISA |
Bioorg Med Chem 18: 3575-87 (2010)
Article DOI: 10.1016/j.bmc.2010.03.052
BindingDB Entry DOI: 10.7270/Q2V69JRB |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50360178

(CHEMBL1929556)Show SMILES COc1ccc(OC)c(Cc2cc3c(Nc4ccc(Cl)cc4F)nc(N)nc3[nH]2)c1 Show InChI InChI=1S/C21H19ClFN5O2/c1-29-14-4-6-18(30-2)11(8-14)7-13-10-15-19(25-13)27-21(24)28-20(15)26-17-5-3-12(22)9-16(17)23/h3-6,8-10H,7H2,1-2H3,(H4,24,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 tyrosine kinase activity in VEGF-stimulated human U251 cells after 60 mins by ELISA |
Bioorg Med Chem 20: 910-4 (2012)
Article DOI: 10.1016/j.bmc.2011.11.058
BindingDB Entry DOI: 10.7270/Q27W6CMN |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM4811
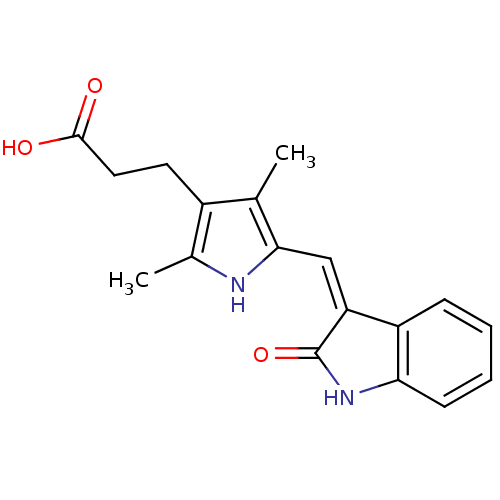
((Z)-3-[2,4-dimethyl-5-(2-oxo-1,2-dihydro-indol-3-y...)Show SMILES Cc1[nH]c(\C=C2/C(=O)Nc3ccccc23)c(C)c1CCC(O)=O Show InChI InChI=1S/C18H18N2O3/c1-10-12(7-8-17(21)22)11(2)19-16(10)9-14-13-5-3-4-6-15(13)20-18(14)23/h3-6,9,19H,7-8H2,1-2H3,(H,20,23)(H,21,22)/b14-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of PDGF-stimulated PDGFRbeta phosphorylation expressed in mouse NIH/3T3 cells by chemiluminescence assay |
Bioorg Med Chem 18: 3575-87 (2010)
Article DOI: 10.1016/j.bmc.2010.03.052
BindingDB Entry DOI: 10.7270/Q2V69JRB |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM1518
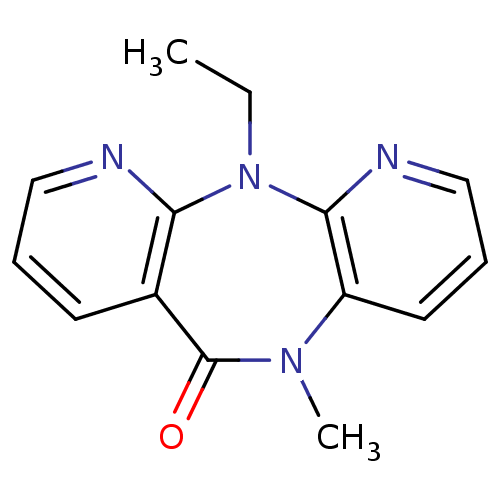
(2-ethyl-9-methyl-2,4,9,15-tetraazatricyclo[9.4.0.0...)Show InChI InChI=1S/C14H14N4O/c1-3-18-12-10(6-4-8-15-12)14(19)17(2)11-7-5-9-16-13(11)18/h4-9H,3H2,1-2H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase derived from HIV patient plasma by LC/MS/MS analysis |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3032

