

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM7840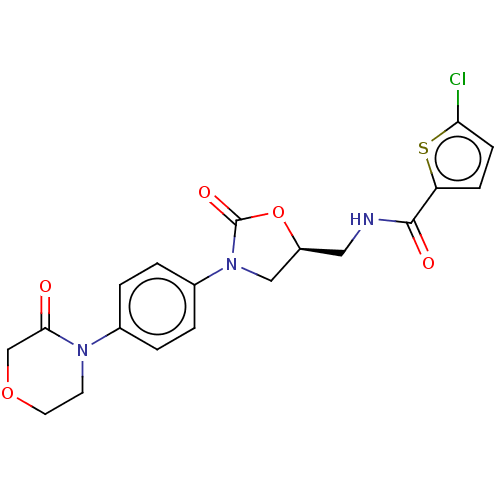 (RIVAROXABAN | US8822458, 44 | US8822458, 97) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of factor-10a (unknown origin) | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50443853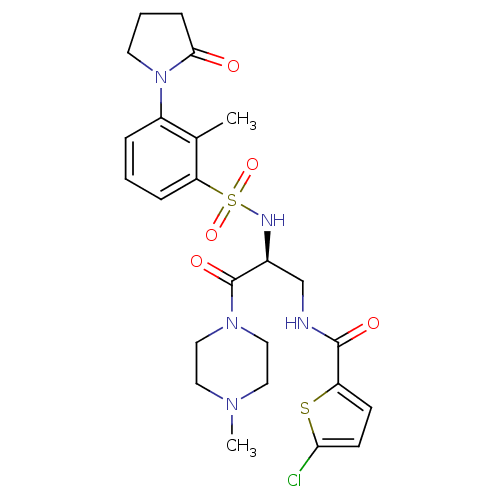 (CHEMBL3091519 | US20230391761, Reference 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacylase sirtuin-5, mitochondrial (Homo sapiens (Human)) | BDBM50255730 (CHEMBL4065067) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Enzymology, Institute of Biochemistry and Biotechnology , Martin-Luther-University Halle-Wittenberg , 06120 Halle/Saale , Germany. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged SIRT5 (34 to 269 residues) expressed in Escherichia coli Transetta(DE3) cells using Benzyl Lys(... | J Med Chem 61: 2460-2471 (2018) Article DOI: 10.1021/acs.jmedchem.7b01648 BindingDB Entry DOI: 10.7270/Q2WW7M4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacylase sirtuin-5, mitochondrial (Homo sapiens (Human)) | BDBM50255693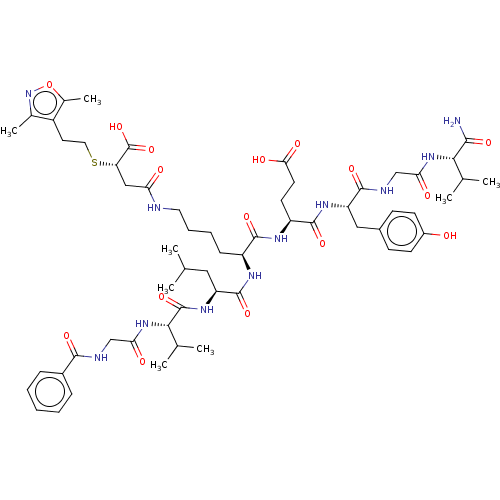 (CHEMBL4073655) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Enzymology, Institute of Biochemistry and Biotechnology , Martin-Luther-University Halle-Wittenberg , 06120 Halle/Saale , Germany. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged SIRT5 (34 to 269 residues) expressed in Escherichia coli Transetta(DE3) cells using Benzyl Lys(... | J Med Chem 61: 2460-2471 (2018) Article DOI: 10.1021/acs.jmedchem.7b01648 BindingDB Entry DOI: 10.7270/Q2WW7M4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacylase sirtuin-5, mitochondrial (Homo sapiens (Human)) | BDBM50255691 (CHEMBL4092751) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Enzymology, Institute of Biochemistry and Biotechnology , Martin-Luther-University Halle-Wittenberg , 06120 Halle/Saale , Germany. