

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate--tRNA ligase (Escherichia coli) | BDBM18122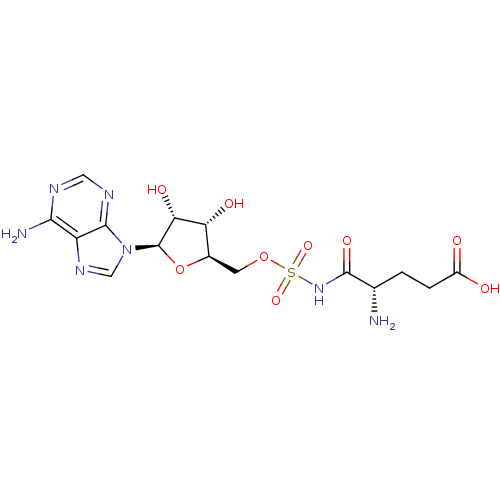 ((4S)-4-amino-5-[({[(2R,3S,4R,5R)-5-(6-amino-9H-pur...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 2.80 | -50.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
CREFSIP | Assay Description The Km and Kappm for the amino acid substrate were first calculated from Lineweaver-Burk plots. The Ki values were calculated from the Kappm vs [I] p... | J Enzyme Inhib Med Chem 20: 61-7 (2005) Article DOI: 10.1080/14756360400002007 BindingDB Entry DOI: 10.7270/Q2X0659C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aspartate--tRNA ligase (Escherichia coli) | BDBM18127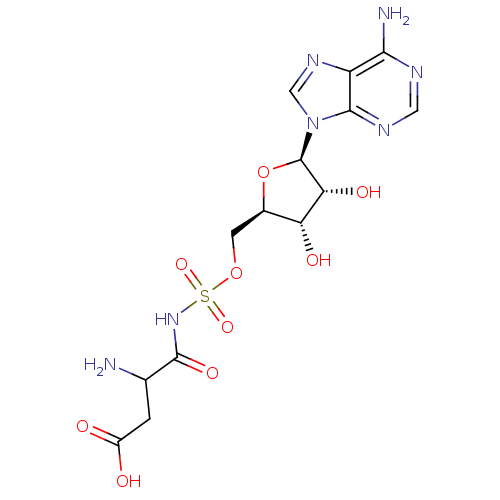 (3-amino-4-[({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 15 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
CREFSIP | Assay Description The Km and Kappm for the amino acid substrate were first calculated from Lineweaver-Burk plots. The Ki values were calculated from the Kappm vs [I] p... | Bioorg Med Chem 13: 69-75 (2005) Article DOI: 10.1016/j.bmc.2004.09.055 BindingDB Entry DOI: 10.7270/Q2S75DM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable glutamate--tRNA ligase, mitochondrial (Mus musculus (mouse)) | BDBM18122 ((4S)-4-amino-5-[({[(2R,3S,4R,5R)-5-(6-amino-9H-pur...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 70 | -41.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 30 |
CREFSIP | Assay Description The Km and Kappm for the amino acid substrate were first calculated from Lineweaver-Burk plots. The Ki values were calculated from the Kappm vs [I] p... | J Enzyme Inhib Med Chem 20: 61-7 (2005) Article DOI: 10.1080/14756360400002007 BindingDB Entry DOI: 10.7270/Q2X0659C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aspartate--tRNA ligase (Escherichia coli) | BDBM18126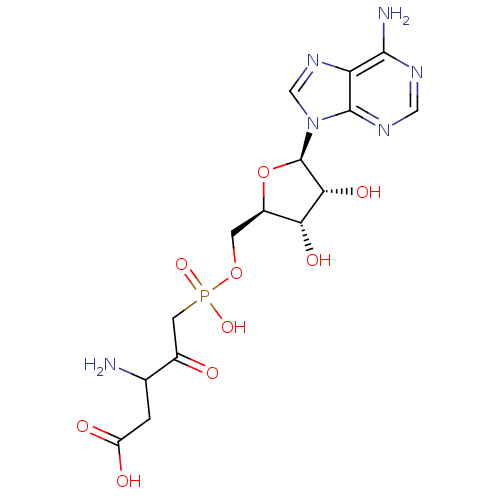 (3-amino-5-({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 123 | -41.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