(CHEMBL1204168 | CHEMBL29197 | N-(3-bromophenyl)-6,...)Show InChI InChI=1S/C16H14BrN3O2/c1-21-14-7-12-13(8-15(14)22-2)18-9-19-16(12)20-11-5-3-4-10(17)6-11/h3-9H,1-2H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR phosphorylation in human A431 cells after 10 mins by FLISA |
Bioorg Med Chem 18: 3575-87 (2010)
Article DOI: 10.1016/j.bmc.2010.03.052
BindingDB Entry DOI: 10.7270/Q2V69JRB |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50318174
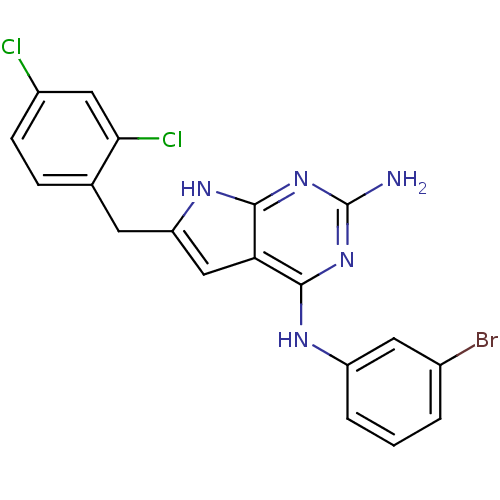
(CHEMBL1095761 | N4-(3-bromophenyl)-6-(2,4-dichloro...)Show SMILES Nc1nc(Nc2cccc(Br)c2)c2cc(Cc3ccc(Cl)cc3Cl)[nH]c2n1 Show InChI InChI=1S/C19H14BrCl2N5/c20-11-2-1-3-13(7-11)24-17-15-9-14(25-18(15)27-19(23)26-17)6-10-4-5-12(21)8-16(10)22/h1-5,7-9H,6H2,(H4,23,24,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR phosphorylation in human A431 cells after 10 mins by FLISA |
Bioorg Med Chem 18: 3575-87 (2010)
Article DOI: 10.1016/j.bmc.2010.03.052
BindingDB Entry DOI: 10.7270/Q2V69JRB |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50318182
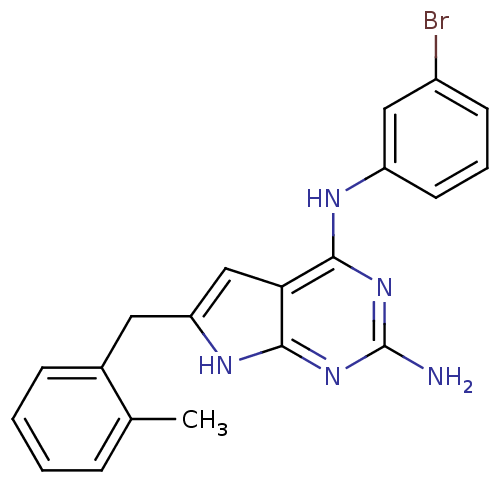
(CHEMBL1095464 | N4-(3-bromophenyl)-6-(2-methylbenz...)Show SMILES Cc1ccccc1Cc1cc2c(Nc3cccc(Br)c3)nc(N)nc2[nH]1 Show InChI InChI=1S/C20H18BrN5/c1-12-5-2-3-6-13(12)9-16-11-17-18(25-20(22)26-19(17)24-16)23-15-8-4-7-14(21)10-15/h2-8,10-11H,9H2,1H3,(H4,22,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 phosphorylation in human U251 cells after 10 mins by FLISA |
Bioorg Med Chem 18: 3575-87 (2010)
Article DOI: 10.1016/j.bmc.2010.03.052
BindingDB Entry DOI: 10.7270/Q2V69JRB |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3032

(CHEMBL1204168 | CHEMBL29197 | N-(3-bromophenyl)-6,...)Show InChI InChI=1S/C16H14BrN3O2/c1-21-14-7-12-13(8-15(14)22-2)18-9-19-16(12)20-11-5-3-4-10(17)6-11/h3-9H,1-2H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR tyrosine kinase activity in EGF-stimulated human A431 cells after 60 mins by ELISA |
Bioorg Med Chem 20: 910-4 (2012)
Article DOI: 10.1016/j.bmc.2011.11.058
BindingDB Entry DOI: 10.7270/Q27W6CMN |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50318183
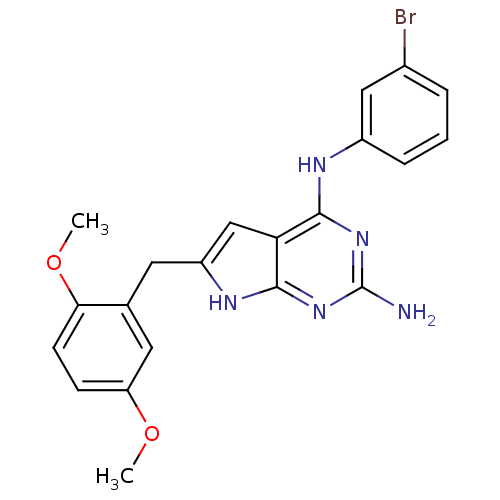
(CHEMBL1095465 | N4-(3-bromophenyl)-6-(2,5-dimethox...)Show SMILES COc1ccc(OC)c(Cc2cc3c(Nc4cccc(Br)c4)nc(N)nc3[nH]2)c1 Show InChI InChI=1S/C21H20BrN5O2/c1-28-16-6-7-18(29-2)12(9-16)8-15-11-17-19(26-21(23)27-20(17)25-15)24-14-5-3-4-13(22)10-14/h3-7,9-11H,8H2,1-2H3,(H4,23,24,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR tyrosine kinase activity in EGF-stimulated human A431 cells after 60 mins by ELISA |
Bioorg Med Chem 20: 910-4 (2012)
Article DOI: 10.1016/j.bmc.2011.11.058
BindingDB Entry DOI: 10.7270/Q27W6CMN |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50318182