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged SIRT5 (34 to 269 residues) expressed in Escherichia coli Transetta(DE3) cells using Benzyl Lys(... | J Med Chem 61: 2460-2471 (2018) Article DOI: 10.1021/acs.jmedchem.7b01648 BindingDB Entry DOI: 10.7270/Q2WW7M4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacylase sirtuin-5, mitochondrial (Homo sapiens (Human)) | BDBM50255690 (CHEMBL4068735) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Enzymology, Institute of Biochemistry and Biotechnology , Martin-Luther-University Halle-Wittenberg , 06120 Halle/Saale , Germany. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal Strep2-tagged SIRT5 expressed in Escherichia coli BL21 (DE3) using Abz-GVLK(glutaryl)AY(NO2)GV-NH2 as subs... | J Med Chem 61: 2460-2471 (2018) Article DOI: 10.1021/acs.jmedchem.7b01648 BindingDB Entry DOI: 10.7270/Q2WW7M4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacylase sirtuin-5, mitochondrial (Homo sapiens (Human)) | BDBM50255692 (CHEMBL4100475) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Enzymology, Institute of Biochemistry and Biotechnology , Martin-Luther-University Halle-Wittenberg , 06120 Halle/Saale , Germany. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal Strep2-tagged SIRT5 expressed in Escherichia coli BL21 (DE3) using Abz-GVLK(glutaryl)AY(NO2)GV-NH2 as subs... | J Med Chem 61: 2460-2471 (2018) Article DOI: 10.1021/acs.jmedchem.7b01648 BindingDB Entry DOI: 10.7270/Q2WW7M4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24732 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | -48.8 | 32 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacylase sirtuin-5, mitochondrial (Homo sapiens (Human)) | BDBM50255710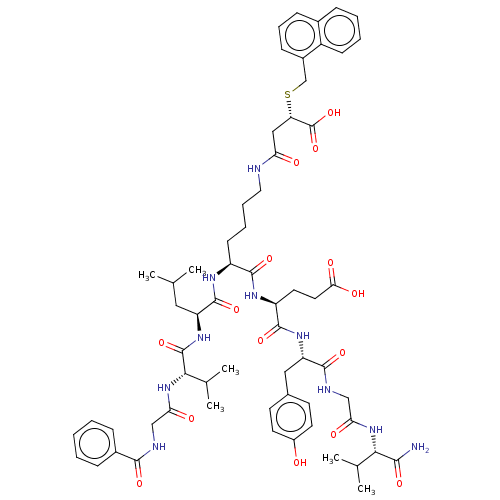 (CHEMBL4091258) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Enzymology, Institute of Biochemistry and Biotechnology , Martin-Luther-University Halle-Wittenberg , 06120 Halle/Saale , Germany. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal Strep2-tagged SIRT5 expressed in Escherichia coli BL21 (DE3) using Abz-GVLK(glutaryl)AY(NO2)GV-NH2 as subs... | J Med Chem 61: 2460-2471 (2018) Article DOI: 10.1021/acs.jmedchem.7b01648 BindingDB Entry DOI: 10.7270/Q2WW7M4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM18071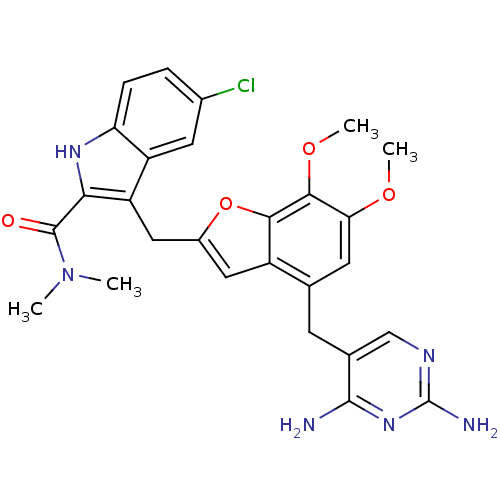 (5-chloro-3-({4-[(2,4-diaminopyrimidin-5-yl)methyl]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | 8 | -47.0 | 50 | n/a | n/a | n/a | n/a | 7.0 | 30 | |
