

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50255365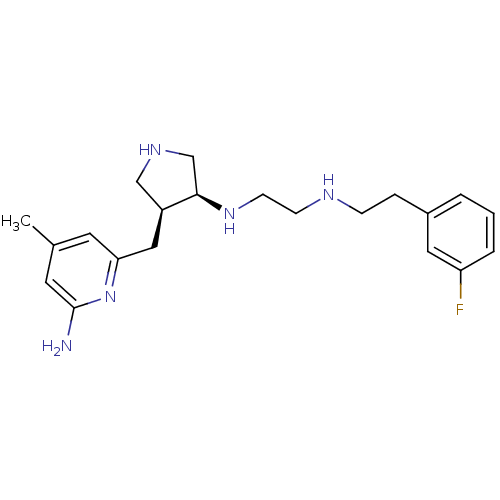 ((+/-)-cis-N1-((3S,4S)-4-((6-amino-4-methylpyridin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of rat recombinant nNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50278675 ((+/-)-cis-6-((4-(2-(3-Fluorophenethylamino)-ethoxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of rat recombinant nNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50278676 ((+/-)-cis-N-(4-((6-Amino-4-methylpyridin-2-yl)-met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of rat recombinant nNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM29234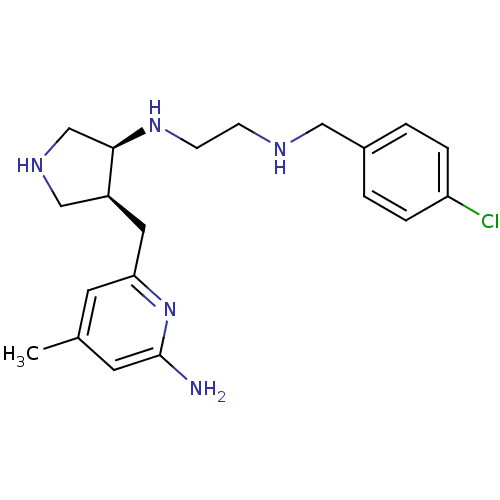 (CHEMBL481815 | US9090589, 6 | aminopyridine-pyrrol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 85 | -41.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California at Irvine | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | J Med Chem 52: 2060-6 (2009) Article DOI: 10.1021/jm900007a BindingDB Entry DOI: 10.7270/Q2HX1B1J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM21961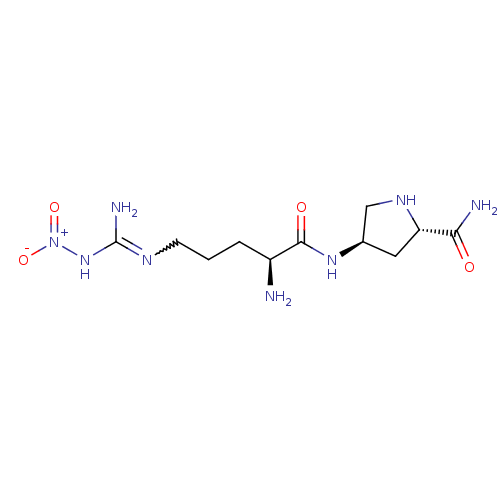 ((2S,4R)-4-[(2S)-2-amino-5-(1-nitrocarbamimidamido)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 100 | -40.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California at Irvine | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | J Med Chem 52: 2060-6 (2009) Article DOI: 10.1021/jm900007a BindingDB Entry DOI: 10.7270/Q2HX1B1J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM21960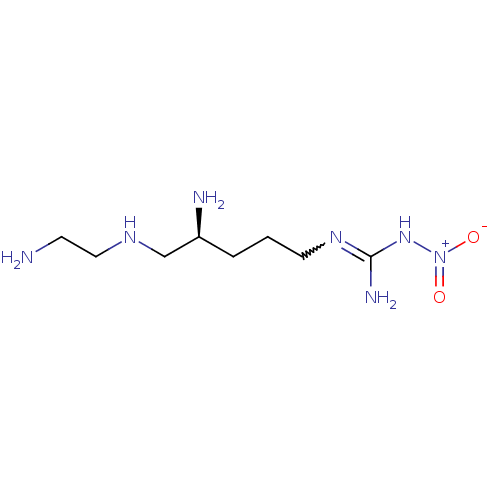 (3-[(4S)-4-amino-5-[(2-aminoethyl)amino]pentyl]-1-n...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 150 | -39.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California at Irvine | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | J Med Chem 52: 2060-6 (2009) Article DOI: 10.1021/jm900007a BindingDB Entry DOI: 10.7270/Q2HX1B1J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM29235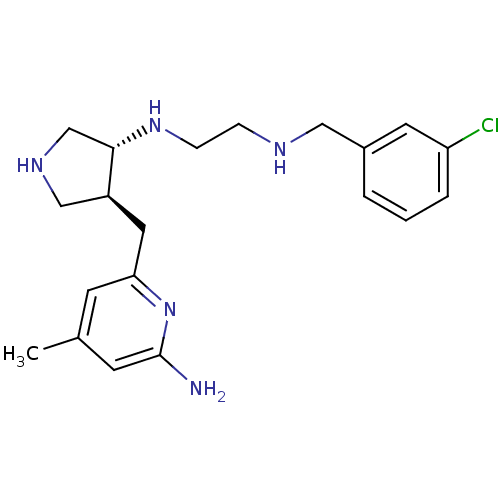 (aminopyridine-pyrrolidine, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 250 | -38.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California at Irvine | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | J Med Chem 52: 2060-6 (2009) Article DOI: 10.1021/jm900007a BindingDB Entry DOI: 10.7270/Q2HX1B1J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM22030 ((2S)-2-amino-N-[(1S)-3-amino-1-(aminocarbonyl)prop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 300 | -37.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California at Irvine | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | J Med Chem 52: 2060-6 (2009) Article DOI: 10.1021/jm900007a BindingDB Entry DOI: 10.7270/Q2HX1B1J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM29233 (CHEMBL508014 | aminopyridine-pyrrolidine, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 388 | -37.