(CHEMBL1095464 | N4-(3-bromophenyl)-6-(2-methylbenz...)Show SMILES Cc1ccccc1Cc1cc2c(Nc3cccc(Br)c3)nc(N)nc2[nH]1 Show InChI InChI=1S/C20H18BrN5/c1-12-5-2-3-6-13(12)9-16-11-17-18(25-20(22)26-19(17)24-16)23-15-8-4-7-14(21)10-15/h2-8,10-11H,9H2,1H3,(H4,22,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 tyrosine kinase activity in VEGF-stimulated human U251 cells after 60 mins by ELISA |
Bioorg Med Chem 20: 910-4 (2012)
Article DOI: 10.1016/j.bmc.2011.11.058
BindingDB Entry DOI: 10.7270/Q27W6CMN |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM1944

(BHAP deriv. | CHEMBL593 | DELAVIRDINE MESYLATE | D...)Show SMILES CC(C)Nc1cccnc1N1CCN(CC1)C(=O)c1cc2cc(NS(C)(=O)=O)ccc2[nH]1 Show InChI InChI=1S/C22H28N6O3S/c1-15(2)24-19-5-4-8-23-21(19)27-9-11-28(12-10-27)22(29)20-14-16-13-17(26-32(3,30)31)6-7-18(16)25-20/h4-8,13-15,24-26H,9-12H2,1-3H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of wild type recombinant HIV1 reverse transcriptase using poly(rA)-oligo(dT) as template/primer |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50318176

(CHEMBL1095131 | N4-(3-Fluorophenyl)-6-(2,4-dichlor...)Show SMILES Nc1nc(Nc2cccc(F)c2)c2cc(Cc3ccc(Cl)cc3Cl)[nH]c2n1 Show InChI InChI=1S/C19H14Cl2FN5/c20-11-5-4-10(16(21)7-11)6-14-9-15-17(26-19(23)27-18(15)25-14)24-13-3-1-2-12(22)8-13/h1-5,7-9H,6H2,(H4,23,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 phosphorylation in human U251 cells after 10 mins by FLISA |
Bioorg Med Chem 18: 3575-87 (2010)
Article DOI: 10.1016/j.bmc.2010.03.052
BindingDB Entry DOI: 10.7270/Q2V69JRB |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM1633

(2-ethyl-10-methyl-2,4,10-triazatricyclo[9.4.0.0^{3...)Show InChI InChI=1S/C15H15N3O/c1-3-18-13-9-5-4-8-12(13)17(2)15(19)11-7-6-10-16-14(11)18/h4-10H,3H2,1-2H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase derived from HIV patient plasma by LC/MS/MS analysis |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50360175
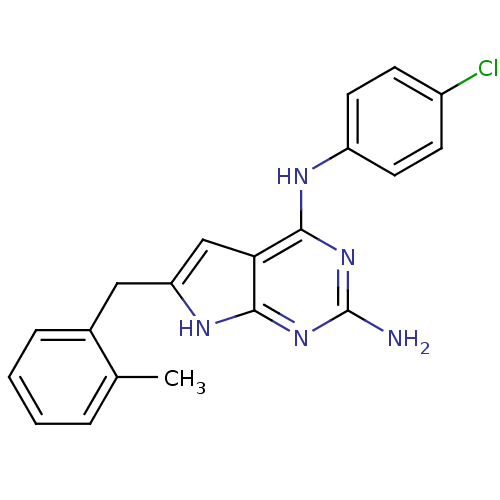
(CHEMBL1929553)Show SMILES Cc1ccccc1Cc1cc2c(Nc3ccc(Cl)cc3)nc(N)nc2[nH]1 Show InChI InChI=1S/C20H18ClN5/c1-12-4-2-3-5-13(12)10-16-11-17-18(25-20(22)26-19(17)24-16)23-15-8-6-14(21)7-9-15/h2-9,11H,10H2,1H3,(H4,22,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 tyrosine kinase activity in VEGF-stimulated human U251 cells after 60 mins by ELISA |
Bioorg Med Chem 20: 910-4 (2012)
Article DOI: 10.1016/j.bmc.2011.11.058
BindingDB Entry DOI: 10.7270/Q27W6CMN |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50318183