ARPIDA AG | Assay Description Activity was measured as a change in absorbance over time at a wavelength of 340 nm (A340), so as to monitor the disappearance of NADPH. After incuba... | Interscience Conference on Antimicrobial Agents in Chemotherapy 1-1 (2006) BindingDB Entry DOI: 10.7270/Q2959FTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24731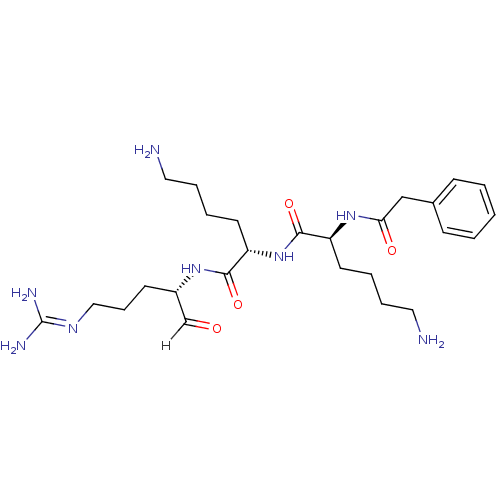 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9 | -47.8 | 51 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Streptococcus pneumoniae (ATCC49619)) | BDBM18071 (5-chloro-3-({4-[(2,4-diaminopyrimidin-5-yl)methyl]...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | 9 | -46.7 | 90 | n/a | n/a | n/a | n/a | 7.0 | 30 | |
ARPIDA AG | Assay Description Activity was measured as a change in absorbance over time at a wavelength of 340 nm (A340), so as to monitor the disappearance of NADPH. After incuba... | Interscience Conference on Antimicrobial Agents in Chemotherapy 1-1 (2006) BindingDB Entry DOI: 10.7270/Q2959FTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24734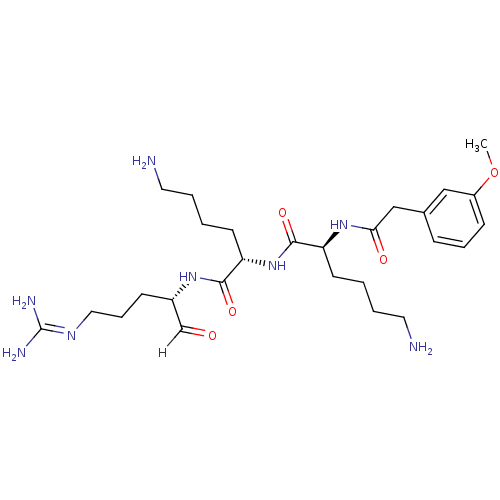 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 11 | -47.3 | 60 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24746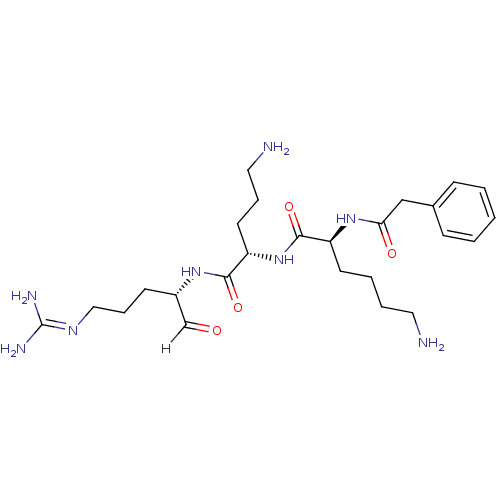 ((2S)-6-amino-N-[(1S)-4-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | -46.8 | 73 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacylase sirtuin-5, mitochondrial (Homo sapiens (Human)) | BDBM50255714 (CHEMBL4064460) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Enzymology, Institute of Biochemistry and Biotechnology , Martin-Luther-University Halle-Wittenberg , 06120 Halle/Saale , Germany. Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant N-terminal Strep2-tagged SIRT5 expressed in Escherichia coli BL21 (DE3) using Abz-GVLK(glutaryl)AY(NO2)GV... | J Med Chem 61: 2460-2471 (2018) Article DOI: 10.1021/acs.jmedchem.7b01648 BindingDB Entry DOI: 10.7270/Q2WW7M4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24739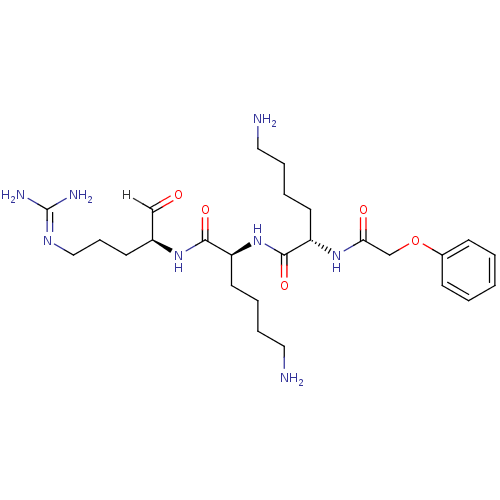 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 19 | -45.8 | 107 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24733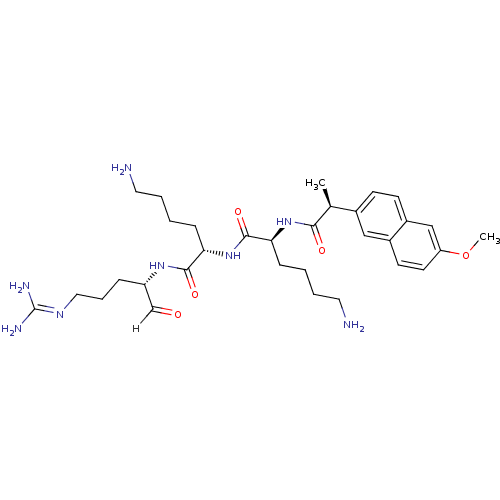 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | -45.7 | 112 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacylase sirtuin-5, mitochondrial (Homo sapiens (Human)) | BDBM50255740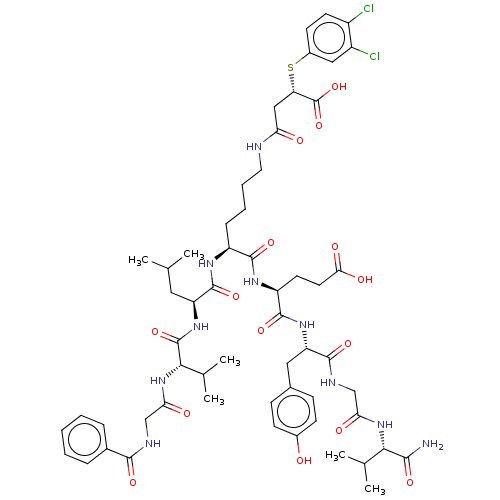 (CHEMBL4100351) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Enzymology, Institute of Biochemistry and Biotechnology , Martin-Luther-University Halle-Wittenberg , 06120 Halle/Saale , Germany. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged SIRT5 (34 to 269 residues) expressed in Escherichia coli Transetta(DE3) cells using Benzyl Lys(... | J Med Chem 61: 2460-2471 (2018) Article DOI: 10.1021/acs.jmedchem.7b01648 BindingDB Entry DOI: 10.7270/Q2WW7M4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24735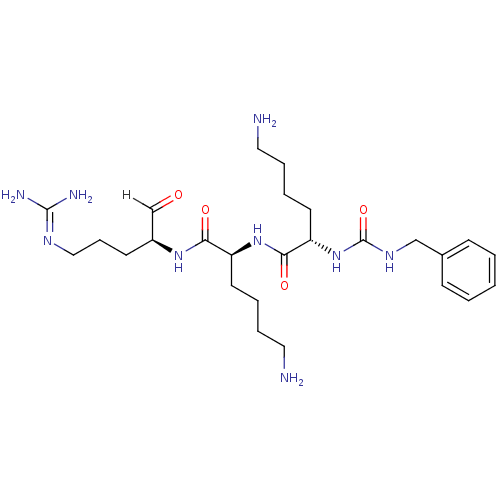 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 26 | -45.0 | 146 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Streptococcus pneumoniae) | BDBM18071 (5-chloro-3-({4-[(2,4-diaminopyrimidin-5-yl)methyl]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | 27 | -43.9 | 50 | n/a | n/a | n/a | n/a | 7.0 | 30 | |
ARPIDA AG | Assay Description Activity was measured as a change in absorbance over time at a wavelength of 340 nm (A340), so as to monitor the disappearance of NADPH. After incuba... | Interscience Conference on Antimicrobial Agents in Chemotherapy 1-1 (2006) BindingDB Entry DOI: 10.7270/Q2959FTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24741 ((2S)-6-amino-N-[(2S)-5-carbamimidamido-1-oxopentan...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 28 | -44.8 | 154 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24736 (Capped tripeptide aldehyde inhibitor, 24 | benzyl ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 40 | -43.9 | 222 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM18069 (5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB | 40 | -42.9 | 600 | n/a | n/a | n/a | n/a | 7.0 | 30 |