CREFSIP | Assay Description The Km and Kappm for the amino acid substrate were first calculated from Lineweaver-Burk plots. The Ki values were calculated from the Kappm vs [I] p... | Bioorg Med Chem 13: 69-75 (2005) Article DOI: 10.1016/j.bmc.2004.09.055 BindingDB Entry DOI: 10.7270/Q2S75DM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamine--tRNA ligase (Homo sapiens (Human)) | BDBM50366674 (CHEMBL609187) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of Glutaminyl-tRNA synthetase with respect to glutamine. | Bioorg Med Chem Lett 10: 2441-4 (2001) BindingDB Entry DOI: 10.7270/Q280533K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamine--tRNA ligase (Homo sapiens (Human)) | BDBM50366674 (CHEMBL609187) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of Glutaminyl-tRNA synthetase for Escherichia coli with respect to ATP. | Bioorg Med Chem Lett 10: 2441-4 (2001) BindingDB Entry DOI: 10.7270/Q280533K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamine--tRNA ligase (Homo sapiens (Human)) | BDBM50366675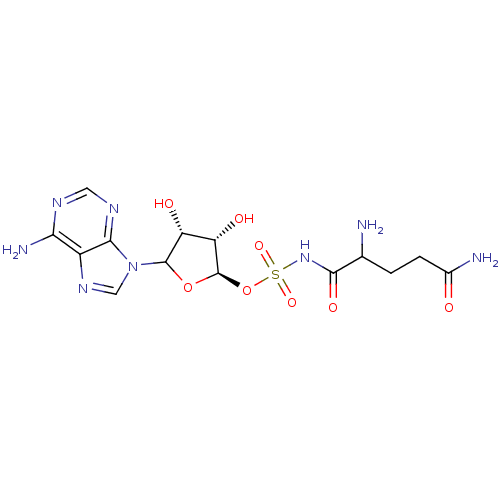 (CHEMBL609496) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval Curated by ChEMBL | Assay Description The compound was evaluated for binding affinity to Glutaminyl-tRNA synthetase with respect to glutamine. | Bioorg Med Chem Lett 10: 2441-4 (2001) BindingDB Entry DOI: 10.7270/Q280533K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamine--tRNA ligase (Homo sapiens (Human)) | BDBM50366673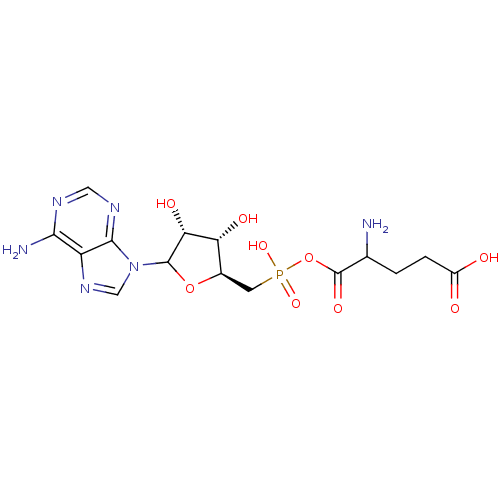 (CHEMBL608302) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Laval Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of Glutaminyl-tRNA synthetase with respect to glutamine. | Bioorg Med Chem Lett 10: 2441-4 (2001) BindingDB Entry DOI: 10.7270/Q280533K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate--tRNA ligase (Escherichia coli) | BDBM18123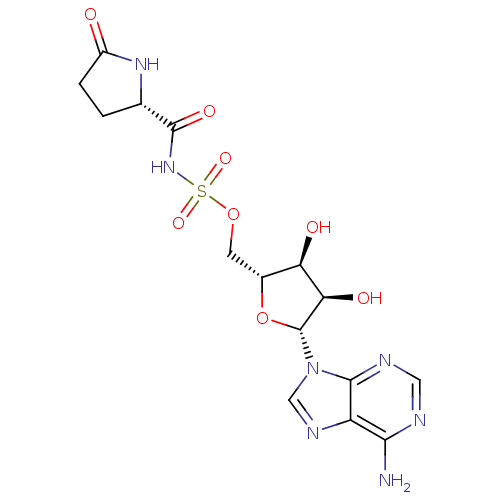 ((5S)-5-{[({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.50E+4 | -28.6 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
