

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM23274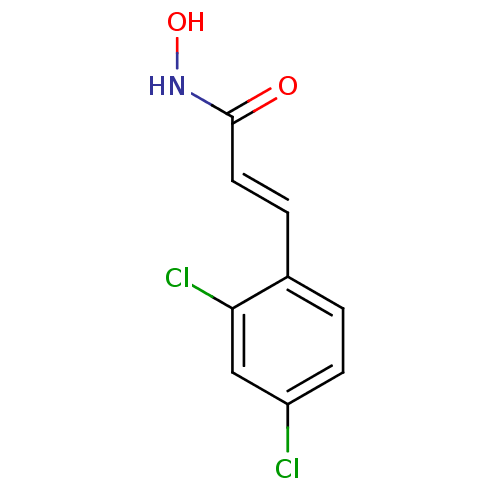 ((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium botulinum truncated BoNT/A light chain (1-425) using truncated SNAP 25 (141-206) peptide as substrate by LC/MS ... | ACS Med Chem Lett 4: 283-287 (2013) Article DOI: 10.1021/ml300428s BindingDB Entry DOI: 10.7270/Q20K29WM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445596 (CHEMBL3103447) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain (1 to 425 amino acids) by LC-MS analysis | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50076267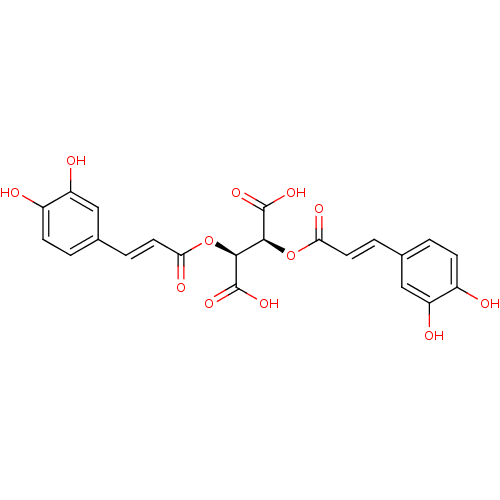 ((2S,3S)-2,3-Bis-[(E)-3-(3,4-dihydroxy-phenyl)-acry...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium botulinum full length BoNT/A light chain (1-448) using truncated SNAP 25 (141-206) peptide as substrate by LC/M... | ACS Med Chem Lett 4: 283-287 (2013) Article DOI: 10.1021/ml300428s BindingDB Entry DOI: 10.7270/Q20K29WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50076267 ((2S,3S)-2,3-Bis-[(E)-3-(3,4-dihydroxy-phenyl)-acry...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Uncompetitive inhibition of Clostridium botulinum truncated BoNT/A light chain (1-425) using truncated SNAP 25 (141-206) peptide as substrate by LC/M... | ACS Med Chem Lett 4: 283-287 (2013) Article DOI: 10.1021/ml300428s BindingDB Entry DOI: 10.7270/Q20K29WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445595 (CHEMBL3103453) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain (1 to 425 amino acids) by LC-MS analysis | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50076267 ((2S,3S)-2,3-Bis-[(E)-3-(3,4-dihydroxy-phenyl)-acry...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Uncompetitive inhibition of Clostridium botulinum full length BoNT/A light chain (1-448) using truncated SNAP 25 (141-206) peptide as substrate by LC... | ACS Med Chem Lett 4: 283-287 (2013) Article DOI: 10.1021/ml300428s BindingDB Entry DOI: 10.7270/Q20K29WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM23274 ((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium botulinum full length BoNT/A light chain (1-448) using truncated SNAP 25 (141-206) peptide as substrate by LC/M... | ACS Med Chem Lett 4: 283-287 (2013) Article DOI: 10.1021/ml300428s BindingDB Entry DOI: 10.7270/Q20K29WM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM43865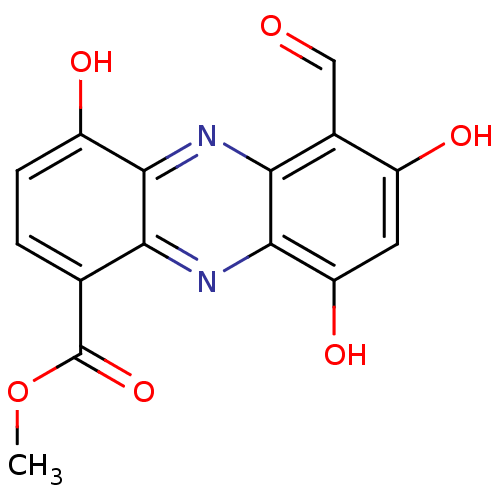 (6-formyl-4,7-dihydroxy-9-keto-5H-phenazine-1-carbo...