ARPIDA AG | Assay Description Activity was measured as a change in absorbance over time at a wavelength of 340 nm (A340), so as to monitor the disappearance of NADPH. After incuba... | Interscience Conference on Antimicrobial Agents in Chemotherapy 1-1 (2006) BindingDB Entry DOI: 10.7270/Q2959FTP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24728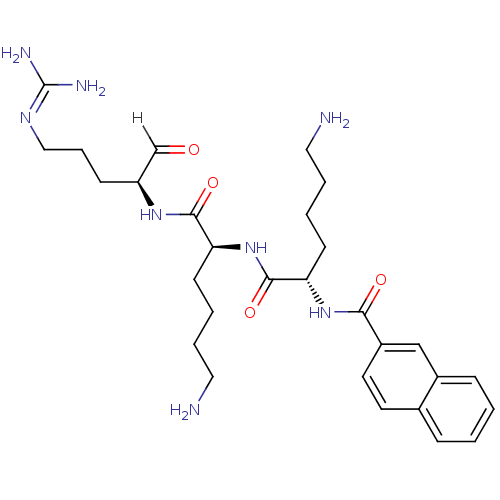 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 41 | -43.9 | 231 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24744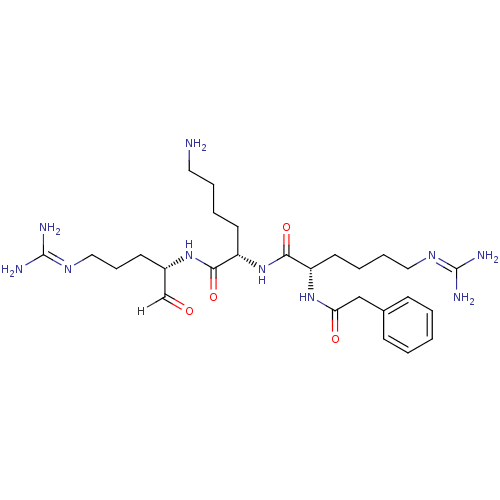 ((2S)-N-[(1S)-5-amino-1-{[(2S)-5-carbamimidamido-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 44 | -43.7 | 245 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24742 ((2S)-6-amino-2-[(2S)-5-amino-2-(1-phenylacetamido)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 46 | -43.6 | 255 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24737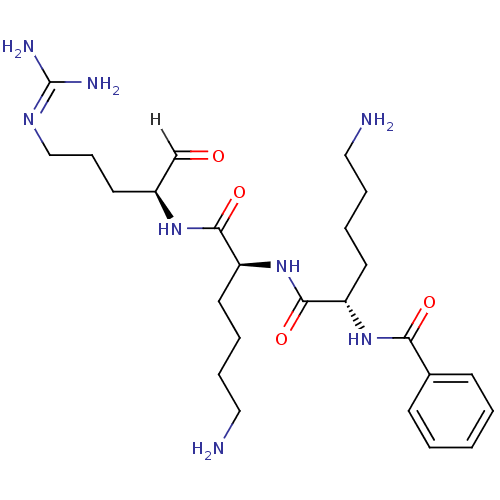 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 49 | -43.4 | 271 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24748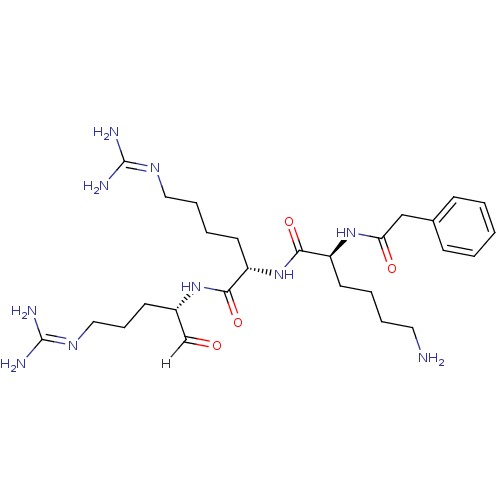 ((2S)-2-[(2S)-6-amino-2-(1-phenylacetamido)hexanami...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 53 | -43.2 | 297 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24745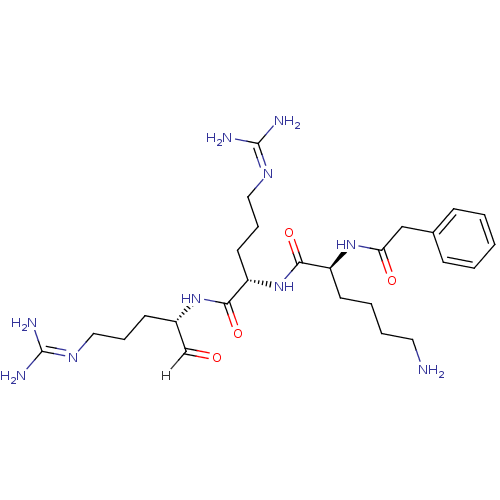 ((2S)-6-amino-N-[(1S)-4-carbamimidamido-1-{[(2S)-5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 58 | -43.0 | 325 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24738 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 81 | -42.1 | 454 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacylase sirtuin-5, mitochondrial (Homo sapiens (Human)) | BDBM50255726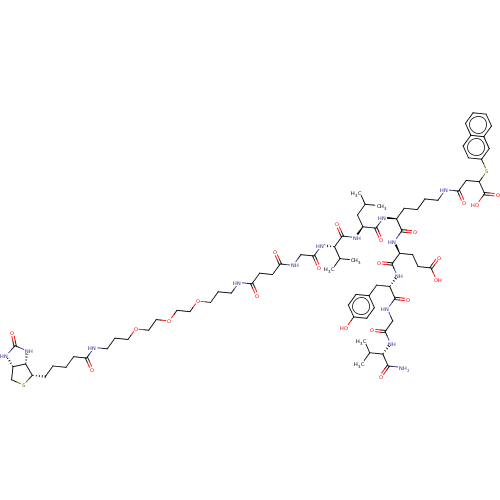 (CHEMBL4085990) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Enzymology, Institute of Biochemistry and Biotechnology , Martin-Luther-University Halle-Wittenberg , 06120 Halle/Saale , Germany. Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant N-terminal Strep2-tagged SIRT5 expressed in Escherichia coli BL21 (DE3) using Abz-GVLK(glutaryl)AY(NO2)GV... | J Med Chem 61: 2460-2471 (2018) Article DOI: 10.1021/acs.jmedchem.7b01648 BindingDB Entry DOI: 10.7270/Q2WW7M4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase [F98Y] (Staphylococcus aureus (subsp. aureus NCTC 8325)) | BDBM18071 (5-chloro-3-({4-[(2,4-diaminopyrimidin-5-yl)methyl]...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | 90 | -40.9 | 900 | n/a | n/a | n/a | n/a | 7.0 | 30 | |
ARPIDA AG | Assay Description Activity was measured as a change in absorbance over time at a wavelength of 340 nm (A340), so as to monitor the disappearance of NADPH. After incuba... | Interscience Conference on Antimicrobial Agents in Chemotherapy 1-1 (2006) BindingDB Entry DOI: 10.7270/Q2959FTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24730 ((2S)-6-amino-2-[(2S)-6-amino-2-[(2E)-3-phenylprop-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 104 | -41.5 | 580 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24743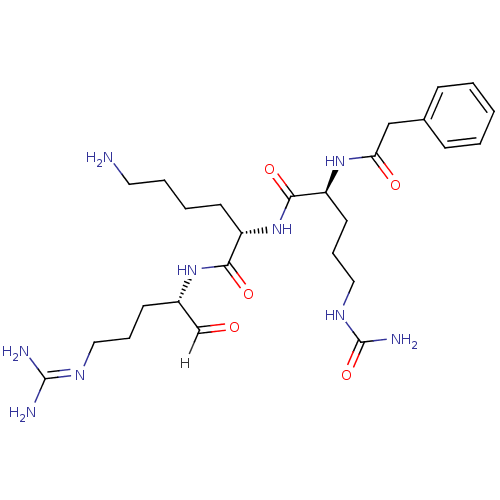 ((2S)-6-amino-N-[(2S)-5-carbamimidamido-1-oxopentan...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 111 | -41.3 | 619 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacylase sirtuin-5, mitochondrial (Homo sapiens (Human)) | BDBM50255644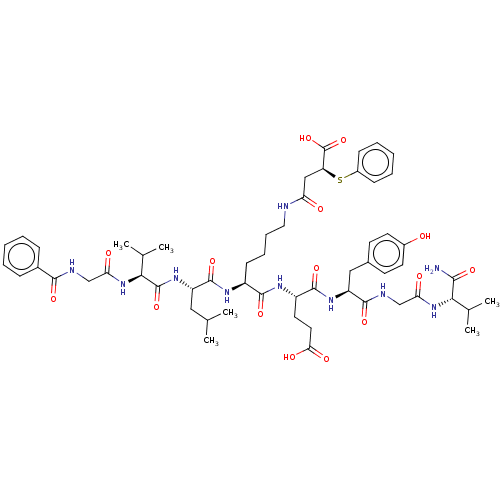 (CHEMBL4079212) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Enzymology, Institute of Biochemistry and Biotechnology , Martin-Luther-University Halle-Wittenberg , 06120 Halle/Saale , Germany. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal Strep2-tagged SIRT5 expressed in Escherichia coli BL21 (DE3) using Abz-GVLK(glutaryl)AY(NO2)GV-NH2 as subs... | J Med Chem 61: 2460-2471 (2018) Article DOI: 10.1021/acs.jmedchem.7b01648 BindingDB Entry DOI: 10.7270/Q2WW7M4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24740 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-5-carbamimid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 160 | -40.3 | 891 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Streptococcus pneumoniae) | BDBM18069 (5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | 240 | -38.4 | 3.00E+3 | n/a | n/a | n/a | n/a | 7.0 | 30 | |