CREFSIP | Assay Description The Km and Kappm for the amino acid substrate were first calculated from Lineweaver-Burk plots. The Ki values were calculated from the Kappm vs [I] p... | J Enzyme Inhib Med Chem 20: 61-7 (2005) Article DOI: 10.1080/14756360400002007 BindingDB Entry DOI: 10.7270/Q2X0659C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate--tRNA ligase (Escherichia coli) | BDBM18118 ((4S)-4-amino-6-({[(2R,3S,4R,5R)-5-(6-amino-9H-puri...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.80E+4 | -28.2 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
CREFSIP | Assay Description The Km and Kappm for the amino acid substrate were first calculated from Lineweaver-Burk plots. The Ki values were calculated from the Kappm vs [I] p... | Bioorg Med Chem 15: 295-304 (2007) Article DOI: 10.1016/j.bmc.2006.09.056 BindingDB Entry DOI: 10.7270/Q21N7ZD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamine--tRNA ligase (Homo sapiens (Human)) | BDBM50290175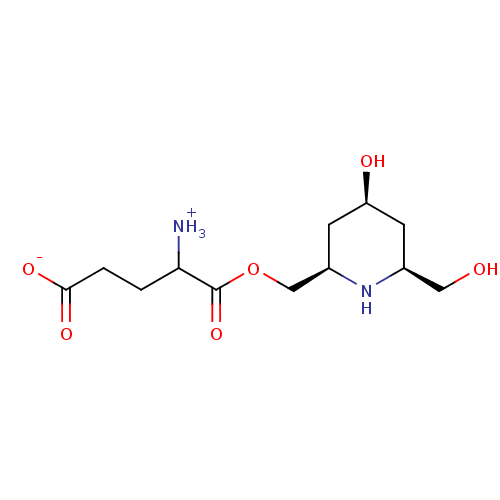 (4-ammonio-5-{[(2R,4S,6S)-4-hydroxy-6-(hydroxymethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition of Escherichia coli glutamyl-t-RNA synthetase | Bioorg Med Chem Lett 7: 2363-2366 (1997) Article DOI: 10.1016/S0960-894X(97)00434-4 BindingDB Entry DOI: 10.7270/Q2CN74D8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM20461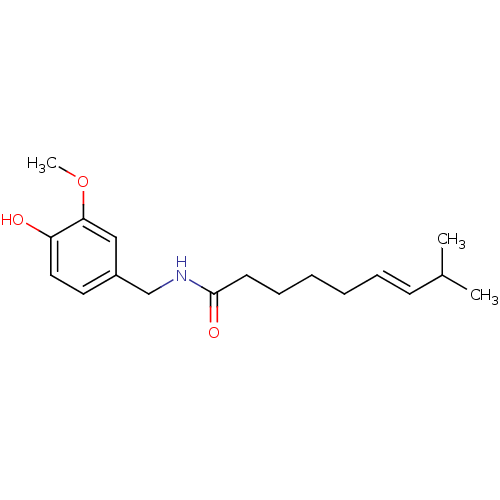 ((6E)-N-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition of aminoacyl tRNA Synthetase | Bioorg Med Chem Lett 7: 2363-2366 (1997) Article DOI: 10.1016/S0960-894X(97)00434-4 BindingDB Entry DOI: 10.7270/Q2CN74D8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aspartate--tRNA ligase (Escherichia coli) | BDBM18124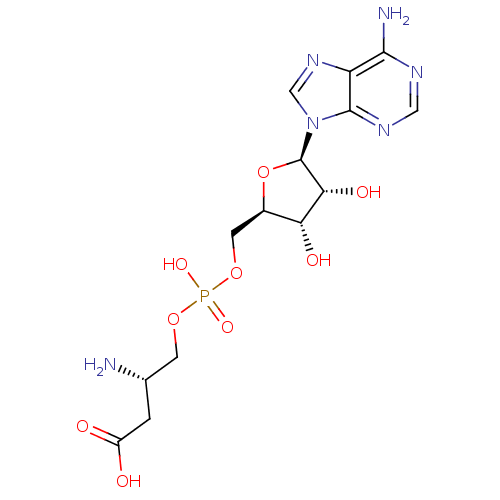 ((3S)-3-amino-4-[({[(2R,3S,4R,5R)-5-(6-amino-9H-pur...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.50E+4 | -25.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
