

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM50001119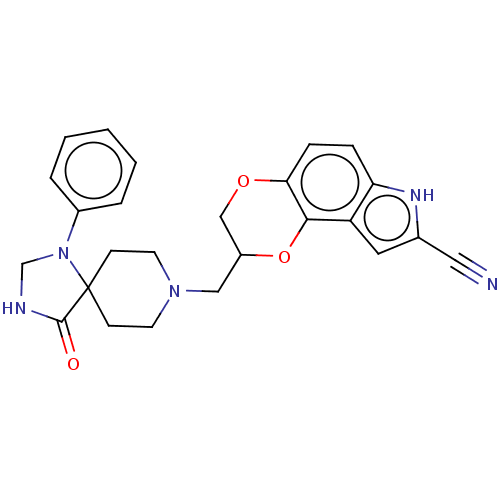 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity towards opioid receptor from rat whole brain using [3H]etorpine (33.2 Ci/mmol,0.2nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50302273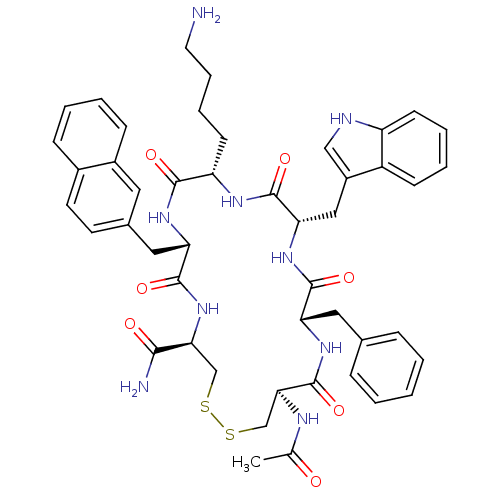 ((4R,7S,10S,13S,16S,19R)-13-((1H-indol-3-yl)methyl)...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]urotensin 2 from rat urotensin 2 receptor expressed in CHOK1 cells by scintillation proximity assay | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50001118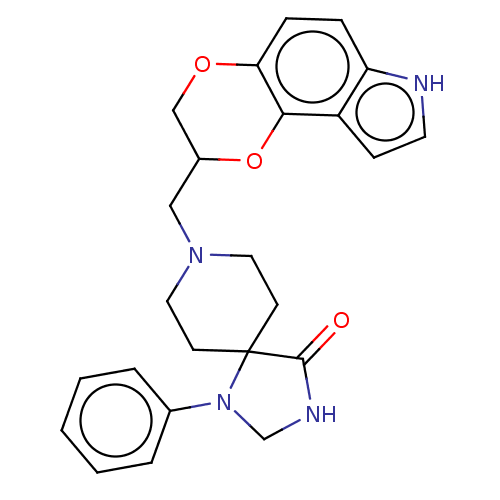 (8-(7,8-Dihydro-3H-6,9-dioxa-3-aza-cyclopenta[a]nap...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity towards Dopamine receptor D2 from mammalian clones expressed in CHO cell membranes using 2 (86.1 Ci/m... | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001117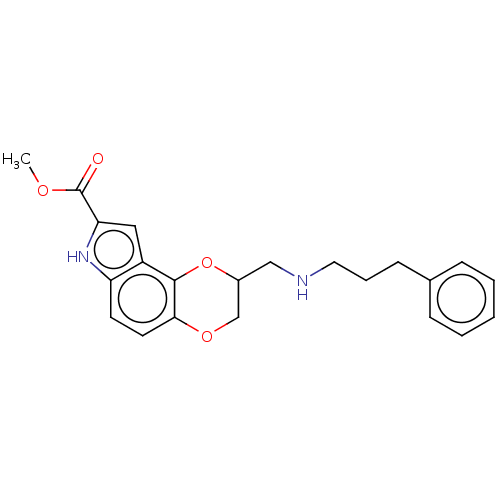 (8-[(3-Phenyl-propylamino)-methyl]-7,8-dihydro-3H-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor expressed in CHO cell membranes | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001113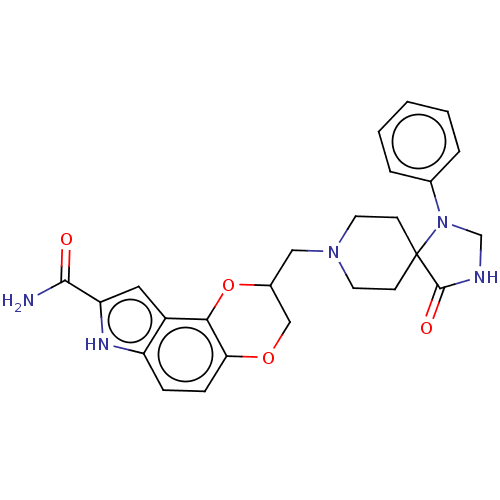 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor expressed in CHO cell membranes | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001118 (8-(7,8-Dihydro-3H-6,9-dioxa-3-aza-cyclopenta[a]nap...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor expressed in CHO cell membranes | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001119 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor expressed in CHO cell membranes | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001112 (8-(2-Hydroxymethyl-7,8-dihydro-3H-6,9-dioxa-3-aza-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor expressed in CHO cell membranes | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50001117 (8-[(3-Phenyl-propylamino)-methyl]-7,8-dihydro-3H-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards Alpha-1 adrenergic receptor from rat whole brain using [3H]prazosin (18.1 Ci/mmol,0.9nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM50001113 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity towards opioid receptor from rat whole brain using [3H]etorpine (33.2 Ci/mmol,0.2nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001116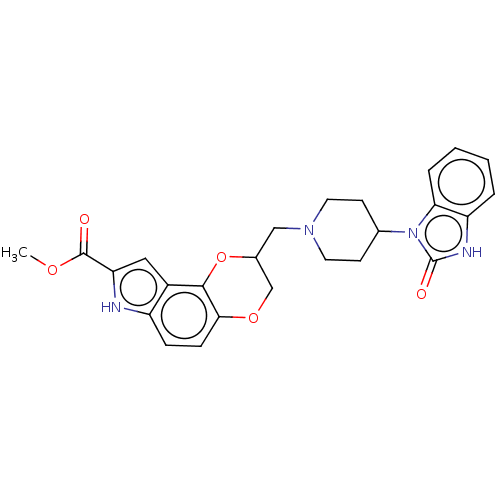 (8-[4-(2-Oxo-2,3-dihydro-benzoimidazol-1-yl)-piperi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor expressed in CHO cell membranes | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50064786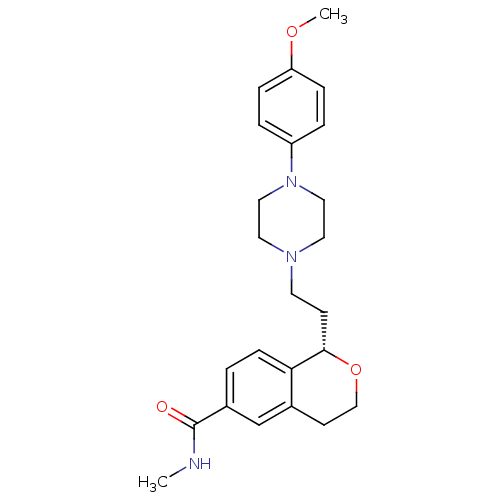 ((S)-1-(2-(4-(4-methoxyphenyl)piperazin-1-yl)ethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description The compound was tested for CNS binding affinity towards 5-hydroxytryptamine 1D receptor from cloned gorilla membranes expressed in cultured HEK 293 ... | J Med Chem 41: 2180-3 (1998) Article DOI: 10.1021/jm980137o BindingDB Entry DOI: 10.7270/Q2NV9HCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001114 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor expressed in CHO cell membranes | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001110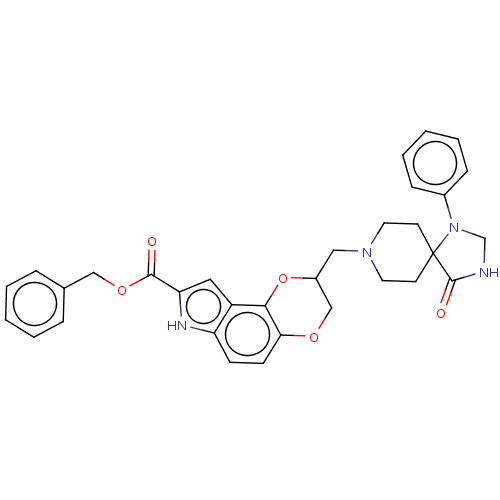 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor expressed in CHO cell membranes | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001111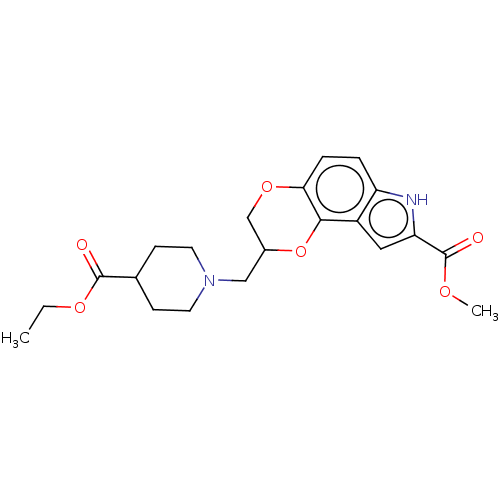 (8-(4-Ethoxycarbonyl-piperidin-1-ylmethyl)-7,8-dihy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor expressed in CHO cell membranes | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (RAT) | BDBM50051562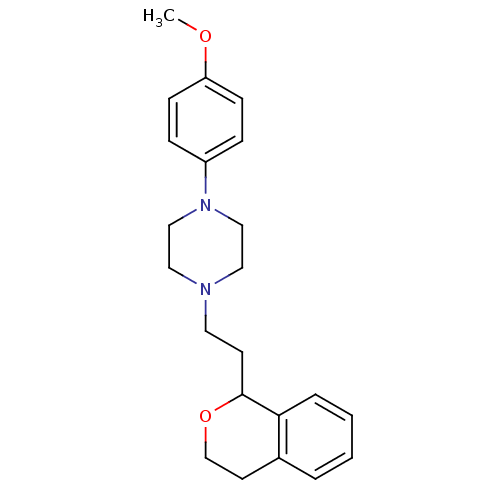 (1-(2-Isochroman-1-yl-ethyl)-4-(4-methoxy-phenyl)-p...) | Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity of [3H]-Spiperone towards Dopamine receptor D4 expressed in cultured cells or from rat whole brain. | J Med Chem 39: 2435-7 (1996) Article DOI: 10.1021/jm960084f BindingDB Entry DOI: 