ARPIDA AG | Assay Description Activity was measured as a change in absorbance over time at a wavelength of 340 nm (A340), so as to monitor the disappearance of NADPH. After incuba... | Interscience Conference on Antimicrobial Agents in Chemotherapy 1-1 (2006) BindingDB Entry DOI: 10.7270/Q2959FTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacylase sirtuin-5, mitochondrial (Homo sapiens (Human)) | BDBM50255725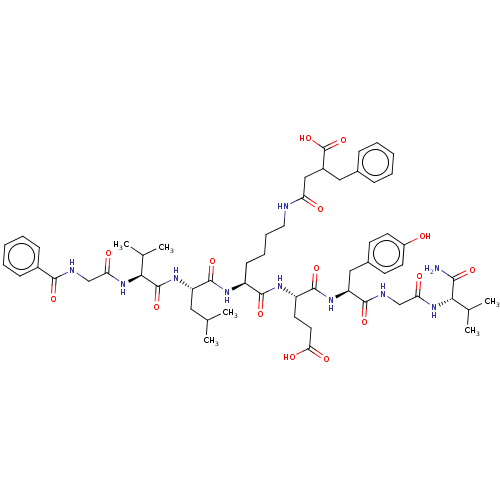 (CHEMBL4068365) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 263 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Enzymology, Institute of Biochemistry and Biotechnology , Martin-Luther-University Halle-Wittenberg , 06120 Halle/Saale , Germany. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal Strep2-tagged SIRT5 expressed in Escherichia coli BL21 (DE3) using Abz-GVLK(glutaryl)AY(NO2)GV-NH2 as subs... | J Med Chem 61: 2460-2471 (2018) Article DOI: 10.1021/acs.jmedchem.7b01648 BindingDB Entry DOI: 10.7270/Q2WW7M4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50112086 (3-({2-[(4-Carbamimidoyl-phenylamino)-methyl]-1-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of factor-10a (unknown origin) | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24747 ((2S)-6-amino-N-[(1S)-1-{[(2S)-5-carbamimidamido-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.07E+3 | -33.7 | 1.16E+4 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50443853 (CHEMBL3091519 | US20230391761, Reference 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of t-PA (unknown origin) | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1424-1463]/[1502-1685] (West Nile virus (WNV)) | BDBM24749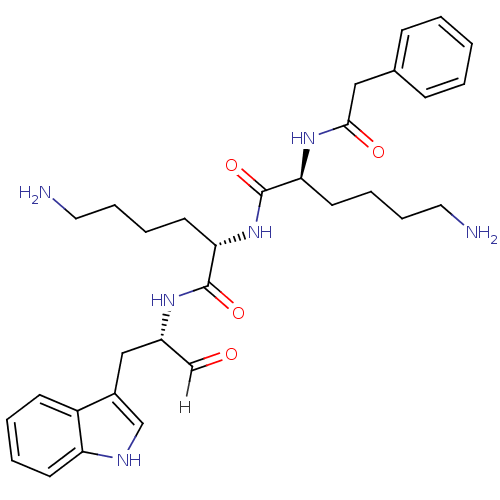 ((2S)-6-amino-N-[(1S)-5-amino-1-{[(2S)-1-(1H-indol-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.60E+3 | -31.7 | 2.57E+4 | n/a | n/a | n/a | n/a | 9.5 | 37 |
