

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM222178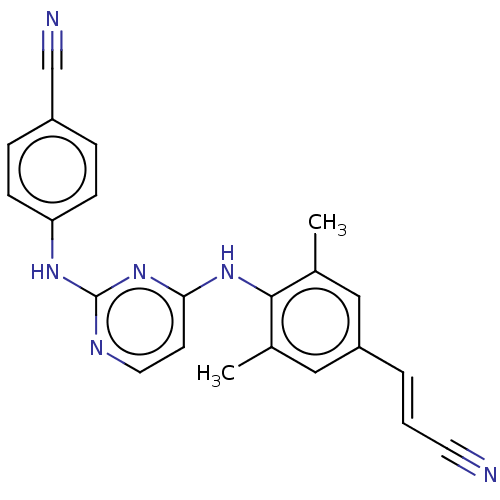 (Rilpivirine) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase p66/p51 Y181C mutant expressed in Escherichia coli BL21 (DE3) pLysS cells preincubated followed ... | Bioorg Med Chem Lett 29: 2182-2188 (2019) Article DOI: 10.1016/j.bmcl.2019.06.047 BindingDB Entry DOI: 10.7270/Q2BR8WKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50134209 (CHEMBL3342974) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of wild-type HIV-1 reverse transcriptase by fluorescence assay | ACS Med Chem Lett 6: 1075-9 (2015) Article DOI: 10.1021/acsmedchemlett.5b00254 BindingDB Entry DOI: 10.7270/Q2M0479N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50386567 (CHEMBL2048437) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced IL8 release pretreated for 30 mins before bzAT... | J Med Chem 55: 3687-98 (2012) Article DOI: 10.1021/jm2012326 BindingDB Entry DOI: 10.7270/Q21G0NB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50134211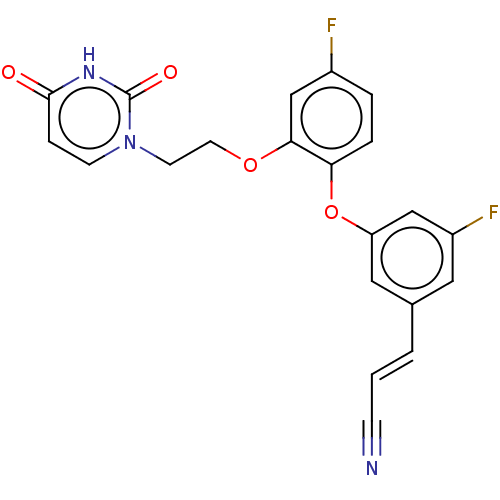 (CHEMBL1923492) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of wild-type HIV-1 reverse transcriptase by fluorescence assay | ACS Med Chem Lett 6: 1075-9 (2015) Article DOI: 10.1021/acsmedchemlett.5b00254 BindingDB Entry DOI: 10.7270/Q2M0479N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50134210 (CHEMBL3342966) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of wild-type HIV-1 reverse transcriptase by fluorescence assay | ACS Med Chem Lett 6: 1075-9 (2015) Article DOI: 10.1021/acsmedchemlett.5b00254 BindingDB Entry DOI: 10.7270/Q2M0479N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50501292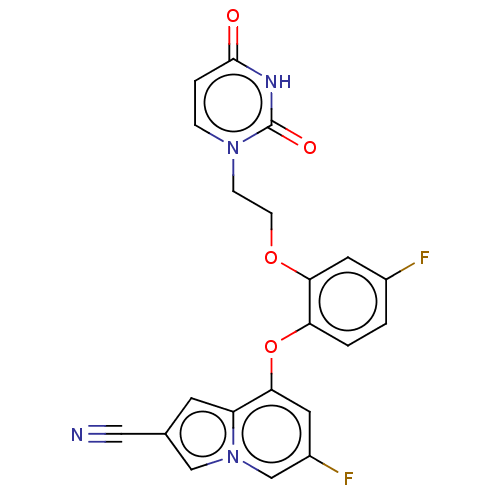 (CHEMBL3342978) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase p66/p51 expressed in Escherichia coli BL21 (DE3) pLysS cells preincubated followed by primer/tem... | Bioorg Med Chem Lett 29: 2182-2188 (2019) Article DOI: 10.1016/j.bmcl.2019.06.047 BindingDB Entry DOI: 10.7270/Q2BR8WKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50386567 (CHEMBL2048437) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced ethidium bromide uptake after 2 hrs by f... | J Med Chem 55: 3687-98 (2012) Article DOI: 10.1021/jm2012326 BindingDB Entry DOI: 10.7270/Q21G0NB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50501292 (CHEMBL3342978) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 reverse transcriptase p66/p51 Y181C mutant expressed in Escherichia coli BL21 (DE3) pLysS cells preincubated followed ... | Bioorg Med Chem Lett 29: 2182-2188 (2019) Article DOI: 10.1016/j.bmcl.2019.06.047 BindingDB Entry DOI: 10.7270/Q2BR8WKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50386566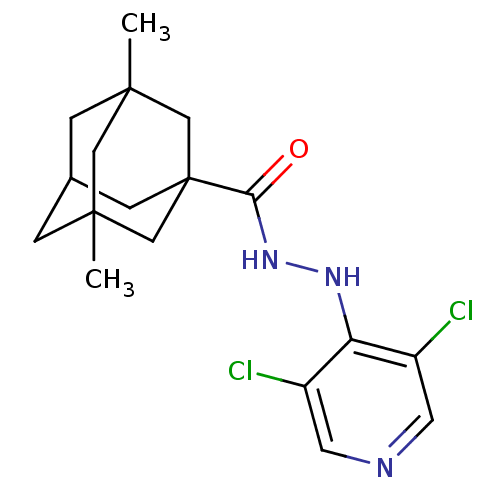 (CHEMBL2048438) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced IL8 release pretreated for 30 mins before bzAT... | J Med Chem 55: 3687-98 (2012) Article DOI: 10.1021/jm2012326 BindingDB Entry DOI: 10.7270/Q21G0NB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM152604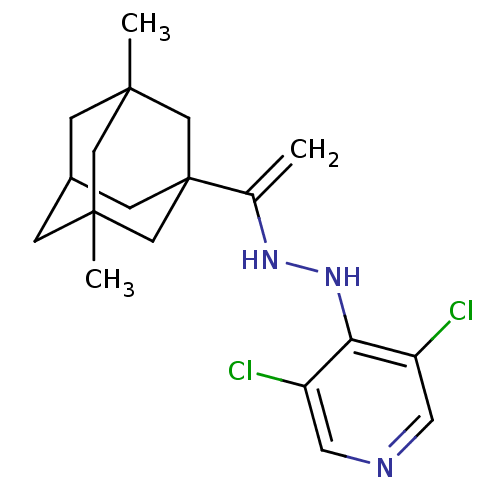 (1-[4-(2,6-dimethylphenyl)but-1-en-2-yl]-3,5-dimeth...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gwangju Institute of Science and Technology | Assay Description hP2X7-expressing HEK 293 cells were re-suspended at 2.5 × 10^6 cells/mL in assay buffer composed of 10 mM HEPES, 5 mM N-methyl-D-glutamine, 5.6 mM KC... | Bioorg Chem 61: 58-65 (2015) Article DOI: 10.1016/j.bioorg.2015.06.003 BindingDB Entry DOI: 10.7270/Q2833QRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50386566 (CHEMBL2048438) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced ethidium bromide uptake after 2 hrs by f... | J Med Chem 55: 3687-98 (2012) Article DOI: 10.1021/jm2012326 BindingDB Entry DOI: 10.7270/Q21G0NB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM222178 (Rilpivirine) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of wild-type HIV-1 reverse transcriptase by fluorescence assay | ACS Med Chem Lett 6: 1075-9 (2015) Article DOI: 10.1021/acsmedchemlett.5b00254 BindingDB Entry DOI: 