10.7270/Q2H1314Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50001119 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 from mammalian clones expressed in CHO cell membranes using 2 (86.1 Ci/mmol,1.7nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001115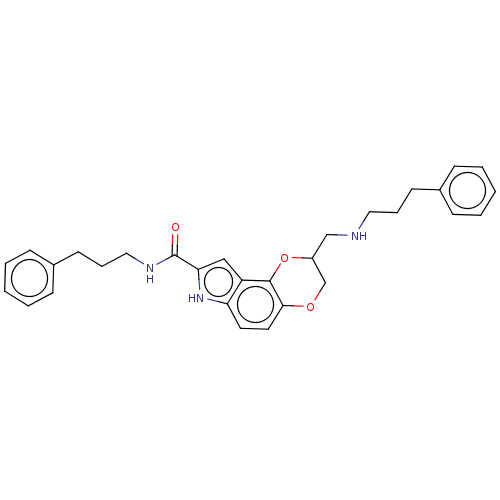 (8-[(3-Phenyl-propylamino)-methyl]-7,8-dihydro-3H-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor expressed in CHO cell membranes | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50001113 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 from mammalian clones expressed in CHO cell membranes using 2 (86.1 Ci/mmol,1.7nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50001116 (8-[4-(2-Oxo-2,3-dihydro-benzoimidazol-1-yl)-piperi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards Alpha-1 adrenergic receptor from rat whole brain using [3H]prazosin (18.1 Ci/mmol,0.9nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50005835 ((3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | DrugBank Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description The compound was tested for CNS binding affinity towards 5-hydroxytryptamine 1D receptor from cloned gorilla membranes expressed in cultured HEK 293 ... | J Med Chem 41: 2180-3 (1998) Article DOI: 10.1021/jm980137o BindingDB Entry DOI: 10.7270/Q2NV9HCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50001112 (8-(2-Hydroxymethyl-7,8-dihydro-3H-6,9-dioxa-3-aza-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 from mammalian clones expressed in CHO cell membranes using 2 (86.1 Ci/mmol,1.7nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (RAT) | BDBM50051561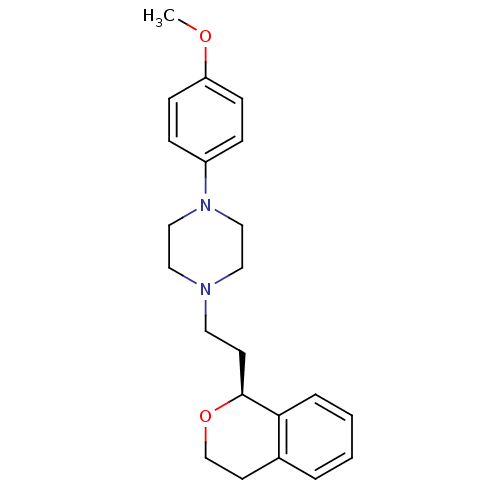 (1-((S)-2-Isochroman-1-yl-ethyl)-4-(4-methoxy-pheny...) | Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity of [3H]-Spiperone towards Dopamine receptor D4 expressed in cultured cells or from rat whole brain. | J Med Chem 39: 2435-7 (1996) Article DOI: 10.1021/jm960084f BindingDB Entry DOI: 10.7270/Q2H1314Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50051562 (1-(2-Isochroman-1-yl-ethyl)-4-(4-methoxy-phenyl)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description The compound was tested for CNS binding affinity towards Dopamine receptor D4 from cloned Human membranes. | J Med Chem 41: 2180-3 (1998) Article DOI: 10.1021/jm980137o BindingDB Entry DOI: 10.7270/Q2NV9HCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (RAT) | BDBM85067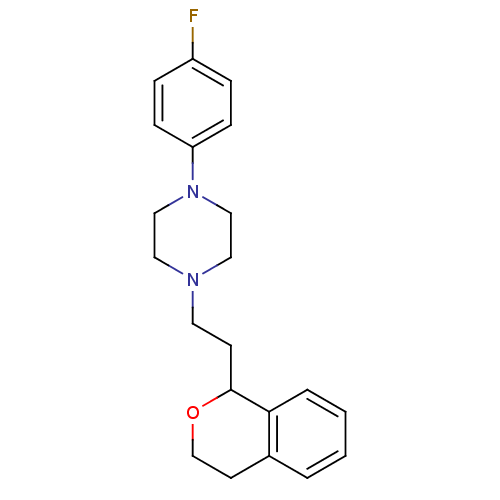 (CAS_170856-41-4 | CHEMBL81330 | PNU 96415E | PNU-9...) | Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity of [3H]-Spiperone towards Dopamine receptor D4 expressed in cultured cells or from rat whole brain. | J Med Chem 39: 2435-7 (1996) Article DOI: 10.1021/jm960084f BindingDB Entry DOI: 10.7270/Q2H1314Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001109 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor expressed in CHO cell membranes | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM50001109 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity towards opioid receptor from rat whole brain using [3H]etorpine (33.2 Ci/mmol,0.2nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50001114 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity towards Dopamine receptor D2 from mammalian clones expressed in CHO cell membranes using [3H]-U-86,17... | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM50001112 (8-(2-Hydroxymethyl-7,8-dihydro-3H-6,9-dioxa-3-aza-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Inhibition of human erythrocyte carbonic anhydrase II | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50064784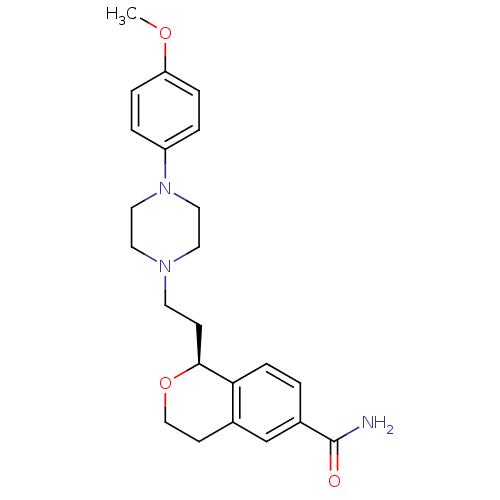 ((S)-1-{2-[4-(4-Methoxy-phenyl)-piperazin-1-yl]-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description The compound was tested for CNS binding affinity towards 5-hydroxytryptamine 1D receptor from cloned gorilla membranes expressed in cultured HEK 293 ... | J Med Chem 41: 2180-3 (1998) Article DOI: 10.1021/jm980137o BindingDB Entry DOI: 10.7270/Q2NV9HCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50302257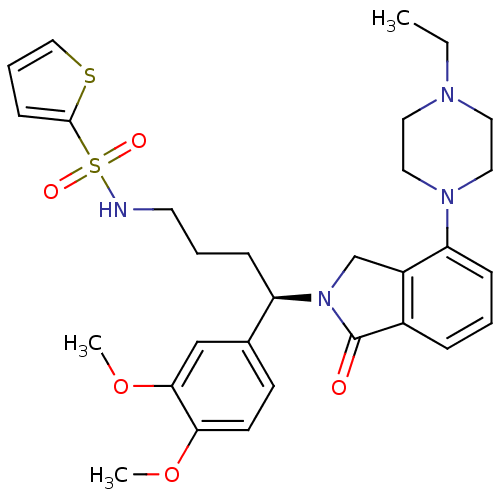 ((R)-N-(4-(3,4-dimethoxyphenyl)-4-(4-(4-ethylpipera...