(CHEMBL1095465 | N4-(3-bromophenyl)-6-(2,5-dimethox...)Show SMILES COc1ccc(OC)c(Cc2cc3c(Nc4cccc(Br)c4)nc(N)nc3[nH]2)c1 Show InChI InChI=1S/C21H20BrN5O2/c1-28-16-6-7-18(29-2)12(9-16)8-15-11-17-19(26-21(23)27-20(17)25-15)24-14-5-3-4-13(22)10-14/h3-7,9-11H,8H2,1-2H3,(H4,23,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 phosphorylation in human U251 cells after 10 mins by FLISA |
Bioorg Med Chem 18: 3575-87 (2010)
Article DOI: 10.1016/j.bmc.2010.03.052
BindingDB Entry DOI: 10.7270/Q2V69JRB |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50360177
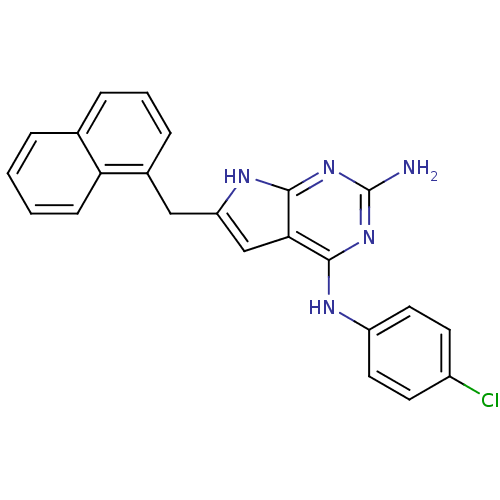
(CHEMBL1929555)Show SMILES Nc1nc(Nc2ccc(Cl)cc2)c2cc(Cc3cccc4ccccc34)[nH]c2n1 Show InChI InChI=1S/C23H18ClN5/c24-16-8-10-17(11-9-16)26-21-20-13-18(27-22(20)29-23(25)28-21)12-15-6-3-5-14-4-1-2-7-19(14)15/h1-11,13H,12H2,(H4,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 tyrosine kinase activity in VEGF-stimulated human U251 cells after 60 mins by ELISA |
Bioorg Med Chem 20: 910-4 (2012)
Article DOI: 10.1016/j.bmc.2011.11.058
BindingDB Entry DOI: 10.7270/Q27W6CMN |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50137120
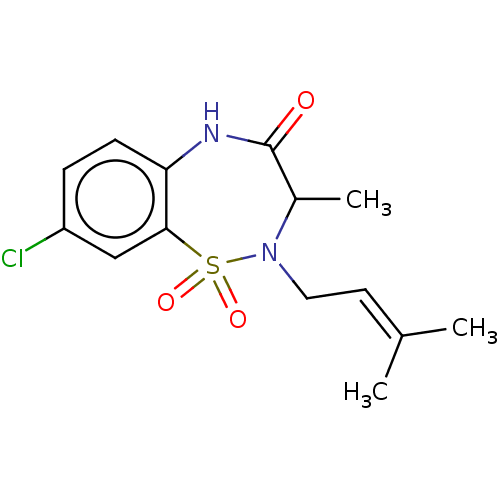
(CHEMBL3753006)Show SMILES [#6]-[#6]-1-[#7](-[#6]\[#6]=[#6](\[#6])-[#6])S(=O)(=O)c2cc(Cl)ccc2-[#7]-[#6]-1=O Show InChI InChI=1S/C14H17ClN2O3S/c1-9(2)6-7-17-10(3)14(18)16-12-5-4-11(15)8-13(12)21(17,19)20/h4-6,8,10H,7H2,1-3H3,(H,16,18) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50137115
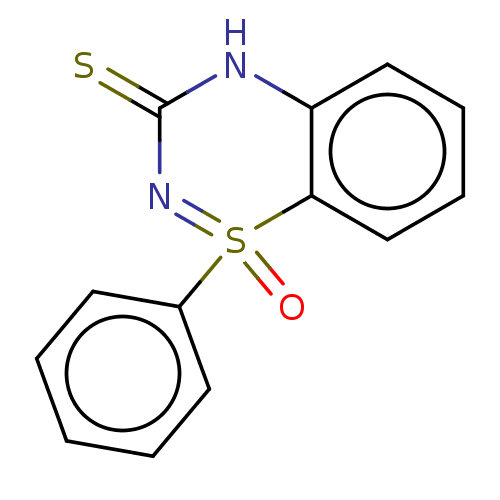
(CHEMBL3754170)Show InChI InChI=1S/C13H10N2OS2/c16-18(10-6-2-1-3-7-10)12-9-5-4-8-11(12)14-13(17)15-18/h1-9H,(H,14,15,16,17) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR phosphorylation in human A431 cells after 10 mins by FLISA |
Bioorg Med Chem 18: 3575-87 (2010)
Article DOI: 10.1016/j.bmc.2010.03.052
BindingDB Entry DOI: 10.7270/Q2V69JRB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR tyrosine kinase activity in EGF-stimulated human A431 cells after 60 mins by ELISA |
Bioorg Med Chem 20: 910-4 (2012)
Article DOI: 10.1016/j.bmc.2011.11.058
BindingDB Entry DOI: 10.7270/Q27W6CMN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50318180