CREFSIP | Assay Description The Km and Kappm for the amino acid substrate were first calculated from Lineweaver-Burk plots. The Ki values were calculated from the Kappm vs [I] p... | Bioorg Med Chem 13: 69-75 (2005) Article DOI: 10.1016/j.bmc.2004.09.055 BindingDB Entry DOI: 10.7270/Q2S75DM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamine--tRNA ligase (Homo sapiens (Human)) | BDBM50290174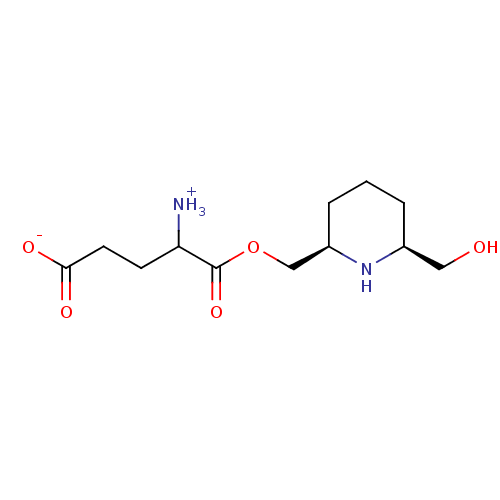 (4-ammonio-5-{[(2R,6S)-6-(hydroxymethyl)piperidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 2.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition of Escherichia coli glutamyl-t-RNA synthetase | Bioorg Med Chem Lett 7: 2363-2366 (1997) Article DOI: 10.1016/S0960-894X(97)00434-4 BindingDB Entry DOI: 10.7270/Q2CN74D8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamine--tRNA ligase (Homo sapiens (Human)) | BDBM50403640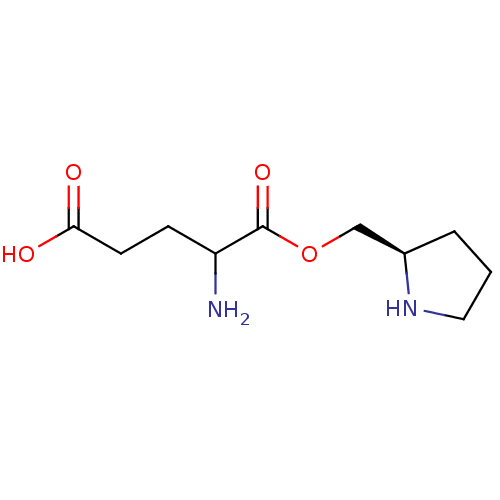 (CHEMBL2115178) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 3.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition of Escherichia coli glutamyl-t-RNA synthetase | Bioorg Med Chem Lett 7: 2363-2366 (1997) Article DOI: 10.1016/S0960-894X(97)00434-4 BindingDB Entry DOI: 10.7270/Q2CN74D8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamine--tRNA ligase (Homo sapiens (Human)) | BDBM50366478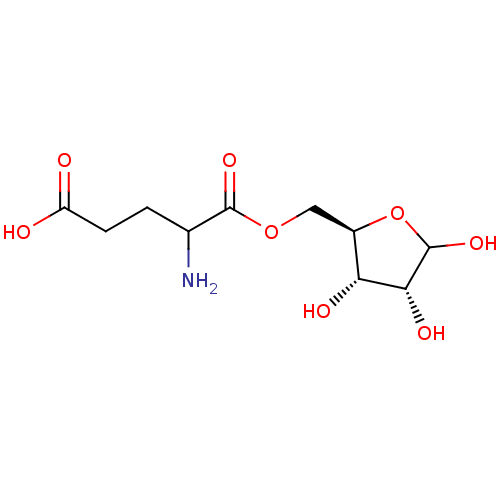 (CHEMBL609498) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition of Escherichia coli glutamyl-t-RNA synthetase | Bioorg Med Chem Lett 7: 2363-2366 (1997) Article DOI: 10.1016/S0960-894X(97)00434-4 BindingDB Entry DOI: 10.7270/Q2CN74D8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamine--tRNA ligase (Escherichia coli) | BDBM18120 (Gln-KPA | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.50E+5 | -18.9 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
CREFSIP | Assay Description The Km and Kappm for the amino acid substrate were first calculated from Lineweaver-Burk plots. The Ki values were calculated from the Kappm vs [I] p... | Bioorg Med Chem 15: 295-304 (2007) Article DOI: 10.1016/j.bmc.2006.09.056 BindingDB Entry DOI: 10.7270/Q21N7ZD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamine--tRNA ligase (Bos taurus (bovine)) | BDBM18118 ((4S)-4-amino-6-({[(2R,3S,4R,5R)-5-(6-amino-9H-puri...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.60E+6 | -15.3 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