10.7270/Q2M0479N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50386572 (CHEMBL2048286) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced IL8 release pretreated for 30 mins before bzAT... | J Med Chem 55: 3687-98 (2012) Article DOI: 10.1021/jm2012326 BindingDB Entry DOI: 10.7270/Q21G0NB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50386573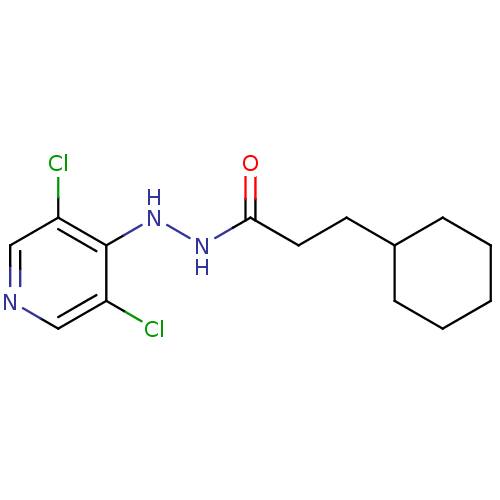 (CHEMBL2048285) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced IL8 release pretreated for 30 mins before bzAT... | J Med Chem 55: 3687-98 (2012) Article DOI: 10.1021/jm2012326 BindingDB Entry DOI: 10.7270/Q21G0NB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50386568 (CHEMBL2048436) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced ethidium bromide uptake after 2 hrs by f... | J Med Chem 55: 3687-98 (2012) Article DOI: 10.1021/jm2012326 BindingDB Entry DOI: 10.7270/Q21G0NB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50087267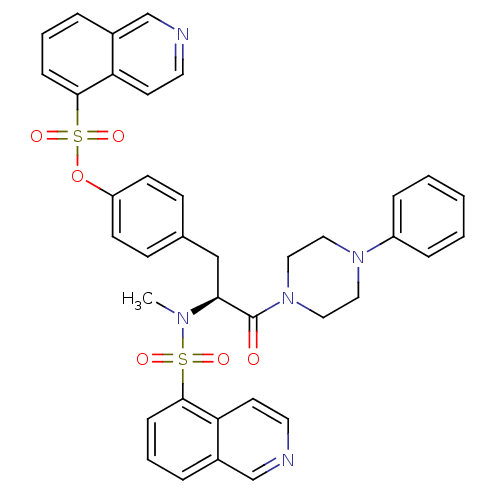 ((1-(N,O-bis(1,5-isoquinolinesulfonyl)-N-methyl-L-t...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced IL8 release pretreated for 30 mins before bzAT... | J Med Chem 55: 3687-98 (2012) Article DOI: 10.1021/jm2012326 BindingDB Entry DOI: 10.7270/Q21G0NB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50485810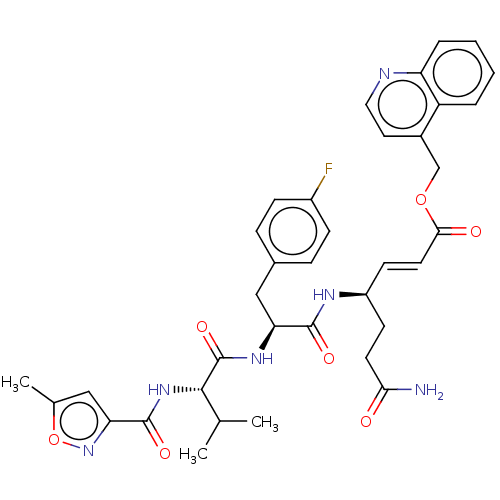 (CHEMBL2164935) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of School of Life Sciences Curated by ChEMBL | Assay Description Inhibition of Coxsackievirus B3 protease 3c using NMA-EALFQGPPVK-DNP as substrate incubated for 5 mins prior to substrate addition measured after 1 h... | Bioorg Med Chem Lett 22: 6952-6 (2012) Article DOI: 10.1016/j.bmcl.2012.08.120 BindingDB Entry DOI: 10.7270/Q2MS3WM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50386568 (CHEMBL2048436) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced IL8 release pretreated for 30 mins before bzAT... | J Med Chem 55: 3687-98 (2012) Article DOI: 10.1021/jm2012326 BindingDB Entry DOI: 10.7270/Q21G0NB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50386573 (CHEMBL2048285) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced ethidium bromide uptake after 2 hrs by f... | J Med Chem 55: 3687-98 (2012) Article DOI: 10.1021/jm2012326 BindingDB Entry DOI: 10.7270/Q21G0NB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50087267 ((1-(N,O-bis(1,5-isoquinolinesulfonyl)-N-methyl-L-t...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gwangju Institute of Science and Technology | Assay Description hP2X7-expressing HEK 293 cells were re-suspended at 2.5 × 10^6 cells/mL in assay buffer composed of 10 mM HEPES, 5 mM N-methyl-D-glutamine, 5.6 mM KC... | Bioorg Chem 61: 58-65 (2015) Article DOI: 10.1016/j.bioorg.2015.06.003 BindingDB Entry DOI: 10.7270/Q2833QRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50485817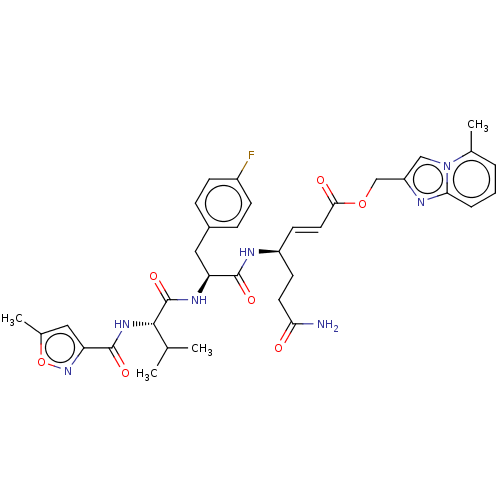 (CHEMBL2164929) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of School of Life Sciences Curated by ChEMBL | Assay Description Inhibition of Coxsackievirus B3 protease 3c using NMA-EALFQGPPVK-DNP as substrate incubated for 5 mins prior to substrate addition measured after 1 h... | Bioorg Med Chem Lett 22: 6952-6 (2012) Article DOI: 10.1016/j.bmcl.2012.08.120 BindingDB Entry DOI: 10.7270/Q2MS3WM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50485807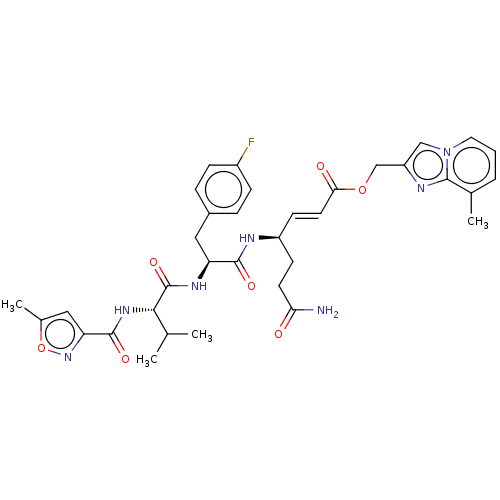 (CHEMBL2164928) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of School of Life Sciences Curated by ChEMBL | Assay Description Inhibition of Coxsackievirus B3 protease 3c using NMA-EALFQGPPVK-DNP as substrate incubated for 5 mins prior to substrate addition measured after 1 h... | Bioorg Med Chem Lett 22: 6952-6 (2012) Article DOI: 10.1016/j.bmcl.2012.08.120 BindingDB Entry DOI: 10.7270/Q2MS3WM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM152611 (N'-(2-Chloro-7H-purin-6-yl)-3,5-dimethyladaman...