(CHEMBL1095463 | N4-(4-Isopropylphenyl)-6-(2,4-dich...)Show SMILES CC(C)c1ccc(Nc2nc(N)nc3[nH]c(Cc4ccc(Cl)cc4Cl)cc23)cc1 Show InChI InChI=1S/C22H21Cl2N5/c1-12(2)13-4-7-16(8-5-13)26-20-18-11-17(27-21(18)29-22(25)28-20)9-14-3-6-15(23)10-19(14)24/h3-8,10-12H,9H2,1-2H3,(H4,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 phosphorylation in human U251 cells after 10 mins by FLISA |
Bioorg Med Chem 18: 3575-87 (2010)
Article DOI: 10.1016/j.bmc.2010.03.052
BindingDB Entry DOI: 10.7270/Q2V69JRB |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM1437
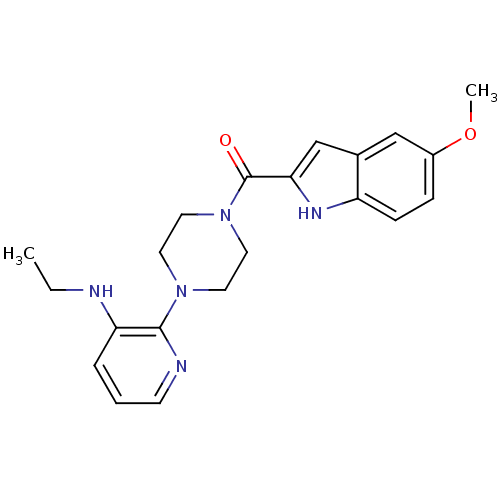
(5-methoxyindole-2-carboxylic acid [N -[3-(aminoeth...)Show SMILES CCNc1cccnc1N1CCN(CC1)C(=O)c1cc2cc(OC)ccc2[nH]1 Show InChI InChI=1S/C21H25N5O2/c1-3-22-18-5-4-8-23-20(18)25-9-11-26(12-10-25)21(27)19-14-15-13-16(28-2)6-7-17(15)24-19/h4-8,13-14,22,24H,3,9-12H2,1-2H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of wild type recombinant HIV1 reverse transcriptase using poly(rA)-oligo(dT) as template/primer |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50318175
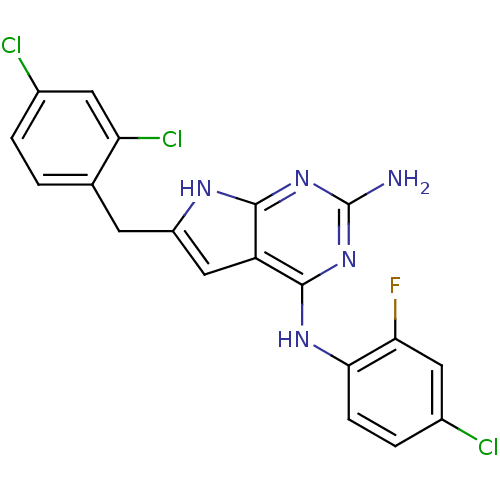
(CHEMBL1095130 | N4-(2-Fluoro-4-chlorophenyl)-6-(2,...)Show SMILES Nc1nc(Nc2ccc(Cl)cc2F)c2cc(Cc3ccc(Cl)cc3Cl)[nH]c2n1 Show InChI InChI=1S/C19H13Cl3FN5/c20-10-2-1-9(14(22)6-10)5-12-8-13-17(25-12)27-19(24)28-18(13)26-16-4-3-11(21)7-15(16)23/h1-4,6-8H,5H2,(H4,24,25,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR phosphorylation in human A431 cells after 10 mins by FLISA |
Bioorg Med Chem 18: 3575-87 (2010)
Article DOI: 10.1016/j.bmc.2010.03.052
BindingDB Entry DOI: 10.7270/Q2V69JRB |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM17747