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California at Irvine | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | J Med Chem 52: 2060-6 (2009) Article DOI: 10.1021/jm900007a BindingDB Entry DOI: 10.7270/Q2HX1B1J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50278744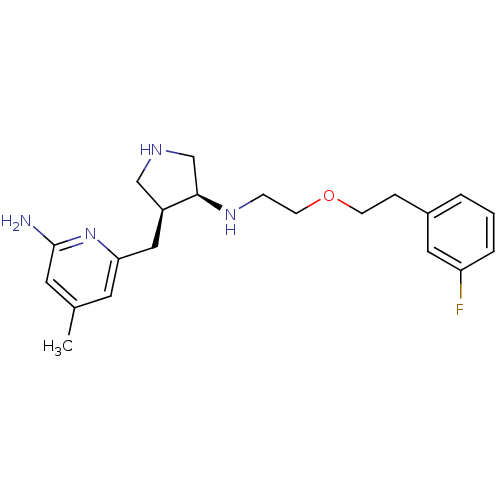 ((+/-)-cis-6-((4-(2-(3-Fluorophenethoxy)ethylamino)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of rat recombinant nNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain [D597N,M336V] (Rattus norvegicus (rat)) | BDBM29234 (CHEMBL481815 | US9090589, 6 | aminopyridine-pyrrol...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.20E+3 | -34.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California at Irvine | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | J Med Chem 52: 2060-6 (2009) Article DOI: 10.1021/jm900007a BindingDB Entry DOI: 10.7270/Q2HX1B1J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50278745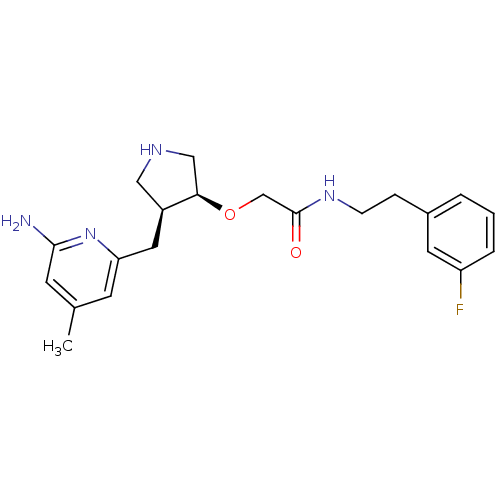 ((+/-)-cis-2-(4-((6-Amino-4-methylpyridin-2-yl)-met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of rat recombinant nNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50278744 ((+/-)-cis-6-((4-(2-(3-Fluorophenethoxy)ethylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant iNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50255365 ((+/-)-cis-N1-((3S,4S)-4-((6-amino-4-methylpyridin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant iNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50278746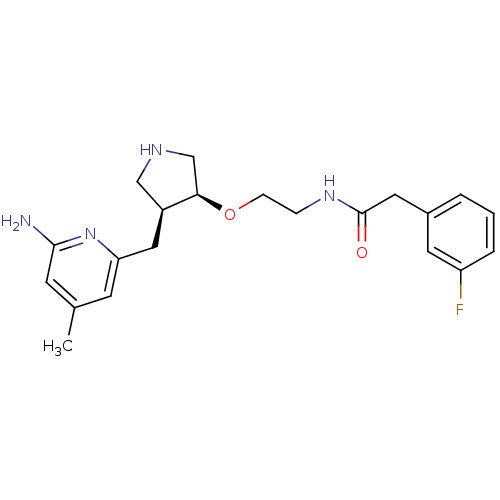 ((+/-)-cis-N-(2-(4-((6-Amino-4-methylpyridin-2-yl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of rat recombinant nNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50278676 ((+/-)-cis-N-(4-((6-Amino-4-methylpyridin-2-yl)-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant iNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain [D597N,M336V] (Rattus norvegicus (rat)) | BDBM29235 (aminopyridine-pyrrolidine, 7) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 6.10E+3 | -30.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California at Irvine | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | J Med Chem 52: 2060-6 (2009) Article DOI: 10.1021/jm900007a BindingDB Entry DOI: 10.7270/Q2HX1B1J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM29232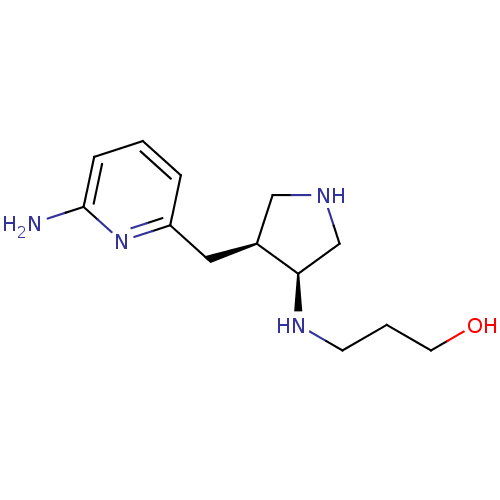 (3-({(3S,4S)-4-[(6-aminopyridin-2-yl)methyl]pyrroli...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 9.40E+3 | -29.