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]urotensin 2 from urotensin 2 receptor in human RMS13 cells by scintillation proximity assay | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50302258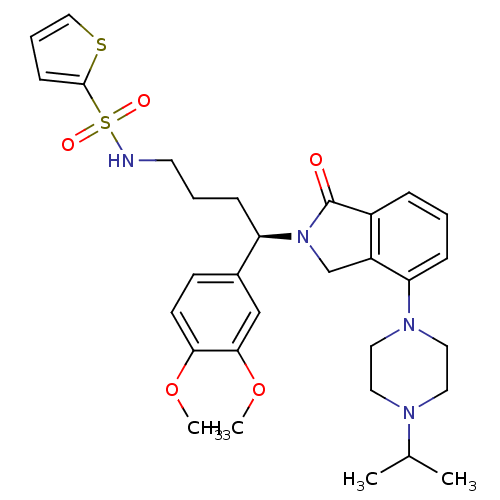 ((R)-N-(4-(3,4-dimethoxyphenyl)-4-(4-(4-isopropylpi...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]urotensin 2 from urotensin 2 receptor in human RMS13 cells by scintillation proximity assay | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM50001116 (8-[4-(2-Oxo-2,3-dihydro-benzoimidazol-1-yl)-piperi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Inhibition of human erythrocyte carbonic anhydrase II | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50001109 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 from mammalian clones expressed in CHO cell membranes using 2 (86.1 Ci/mmol,1.7nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50001110 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 from mammalian clones expressed in CHO cell membranes using 2 (86.1 Ci/mmol,1.7nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50001119 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards Alpha-1 adrenergic receptor from rat whole brain using [3H]prazosin (18.1 Ci/mmol,0.9nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Gorilla gorilla gorilla) | BDBM50005835 ((3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methy...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description The compound was tested for CNS binding affinity towards 5-hydroxytryptamine 1B receptor from cloned gorilla membranes expressed in cultured HEK 293 ... | J Med Chem 41: 2180-3 (1998) Article DOI: 10.1021/jm980137o BindingDB Entry DOI: 10.7270/Q2NV9HCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM50001114 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Inhibition