((3Z)-3-{[4-(dimethylamino)phenyl]methylidene}-2,3-...)Show InChI InChI=1S/C17H16N2O/c1-19(2)13-9-7-12(8-10-13)11-15-14-5-3-4-6-16(14)18-17(15)20/h3-11H,1-2H3,(H,18,20)/b15-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta phosphorylation in human SF539 cells after 10 mins by FLISA |
Bioorg Med Chem 18: 3575-87 (2010)
Article DOI: 10.1016/j.bmc.2010.03.052
BindingDB Entry DOI: 10.7270/Q2V69JRB |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM17747

((3Z)-3-{[4-(dimethylamino)phenyl]methylidene}-2,3-...)Show InChI InChI=1S/C17H16N2O/c1-19(2)13-9-7-12(8-10-13)11-15-14-5-3-4-6-16(14)18-17(15)20/h3-11H,1-2H3,(H,18,20)/b15-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta tyrosine kinase activity in PDGF-BB-stimulated human SF-539 cells after 60 mins by ELISA |
Bioorg Med Chem 20: 910-4 (2012)
Article DOI: 10.1016/j.bmc.2011.11.058
BindingDB Entry DOI: 10.7270/Q27W6CMN |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM4811

((Z)-3-[2,4-dimethyl-5-(2-oxo-1,2-dihydro-indol-3-y...)Show SMILES Cc1[nH]c(\C=C2/C(=O)Nc3ccccc23)c(C)c1CCC(O)=O Show InChI InChI=1S/C18H18N2O3/c1-10-12(7-8-17(21)22)11(2)19-16(10)9-14-13-5-3-4-6-15(13)20-18(14)23/h3-6,9,19H,7-8H2,1-2H3,(H,20,23)(H,21,22)/b14-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 phosphorylation in HUVEC by chemiluminescence assay |
Bioorg Med Chem 18: 3575-87 (2010)
Article DOI: 10.1016/j.bmc.2010.03.052
BindingDB Entry DOI: 10.7270/Q2V69JRB |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50360174
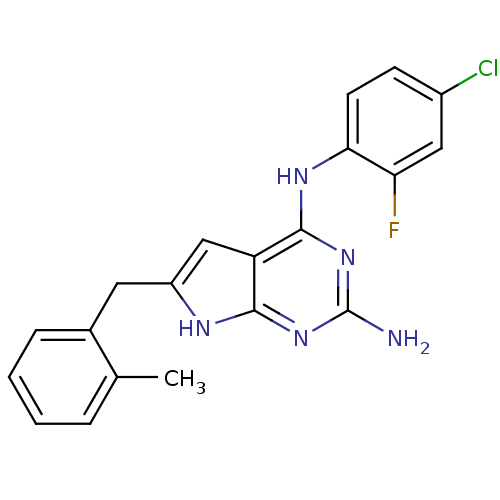
(CHEMBL1929552)Show SMILES Cc1ccccc1Cc1cc2c(Nc3ccc(Cl)cc3F)nc(N)nc2[nH]1 Show InChI InChI=1S/C20H17ClFN5/c1-11-4-2-3-5-12(11)8-14-10-15-18(24-14)26-20(23)27-19(15)25-17-7-6-13(21)9-16(17)22/h2-7,9-10H,8H2,1H3,(H4,23,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 tyrosine kinase activity in VEGF-stimulated human U251 cells after 60 mins by ELISA |
Bioorg Med Chem 20: 910-4 (2012)
Article DOI: 10.1016/j.bmc.2011.11.058
BindingDB Entry DOI: 10.7270/Q27W6CMN |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50524091
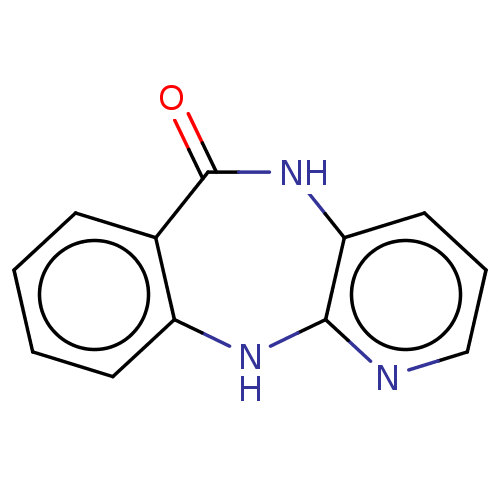
(CHEMBL307792)Show InChI InChI=1S/C12H9N3O/c16-12-8-4-1-2-5-9(8)14-11-10(15-12)6-3-7-13-11/h1-7H,(H,13,14)(H,15,16) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase derived from HIV patient plasma by LC/MS/MS analysis |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM1944