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 176 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gwangju Institute of Science and Technology | Assay Description hP2X7-expressing HEK 293 cells were re-suspended at 2.5 × 10^6 cells/mL in assay buffer composed of 10 mM HEPES, 5 mM N-methyl-D-glutamine, 5.6 mM KC... | Bioorg Chem 61: 58-65 (2015) Article DOI: 10.1016/j.bioorg.2015.06.003 BindingDB Entry DOI: 10.7270/Q2833QRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50485814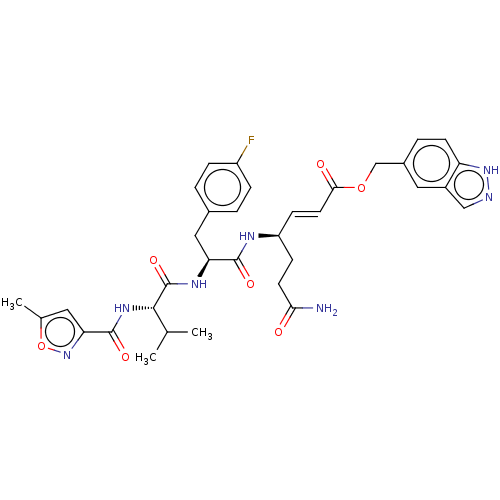 (CHEMBL2164927) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of School of Life Sciences Curated by ChEMBL | Assay Description Inhibition of Coxsackievirus B3 protease 3c using NMA-EALFQGPPVK-DNP as substrate incubated for 5 mins prior to substrate addition measured after 1 h... | Bioorg Med Chem Lett 22: 6952-6 (2012) Article DOI: 10.1016/j.bmcl.2012.08.120 BindingDB Entry DOI: 10.7270/Q2MS3WM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50386572 (CHEMBL2048286) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced ethidium bromide uptake after 2 hrs by f... | J Med Chem 55: 3687-98 (2012) Article DOI: 10.1021/jm2012326 BindingDB Entry DOI: 10.7270/Q21G0NB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50324675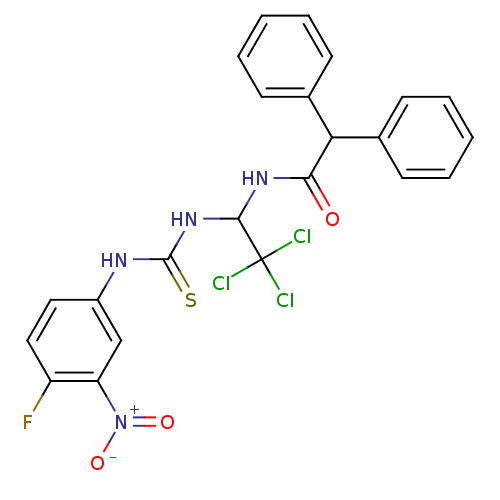 (2,2-diphenyl-N-(2,2,2-trichloro-1-(3-(4-fluoro-3-n...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Advanced Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of ataxia telangiectasia-mutated | Nat Chem Biol 2: 369-74 (2006) Article DOI: 10.1038/nchembio800 BindingDB Entry DOI: 10.7270/Q2Q52PTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50324675 (2,2-diphenyl-N-(2,2,2-trichloro-1-(3-(4-fluoro-3-n...) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Advanced Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of Rad3-related (ATR) protein-mediated p53 phosphorylation | Nat Chem Biol 2: 369-74 (2006) Article DOI: 10.1038/nchembio800 BindingDB Entry DOI: 10.7270/Q2Q52PTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50386563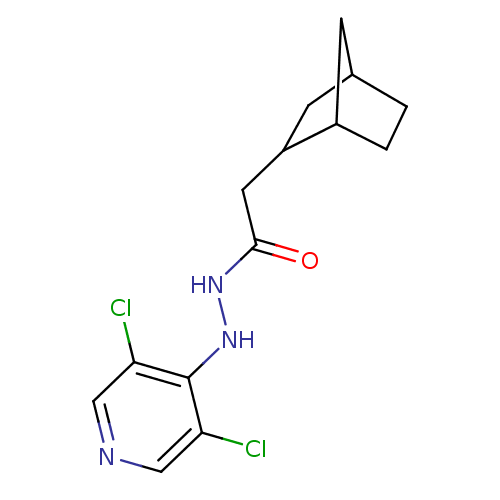 (CHEMBL2048441) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced ethidium bromide uptake after 2 hrs by f... | J Med Chem 55: 3687-98 (2012) Article DOI: 10.1021/jm2012326 