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California at Irvine | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | J Med Chem 52: 2060-6 (2009) Article DOI: 10.1021/jm900007a BindingDB Entry DOI: 10.7270/Q2HX1B1J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50278675 ((+/-)-cis-6-((4-(2-(3-Fluorophenethylamino)-ethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant iNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50278676 ((+/-)-cis-N-(4-((6-Amino-4-methylpyridin-2-yl)-met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of bovine recombinant eNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50255365 ((+/-)-cis-N1-((3S,4S)-4-((6-amino-4-methylpyridin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of bovine recombinant eNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50278675 ((+/-)-cis-6-((4-(2-(3-Fluorophenethylamino)-ethoxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of bovine recombinant eNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50278744 ((+/-)-cis-6-((4-(2-(3-Fluorophenethoxy)ethylamino)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of bovine recombinant eNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain [D597N,M336V] (Rattus norvegicus (rat)) | BDBM29233 (CHEMBL508014 | aminopyridine-pyrrolidine, 5) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 3.67E+4 | -25.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California at Irvine | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | J Med Chem 52: 2060-6 (2009) Article DOI: 10.1021/jm900007a BindingDB Entry DOI: 10.7270/Q2HX1B1J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain [D597N,M336V] (Rattus norvegicus (rat)) | BDBM29232 (3-({(3S,4S)-4-[(6-aminopyridin-2-yl)methyl]pyrroli...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 4.92E+4 | -25.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California at Irvine | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | J Med Chem 52: 2060-6 (2009) Article DOI: 10.1021/jm900007a BindingDB Entry DOI: 10.7270/Q2HX1B1J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50278745 ((+/-)-cis-2-(4-((6-Amino-4-methylpyridin-2-yl)-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant iNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50278746 ((+/-)-cis-N-(2-(4-((6-Amino-4-methylpyridin-2-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant iNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM21960 (3-[(4S)-4-amino-5-[(2-aminoethyl)amino]pentyl]-1-n...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 8.00E+4 | -23.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California at Irvine | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | J Med Chem 52: 2060-6 (2009) Article DOI: 10.1021/jm900007a BindingDB Entry DOI: 10.7270/Q2HX1B1J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM29234 (CHEMBL481815 | US9090589, 6 | aminopyridine-pyrrol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 8.52E+4 | -23.