(BHAP deriv. | CHEMBL593 | DELAVIRDINE MESYLATE | D...)Show SMILES CC(C)Nc1cccnc1N1CCN(CC1)C(=O)c1cc2cc(NS(C)(=O)=O)ccc2[nH]1 Show InChI InChI=1S/C22H28N6O3S/c1-15(2)24-19-5-4-8-23-21(19)27-9-11-28(12-10-27)22(29)20-14-16-13-17(26-32(3,30)31)6-7-18(16)25-20/h4-8,13-15,24-26H,9-12H2,1-3H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of wild type recombinant HIV1 reverse transcriptase Y181C mutant using poly(rA)-oligo(dT) as template/primer |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50318183

(CHEMBL1095465 | N4-(3-bromophenyl)-6-(2,5-dimethox...)Show SMILES COc1ccc(OC)c(Cc2cc3c(Nc4cccc(Br)c4)nc(N)nc3[nH]2)c1 Show InChI InChI=1S/C21H20BrN5O2/c1-28-16-6-7-18(29-2)12(9-16)8-15-11-17-19(26-21(23)27-20(17)25-15)24-14-5-3-4-13(22)10-14/h3-7,9-11H,8H2,1-2H3,(H4,23,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta phosphorylation in human SF539 cells after 10 mins by FLISA |
Bioorg Med Chem 18: 3575-87 (2010)
Article DOI: 10.1016/j.bmc.2010.03.052
BindingDB Entry DOI: 10.7270/Q2V69JRB |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50360177

(CHEMBL1929555)Show SMILES Nc1nc(Nc2ccc(Cl)cc2)c2cc(Cc3cccc4ccccc34)[nH]c2n1 Show InChI InChI=1S/C23H18ClN5/c24-16-8-10-17(11-9-16)26-21-20-13-18(27-22(20)29-23(25)28-21)12-15-6-3-5-14-4-1-2-7-19(14)15/h1-11,13H,12H2,(H4,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta tyrosine kinase activity in PDGF-BB-stimulated human SF-539 cells after 60 mins by ELISA |
Bioorg Med Chem 20: 910-4 (2012)
Article DOI: 10.1016/j.bmc.2011.11.058
BindingDB Entry DOI: 10.7270/Q27W6CMN |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50318182

(CHEMBL1095464 | N4-(3-bromophenyl)-6-(2-methylbenz...)Show SMILES Cc1ccccc1Cc1cc2c(Nc3cccc(Br)c3)nc(N)nc2[nH]1 Show InChI InChI=1S/C20H18BrN5/c1-12-5-2-3-6-13(12)9-16-11-17-18(25-20(22)26-19(17)24-16)23-15-8-4-7-14(21)10-15/h2-8,10-11H,9H2,1H3,(H4,22,23,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR phosphorylation in human A431 cells after 10 mins by FLISA |
Bioorg Med Chem 18: 3575-87 (2010)
Article DOI: 10.1016/j.bmc.2010.03.052
BindingDB Entry DOI: 10.7270/Q2V69JRB |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50318182