BindingDB Entry DOI: 10.7270/Q21G0NB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50485811 (CHEMBL2164932) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of School of Life Sciences Curated by ChEMBL | Assay Description Inhibition of Coxsackievirus B3 protease 3c using NMA-EALFQGPPVK-DNP as substrate incubated for 5 mins prior to substrate addition measured after 1 h... | Bioorg Med Chem Lett 22: 6952-6 (2012) Article DOI: 10.1016/j.bmcl.2012.08.120 BindingDB Entry DOI: 10.7270/Q2MS3WM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50485808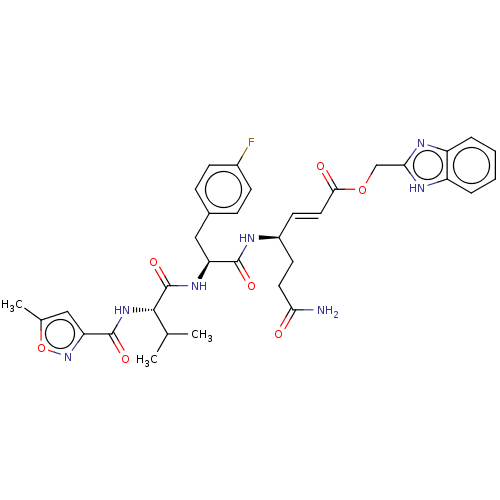 (CHEMBL2164937) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of School of Life Sciences Curated by ChEMBL | Assay Description Inhibition of Coxsackievirus B3 protease 3c using NMA-EALFQGPPVK-DNP as substrate incubated for 5 mins prior to substrate addition measured after 1 h... | Bioorg Med Chem Lett 22: 6952-6 (2012) Article DOI: 10.1016/j.bmcl.2012.08.120 BindingDB Entry DOI: 10.7270/Q2MS3WM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50485815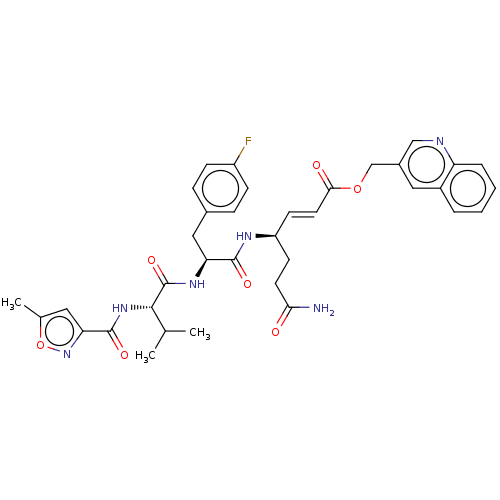 (CHEMBL2164933) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of School of Life Sciences Curated by ChEMBL | Assay Description Inhibition of Coxsackievirus B3 protease 3c using NMA-EALFQGPPVK-DNP as substrate incubated for 5 mins prior to substrate addition measured after 1 h... | Bioorg Med Chem Lett 22: 6952-6 (2012) Article DOI: 10.1016/j.bmcl.2012.08.120 BindingDB Entry DOI: 10.7270/Q2MS3WM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50485819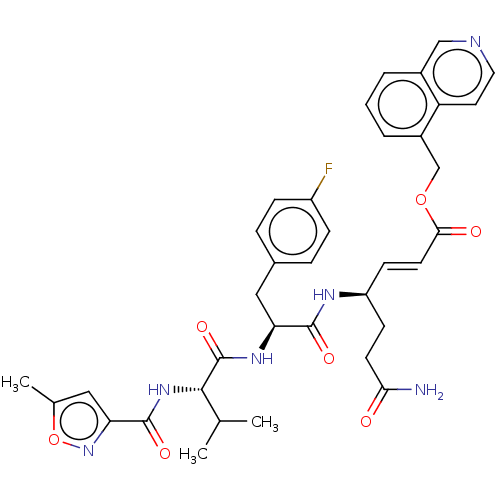 (CHEMBL2164934) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of School of Life Sciences Curated by ChEMBL | Assay Description Inhibition of Coxsackievirus B3 protease 3c using NMA-EALFQGPPVK-DNP as substrate incubated for 5 mins prior to substrate addition measured after 1 h... | Bioorg Med Chem Lett 22: 6952-6 (2012) Article DOI: 10.1016/j.bmcl.2012.08.120 BindingDB Entry DOI: 10.7270/Q2MS3WM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50087267 ((1-(N,O-bis(1,5-isoquinolinesulfonyl)-N-methyl-L-t...