of human erythrocyte carbonic anhydrase II | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50051562 (1-(2-Isochroman-1-yl-ethyl)-4-(4-methoxy-phenyl)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description The compound was tested for CNS binding affinity towards 5-hydroxytryptamine 1D receptor from cloned gorilla membranes expressed in cultured HEK 293 ... | J Med Chem 41: 2180-3 (1998) Article DOI: 10.1021/jm980137o BindingDB Entry DOI: 10.7270/Q2NV9HCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50001116 (8-[4-(2-Oxo-2,3-dihydro-benzoimidazol-1-yl)-piperi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 from mammalian clones expressed in CHO cell membranes using 2 (86.1 Ci/mmol,1.7nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (RAT) | BDBM50051565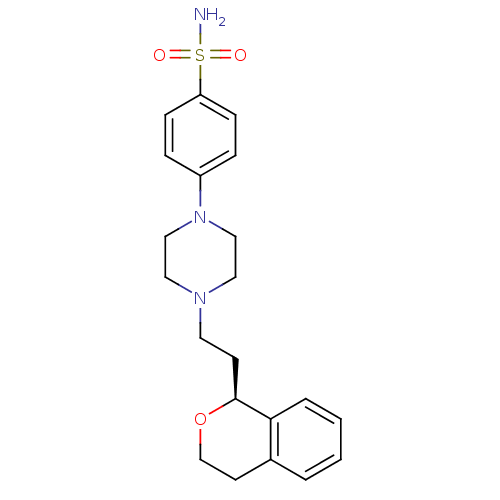 (4-[4-((S)-2-Isochroman-1-yl-ethyl)-piperazin-1-yl]...) | Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity of [3H]-Spiperone towards Dopamine receptor D4 expressed in cultured cells or from rat whole brain. | J Med Chem 39: 2435-7 (1996) Article DOI: 10.1021/jm960084f BindingDB Entry DOI: 10.7270/Q2H1314Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50249878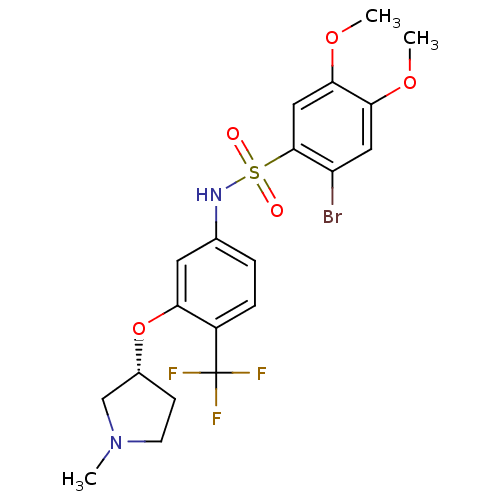 ((R)-2-bromo-4,5-dimethoxy-N-(3-(1-methylpyrrolidin...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]urotensin 2 from urotensin 2 receptor in human RMS13 cells by scintillation proximity assay | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50302271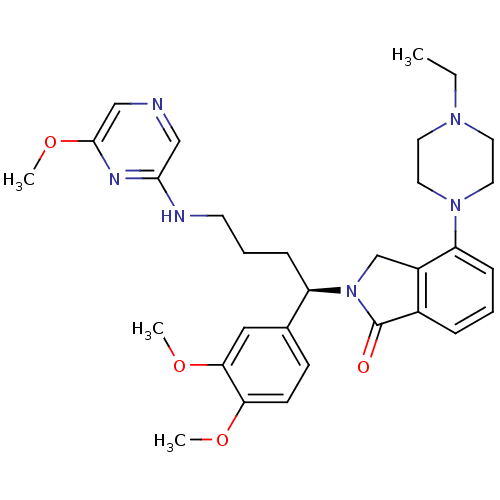 ((R)-2-(1-(3,4-dimethoxyphenyl)-4-(6-methoxypyrazin...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]urotensin 2 from urotensin 2 receptor in human RMS13 cells by scintillation proximity assay | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50064785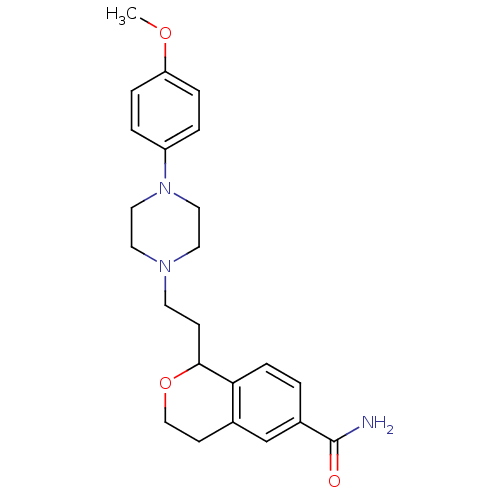 (1-{2-[4-(4-Methoxy-phenyl)-piperazin-1-yl]-ethyl}-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description The compound was tested for CNS binding affinity