(CHEMBL1095464 | N4-(3-bromophenyl)-6-(2-methylbenz...)Show SMILES Cc1ccccc1Cc1cc2c(Nc3cccc(Br)c3)nc(N)nc2[nH]1 Show InChI InChI=1S/C20H18BrN5/c1-12-5-2-3-6-13(12)9-16-11-17-18(25-20(22)26-19(17)24-16)23-15-8-4-7-14(21)10-15/h2-8,10-11H,9H2,1H3,(H4,22,23,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR tyrosine kinase activity in EGF-stimulated human A431 cells after 60 mins by ELISA |
Bioorg Med Chem 20: 910-4 (2012)
Article DOI: 10.1016/j.bmc.2011.11.058
BindingDB Entry DOI: 10.7270/Q27W6CMN |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50524093
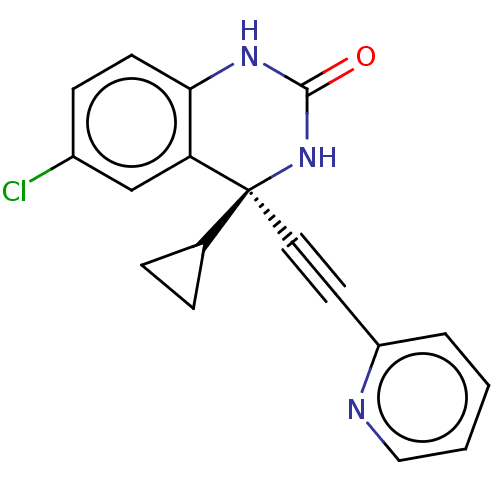
(CHEMBL311050)Show SMILES Clc1ccc2NC(=O)N[C@@](C#Cc3ccccn3)(C3CC3)c2c1 Show InChI InChI=1S/C18H14ClN3O/c19-13-6-7-16-15(11-13)18(12-4-5-12,22-17(23)21-16)9-8-14-3-1-2-10-20-14/h1-3,6-7,10-12H,4-5H2,(H2,21,22,23)/t18-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
J Med Chem 62: 4851-4883 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00843
BindingDB Entry DOI: 10.7270/Q2F76H0J |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM4810
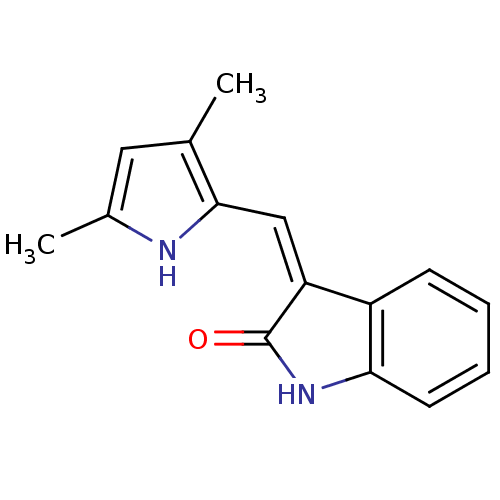
((3Z)-3-[(3,5-dimethyl-1H-pyrrol-2-yl)methylidene]-...)Show InChI InChI=1S/C15H14N2O/c1-9-7-10(2)16-14(9)8-12-11-5-3-4-6-13(11)17-15(12)18/h3-8,16H,1-2H3,(H,17,18)/b12-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 phosphorylation in human U251 cells after 10 mins by FLISA |
Bioorg Med Chem 18: 3575-87 (2010)
Article DOI: 10.1016/j.bmc.2010.03.052
BindingDB Entry DOI: 10.7270/Q2V69JRB |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM4810

((3Z)-3-[(3,5-dimethyl-1H-pyrrol-2-yl)methylidene]-...)Show InChI InChI=1S/C15H14N2O/c1-9-7-10(2)16-14(9)8-12-11-5-3-4-6-13(11)17-15(12)18/h3-8,16H,1-2H3,(H,17,18)/b12-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 tyrosine kinase activity in VEGF-stimulated human U251 cells after 60 mins by ELISA |
Bioorg Med Chem 20: 910-4 (2012)
Article DOI: 10.1016/j.bmc.2011.11.058
BindingDB Entry DOI: 10.7270/Q27W6CMN |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM4814

(CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta tyrosine kinase activity in PDGF-BB-stimulated human SF-539 cells after 60 mins by ELISA |
Bioorg Med Chem 20: 910-4 (2012)
Article DOI: 10.1016/j.bmc.2011.11.058
BindingDB Entry DOI: 10.7270/Q27W6CMN |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM4814

(CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta phosphorylation in human SF539 cells after 10 mins by FLISA |
Bioorg Med Chem 18: 3575-87 (2010)
Article DOI: 10.1016/j.bmc.2010.03.052
BindingDB Entry DOI: 10.7270/Q2V69JRB |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50318183

(CHEMBL1095465 | N4-(3-bromophenyl)-6-(2,5-dimethox...)Show SMILES COc1ccc(OC)c(Cc2cc3c(Nc4cccc(Br)c4)nc(N)nc3[nH]2)c1 Show InChI InChI=1S/C21H20BrN5O2/c1-28-16-6-7-18(29-2)12(9-16)8-15-11-17-19(26-21(23)27-20(17)25-15)24-14-5-3-4-13(22)10-14/h3-7,9-11H,8H2,1-2H3,(H4,23,24,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR phosphorylation in human A431 cells after 10 mins by FLISA |
Bioorg Med Chem 18: 3575-87 (2010)
Article DOI: 10.1016/j.bmc.2010.03.052
BindingDB Entry DOI: 10.7270/Q2V69JRB |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50360177

(CHEMBL1929555)Show SMILES Nc1nc(Nc2ccc(Cl)cc2)c2cc(Cc3cccc4ccccc34)[nH]c2n1 Show InChI InChI=1S/C23H18ClN5/c24-16-8-10-17(11-9-16)26-21-20-13-18(27-22(20)29-23(25)28-21)12-15-6-3-5-14-4-1-2-7-19(14)15/h1-11,13H,12H2,(H4,25,26,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR tyrosine kinase activity in EGF-stimulated human A431 cells after 60 mins by ELISA |
Bioorg Med Chem 20: 910-4 (2012)
Article DOI: 10.1016/j.bmc.2011.11.058
BindingDB Entry DOI: 10.7270/Q27W6CMN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data