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Uncompetitive inhibition of Clostridium botulinum truncated BoNT/A light chain (1-425) using truncated SNAP 25 (141-206) peptide as substrate by LC/M... | ACS Med Chem Lett 4: 283-287 (2013) Article DOI: 10.1021/ml300428s BindingDB Entry DOI: 10.7270/Q20K29WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50076267 ((2S,3S)-2,3-Bis-[(E)-3-(3,4-dihydroxy-phenyl)-acry...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium botulinum truncated BoNT/A light chain (1-425) using truncated SNAP 25 (141-206) peptide as substrate by LC/MS ... | ACS Med Chem Lett 4: 283-287 (2013) Article DOI: 10.1021/ml300428s BindingDB Entry DOI: 10.7270/Q20K29WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM43865 (6-formyl-4,7-dihydroxy-9-keto-5H-phenazine-1-carbo...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium botulinum truncated BoNT/A light chain (1-425) using truncated SNAP 25 (141-206) peptide as substrate by LC/MS ... | ACS Med Chem Lett 4: 283-287 (2013) Article DOI: 10.1021/ml300428s BindingDB Entry DOI: 10.7270/Q20K29WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM43865 (6-formyl-4,7-dihydroxy-9-keto-5H-phenazine-1-carbo...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium botulinum full length BoNT/A light chain (1-448) using truncated SNAP 25 (141-206) peptide as substrate by LC/M... | ACS Med Chem Lett 4: 283-287 (2013) Article DOI: 10.1021/ml300428s BindingDB Entry DOI: 10.7270/Q20K29WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445595 (CHEMBL3103453) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain (1 to 425 amino acids) by FRET method | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445596 (CHEMBL3103447) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain (1 to 425 amino acids) by FRET method | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445604 (CHEMBL3103448) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain (1 to 425 amino acids) by FRET method | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445602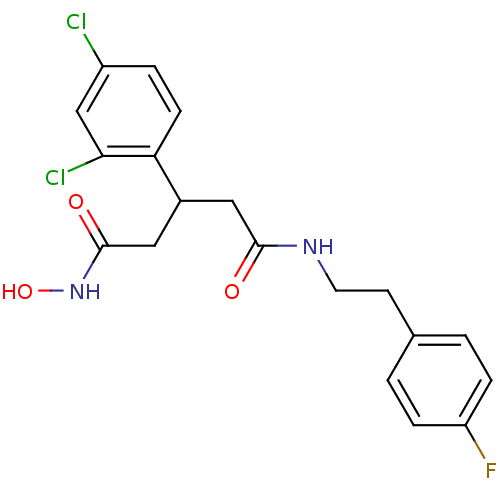 (CHEMBL3103450) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain (1 to 425 amino acids) by FRET method | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445599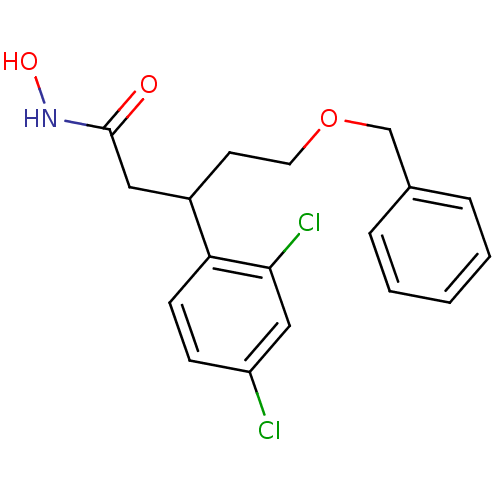 (CHEMBL3103454) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain (1 to 425 amino acids) by FRET method | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445598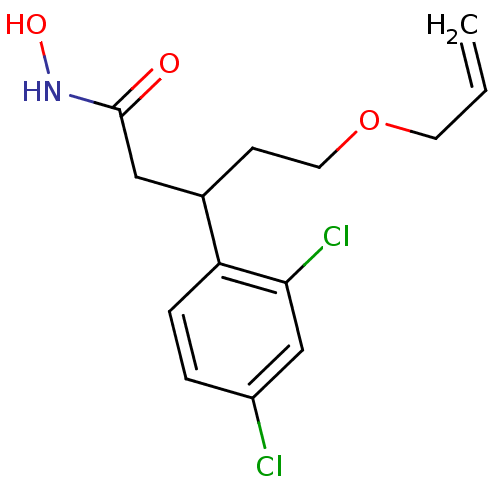 (CHEMBL3103455) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain (1 to 425 amino acids) by FRET method | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445603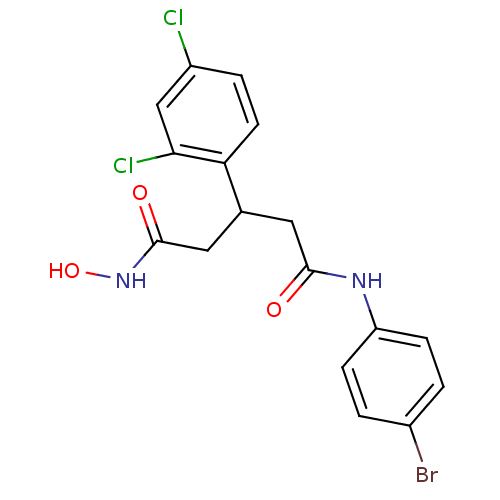 (CHEMBL3103449) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain (1 to 425 amino acids) by FRET method | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445601 (CHEMBL3103451) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain (1 to 425 amino acids) by FRET method | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445600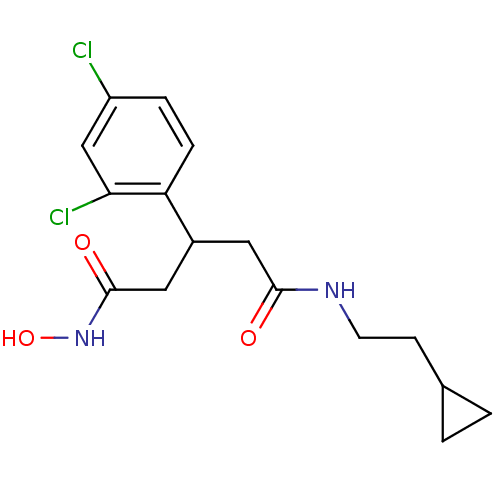 (CHEMBL3103452) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain (1 to 425 amino acids) by FRET method | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445597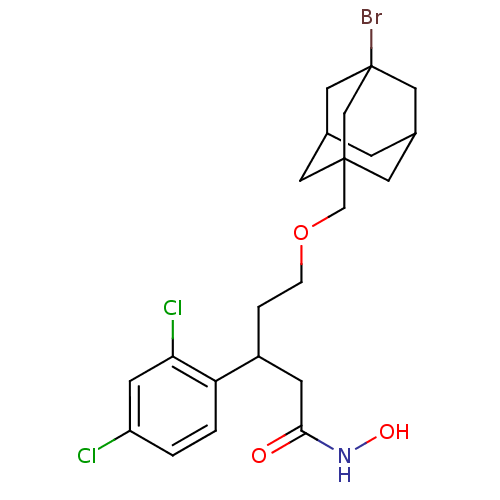 (CHEMBL3103456) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain (1 to 425 amino acids) by FRET method | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445594 (CHEMBL3103459) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum Hall A hyper BoNT/A light chain expressed in human induced pluripotent stem cell-derived neurons after 8 hrs by W... | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445593 (CHEMBL3103460) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.09E+5 | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum Hall A hyper BoNT/A light chain expressed in human induced pluripotent stem cell-derived neurons after 8 hrs by W... | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445592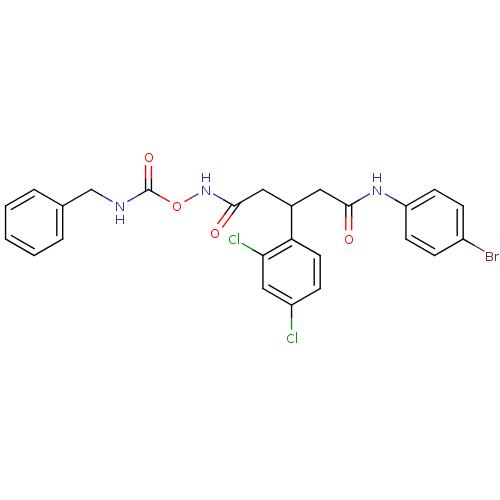 (CHEMBL3103461) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.98E+5 | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum Hall A hyper BoNT/A light chain expressed in human induced pluripotent stem cell-derived neurons after 8 hrs by W... | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445591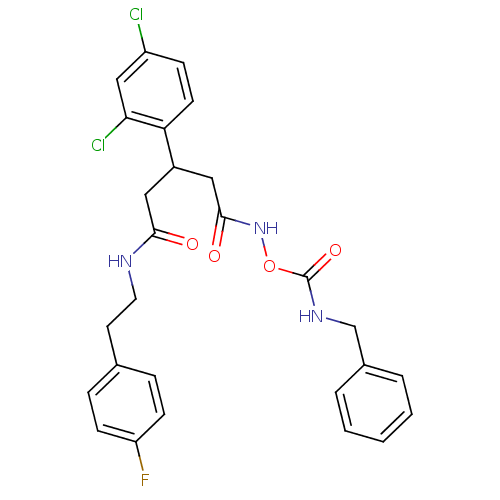 (CHEMBL3103462) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum Hall A hyper BoNT/A light chain expressed in human induced pluripotent stem cell-derived neurons after 8 hrs by W... | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445590 (CHEMBL3103445) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum Hall A hyper BoNT/A light chain expressed in human induced pluripotent stem cell-derived neurons after 8 hrs by W... | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||