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California at Irvine | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | J Med Chem 52: 2060-6 (2009) Article DOI: 10.1021/jm900007a BindingDB Entry DOI: 10.7270/Q2HX1B1J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM29235 (aminopyridine-pyrrolidine, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 9.52E+4 | -23.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California at Irvine | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | J Med Chem 52: 2060-6 (2009) Article DOI: 10.1021/jm900007a BindingDB Entry DOI: 10.7270/Q2HX1B1J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM22030 ((2S)-2-amino-N-[(1S)-3-amino-1-(aminocarbonyl)prop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.07E+5 | -23.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California at Irvine | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | J Med Chem 52: 2060-6 (2009) Article DOI: 10.1021/jm900007a BindingDB Entry DOI: 10.7270/Q2HX1B1J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50278746 ((+/-)-cis-N-(2-(4-((6-Amino-4-methylpyridin-2-yl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.07E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of bovine recombinant eNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM21961 ((2S,4R)-4-[(2S)-2-amino-5-(1-nitrocarbamimidamido)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.10E+5 | -23.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California at Irvine | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | J Med Chem 52: 2060-6 (2009) Article DOI: 10.1021/jm900007a BindingDB Entry DOI: 10.7270/Q2HX1B1J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50278745 ((+/-)-cis-2-(4-((6-Amino-4-methylpyridin-2-yl)-met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of bovine recombinant eNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assay | Bioorg Med Chem 17: 2371-80 (2009) Article DOI: 10.1016/j.bmc.2009.02.017 BindingDB Entry DOI: 10.7270/Q2M32VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM29232 (3-({(3S,4S)-4-[(6-aminopyridin-2-yl)methyl]pyrroli...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 3.67E+5 | -19.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California at Irvine | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | J Med Chem 52: 2060-6 (2009) Article DOI: 10.1021/jm900007a BindingDB Entry DOI: 10.7270/Q2HX1B1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM29233 (CHEMBL508014 | aminopyridine-pyrrolidine, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 4.17E+5 | -19.