CREFSIP | Assay Description The Km and Kappm for the amino acid substrate were first calculated from Lineweaver-Burk plots. The Ki values were calculated from the Kappm vs [I] p... | Bioorg Med Chem 15: 295-304 (2007) Article DOI: 10.1016/j.bmc.2006.09.056 BindingDB Entry DOI: 10.7270/Q21N7ZD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamine--tRNA ligase (Escherichia coli) | BDBM18118 ((4S)-4-amino-6-({[(2R,3S,4R,5R)-5-(6-amino-9H-puri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.80E+6 | -15.2 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
CREFSIP | Assay Description The Km and Kappm for the amino acid substrate were first calculated from Lineweaver-Burk plots. The Ki values were calculated from the Kappm vs [I] p... | Bioorg Med Chem 15: 295-304 (2007) Article DOI: 10.1016/j.bmc.2006.09.056 BindingDB Entry DOI: 10.7270/Q21N7ZD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate--tRNA ligase (Escherichia coli) | BDBM18120 (Gln-KPA | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.90E+6 | -15.1 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
CREFSIP | Assay Description The Km and Kappm for the amino acid substrate were first calculated from Lineweaver-Burk plots. The Ki values were calculated from the Kappm vs [I] p... | Bioorg Med Chem 15: 295-304 (2007) Article DOI: 10.1016/j.bmc.2006.09.056 BindingDB Entry DOI: 10.7270/Q21N7ZD3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamine--tRNA ligase (Homo sapiens (Human)) | BDBM50403639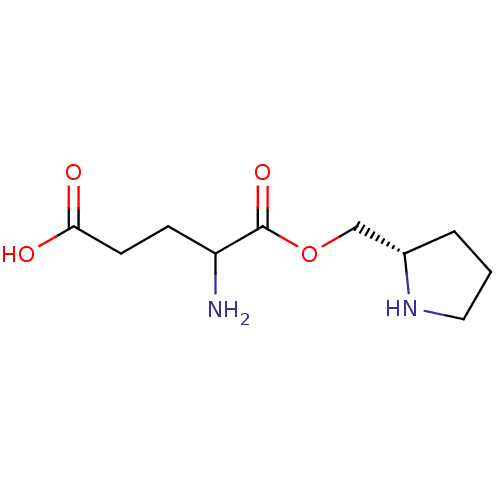 (CHEMBL2114154) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 1.14E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition of Escherichia coli glutamyl-t-RNA synthetase | Bioorg Med Chem Lett 7: 2363-2366 (1997) Article DOI: 10.1016/S0960-894X(97)00434-4 BindingDB Entry DOI: 10.7270/Q2CN74D8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||