towards 5-hydroxytryptamine 1D receptor from cloned gorilla membranes expressed in cultured HEK 293 ... | J Med Chem 41: 2180-3 (1998) Article DOI: 10.1021/jm980137o BindingDB Entry DOI: 10.7270/Q2NV9HCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50001112 (8-(2-Hydroxymethyl-7,8-dihydro-3H-6,9-dioxa-3-aza-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards Alpha-1 adrenergic receptor from rat whole brain using [3H]prazosin (18.1 Ci/mmol,0.9nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (RAT) | BDBM50001884 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...) | Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Binding affinity of [3H]-spiperone towards cloned mammalian Dopamine receptor D4 expressed in cultured cells or from rat whole brain | J Med Chem 39: 2435-7 (1996) Article DOI: 10.1021/jm960084f BindingDB Entry DOI: 10.7270/Q2H1314Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50001114 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity towards Alpha-1 adrenergic receptor from rat whole brain using [3H]prazosin as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50001115 (8-[(3-Phenyl-propylamino)-methyl]-7,8-dihydro-3H-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards Alpha-1 adrenergic receptor from rat whole brain using [3H]prazosin (18.1 Ci/mmol,0.9nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50302267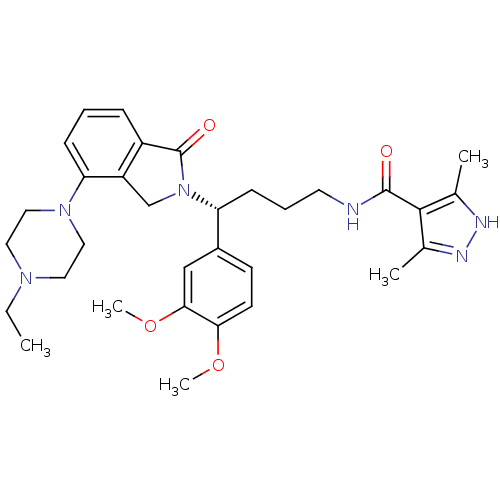 ((R)-N-(4-(3,4-dimethoxyphenyl)-4-(4-(4-ethylpipera...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]urotensin 2 from urotensin 2 receptor in human RMS13 cells by scintillation proximity assay | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50302264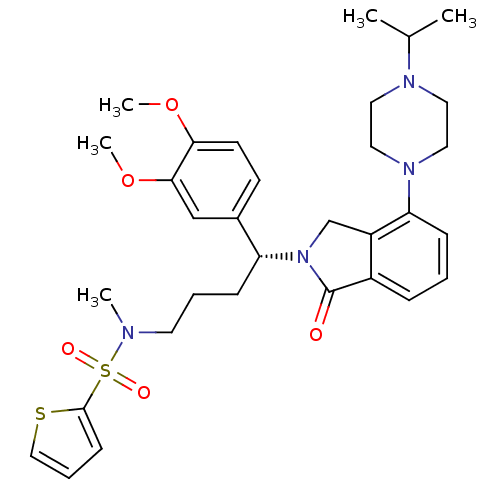 ((R)-N-(4-(3,4-dimethoxyphenyl)-4-(4-(4-isopropylpi...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]urotensin 2 from urotensin 2 receptor in human RMS13 cells by scintillation proximity assay | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 395 total ) | Next | Last >> |