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California at Irvine | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | J Med Chem 52: 2060-6 (2009) Article DOI: 10.1021/jm900007a BindingDB Entry DOI: 10.7270/Q2HX1B1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase/Collagenase 3/Interstitial collagenase/Matrix metalloproteinase-9/Neutrophil collagenase (Homo sapiens (Human)) | BDBM50290085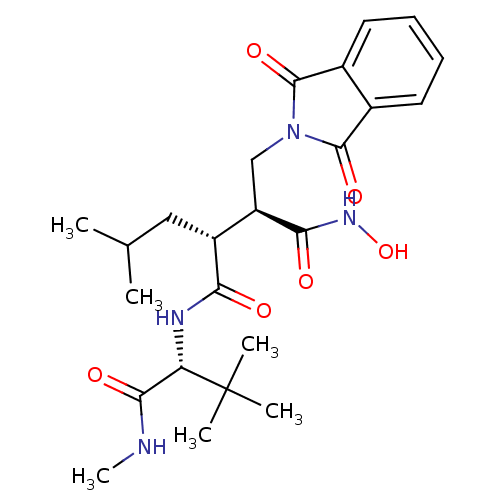 ((2S,3R)-N*4*-((R)-2,2-Dimethyl-1-methylcarbamoyl-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluation for functional inhibitory potency prevention of ET-1 induced constriction of rat aortic rings (ETA receptors) | Bioorg Med Chem Lett 7: 2299-2302 (1997) Article DOI: 10.1016/S0960-894X(97)00416-2 BindingDB Entry DOI: 10.7270/Q26T0MM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase/Collagenase 3/Interstitial collagenase/Matrix metalloproteinase-9/Neutrophil collagenase (Homo sapiens (Human)) | BDBM50290088 ((2S,3R)-N*1*-((R)-2,2-Dimethyl-1-methylcarbamoyl-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluation for functional inhibitory potency prevention of ET-1 induced constriction of rat aortic rings (ETA receptors) | Bioorg Med Chem Lett 7: 2299-2302 (1997) Article DOI: 10.1016/S0960-894X(97)00416-2 BindingDB Entry DOI: 10.7270/Q26T0MM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50290089 ((2R,3R)-N*1*-[2,2-Dimethyl-1-((R)-methylcarbamoyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human gelatinase B, MMP9 | Bioorg Med Chem Lett 7: 2299-2302 (1997) Article DOI: 10.1016/S0960-894X(97)00416-2 BindingDB Entry DOI: 10.7270/Q26T0MM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase/Collagenase 3/Interstitial collagenase/Matrix metalloproteinase-9/Neutrophil collagenase (Homo sapiens (Human)) | BDBM50290089 ((2R,3R)-N*1*-[2,2-Dimethyl-1-((R)-methylcarbamoyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluation for functional inhibitory potency prevention of ET-1 induced constriction of rat aortic rings (ETA receptors) | Bioorg Med Chem Lett 7: 2299-2302 (1997) Article DOI: 10.1016/S0960-894X(97)00416-2 BindingDB Entry DOI: 10.7270/Q26T0MM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50290088 ((2S,3R)-N*1*-((R)-2,2-Dimethyl-1-methylcarbamoyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human gelatinase B, MMP9 | Bioorg Med Chem Lett 7: 2299-2302 (1997) Article DOI: 10.1016/S0960-894X(97)00416-2 BindingDB Entry DOI: 10.7270/Q26T0MM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Rattus norvegicus (Rat)) | BDBM50282632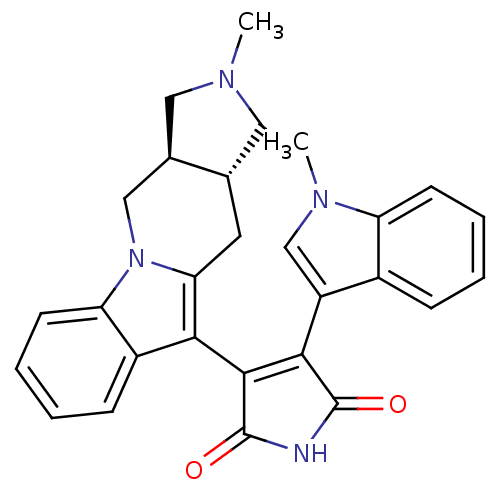 (3-((3aR,10aR)-2-Methyl-2,3,3a,4,10,10a-hexahydro-1...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.85 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Protein kinase C alpha | Bioorg Med Chem Lett 4: 1303-1308 (1994) Article DOI: 10.1016/S0960-894X(01)80349-8 BindingDB Entry DOI: 10.7270/Q27H1JJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2714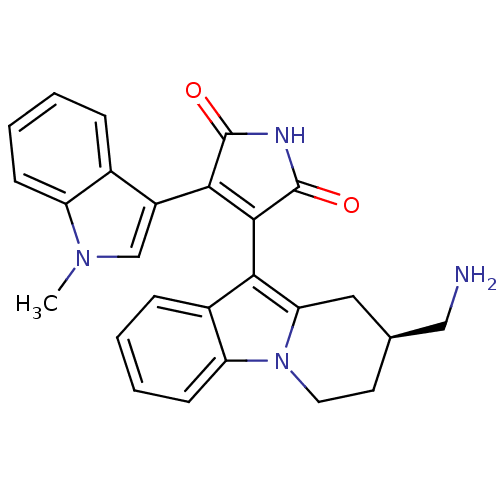 (3-[(2S)-2-(aminomethyl)-1H,2H,3H,4H-pyrido[1,2-a]i...