University of Queensland | Assay Description Inhibition of WNV protease activity was measured using recombinant WNV protease, which first incubated with inhibitor in 96-well plates. Catalysis wa... | J Med Chem 51: 5714-21 (2008) Article DOI: 10.1021/jm800503y BindingDB Entry DOI: 10.7270/Q27P8WPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase [F98Y] (Staphylococcus aureus (subsp. aureus NCTC 8325)) | BDBM18069 (5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB | 5.50E+3 | -30.5 | 4.50E+4 | n/a | n/a | n/a | n/a | 7.0 | 30 |
ARPIDA AG | Assay Description Activity was measured as a change in absorbance over time at a wavelength of 340 nm (A340), so as to monitor the disappearance of NADPH. After incuba... | Interscience Conference on Antimicrobial Agents in Chemotherapy 1-1 (2006) BindingDB Entry DOI: 10.7270/Q2959FTP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Streptococcus pneumoniae (ATCC49619)) | BDBM18069 (5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | 5.60E+3 | -30.5 | 4.20E+4 | n/a | n/a | n/a | n/a | 7.0 | 30 | |
ARPIDA AG | Assay Description Activity was measured as a change in absorbance over time at a wavelength of 340 nm (A340), so as to monitor the disappearance of NADPH. After incuba... | Interscience Conference on Antimicrobial Agents in Chemotherapy 1-1 (2006) BindingDB Entry DOI: 10.7270/Q2959FTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50443855 (CHEMBL3091517) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of factor-10a (unknown origin) | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50443853 (CHEMBL3091519 | US20230391761, Reference 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of plasmin (unknown origin) | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50443857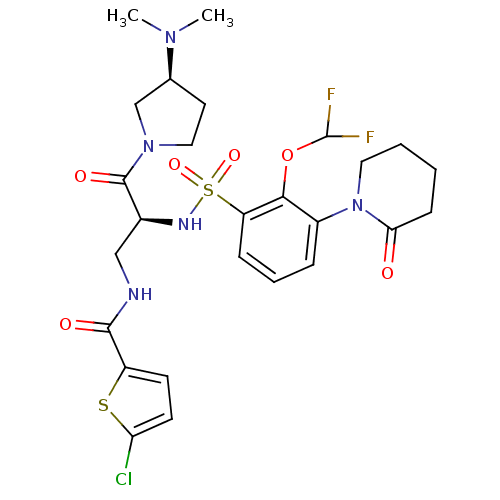 (CHEMBL3091501) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50443858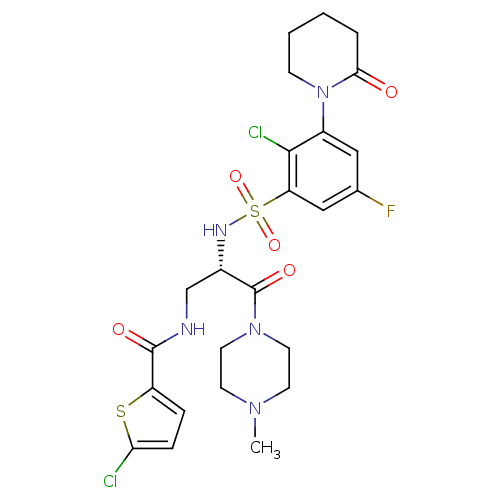 (CHEMBL3091502) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50443846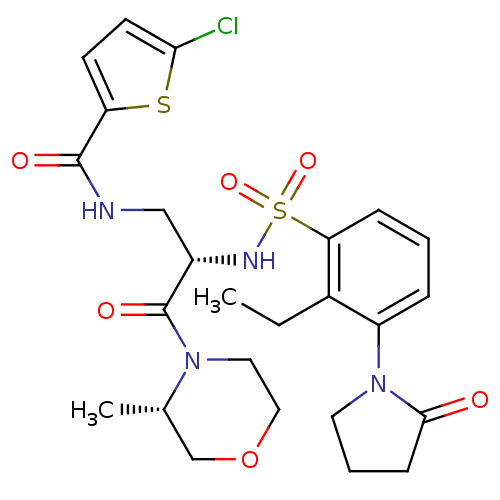 (CHEMBL3091527) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50443847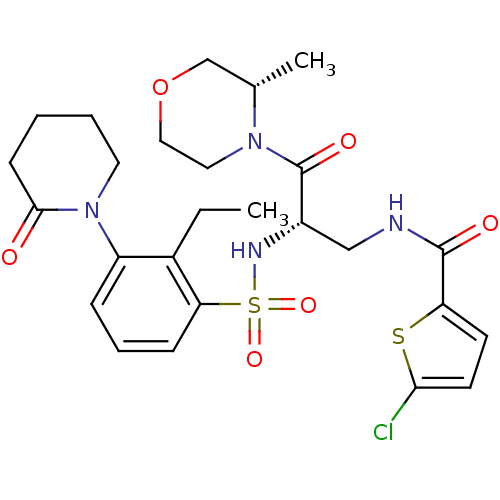 (CHEMBL3091526) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 190 total ) | Next | Last >> |