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 36: 21-9 (1993) Article DOI: 10.1021/jm00053a003 BindingDB Entry DOI: 10.7270/Q29P2ZTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50290085 ((2S,3R)-N*4*-((R)-2,2-Dimethyl-1-methylcarbamoyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human gelatinase B, MMP9 | Bioorg Med Chem Lett 7: 2299-2302 (1997) Article DOI: 10.1016/S0960-894X(97)00416-2 BindingDB Entry DOI: 10.7270/Q26T0MM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase/Collagenase 3/Interstitial collagenase/Matrix metalloproteinase-9/Neutrophil collagenase (Homo sapiens (Human)) | BDBM50290086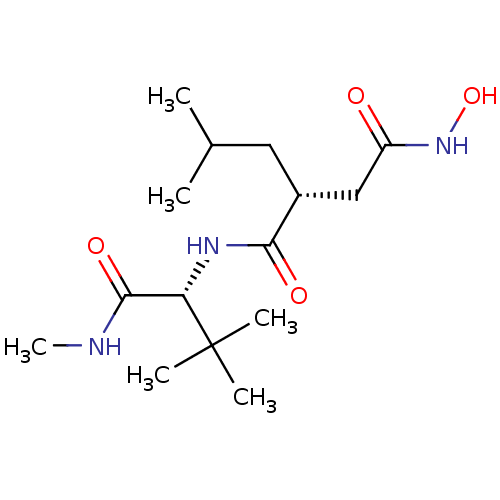 ((R)-N*1*-[2,2-Dimethyl-1-((R)-methylcarbamoyl)-pro...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluation for functional inhibitory potency prevention of ET-1 induced constriction of rat aortic rings (ETA receptors) | Bioorg Med Chem Lett 7: 2299-2302 (1997) Article DOI: 10.1016/S0960-894X(97)00416-2 BindingDB Entry DOI: 10.7270/Q26T0MM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM50282632 (3-((3aR,10aR)-2-Methyl-2,3,3a,4,10,10a-hexahydro-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Protein kinase C beta 1 | Bioorg Med Chem Lett 4: 1303-1308 (1994) Article DOI: 10.1016/S0960-894X(01)80349-8 BindingDB Entry DOI: 10.7270/Q27H1JJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C gamma type (Rattus norvegicus) | BDBM50282632 (3-((3aR,10aR)-2-Methyl-2,3,3a,4,10,10a-hexahydro-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Protein kinase C gamma (PKC) | Bioorg Med Chem Lett 4: 1303-1308 (1994) Article DOI: 10.1016/S0960-894X(01)80349-8 BindingDB Entry DOI: 10.7270/Q27H1JJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM50282632 (3-((3aR,10aR)-2-Methyl-2,3,3a,4,10,10a-hexahydro-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Protein kinase C beta | Bioorg Med Chem Lett 4: 1303-1308 (1994) Article DOI: 10.1016/S0960-894X(01)80349-8 BindingDB Entry DOI: 10.7270/Q27H1JJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase/Collagenase 3/Interstitial collagenase/Matrix metalloproteinase-9/Neutrophil collagenase (Homo sapiens (Human)) | BDBM50290090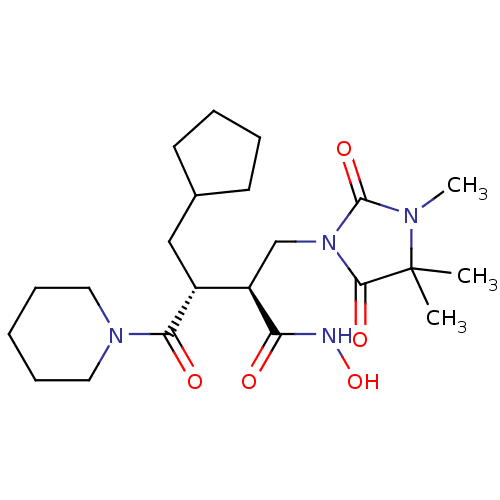 ((2S,3R)-3-Cyclopentylmethyl-N-hydroxy-4-oxo-4-pipe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluation for functional inhibitory potency prevention of ET-1 induced constriction of rat aortic rings (ETA receptors) | Bioorg Med Chem Lett 7: 2299-2302 (1997) Article DOI: 10.1016/S0960-894X(97)00416-2 BindingDB Entry DOI: 10.7270/Q26T0MM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM2700 (3-[2-(aminomethyl)-1H,2H,3H,4H-pyrido[1,2-a]indol-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Roche Products Limited | Assay Description The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his... | J Med Chem 36: 21-9 (1993) Article DOI: 10.1021/jm00053a003 BindingDB Entry DOI: 10.7270/Q29P2ZTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 274 total ) | Next | Last >> |