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced ethidium bromide uptake after 2 hrs by f... | J Med Chem 55: 3687-98 (2012) Article DOI: 10.1021/jm2012326 BindingDB Entry DOI: 10.7270/Q21G0NB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50485809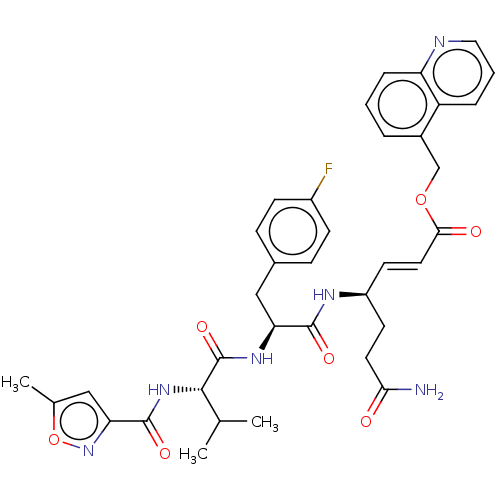 (CHEMBL2164936) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of School of Life Sciences Curated by ChEMBL | Assay Description Inhibition of Coxsackievirus B3 protease 3c using NMA-EALFQGPPVK-DNP as substrate incubated for 5 mins prior to substrate addition measured after 1 h... | Bioorg Med Chem Lett 22: 6952-6 (2012) Article DOI: 10.1016/j.bmcl.2012.08.120 BindingDB Entry DOI: 10.7270/Q2MS3WM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50386562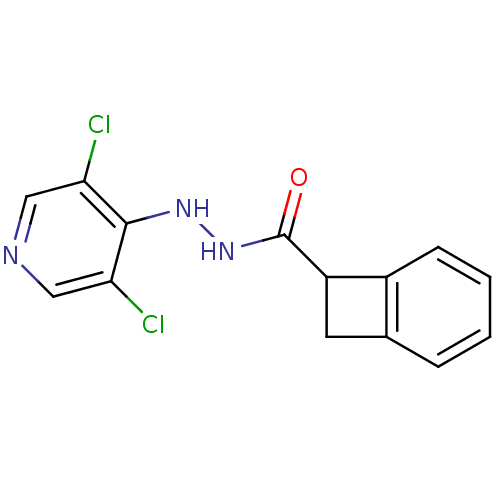 (CHEMBL2048279) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced ethidium bromide uptake after 2 hrs by f... | J Med Chem 55: 3687-98 (2012) Article DOI: 10.1021/jm2012326 BindingDB Entry DOI: 10.7270/Q21G0NB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50386592 (CHEMBL2048251) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced IL8 release pretreated for 30 mins before bzAT... | J Med Chem 55: 3687-98 (2012) Article DOI: 10.1021/jm2012326 BindingDB Entry DOI: 10.7270/Q21G0NB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50386569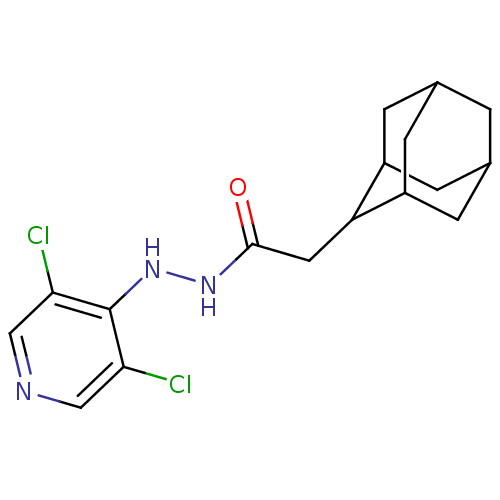 (CHEMBL2048435) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced ethidium bromide uptake after 2 hrs by f... | J Med Chem 55: 3687-98 (2012) Article DOI: 10.1021/jm2012326 BindingDB Entry DOI: 10.7270/Q21G0NB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50386570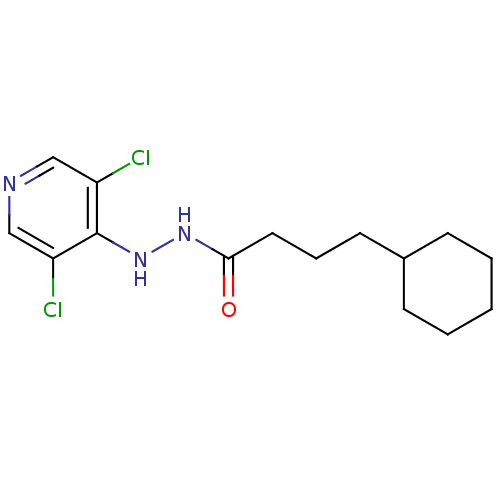 (CHEMBL2048434) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced ethidium bromide uptake after 2 hrs by f... | J Med Chem 55: 3687-98 (2012) Article DOI: 10.1021/jm2012326 BindingDB Entry DOI: 10.7270/Q21G0NB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50386578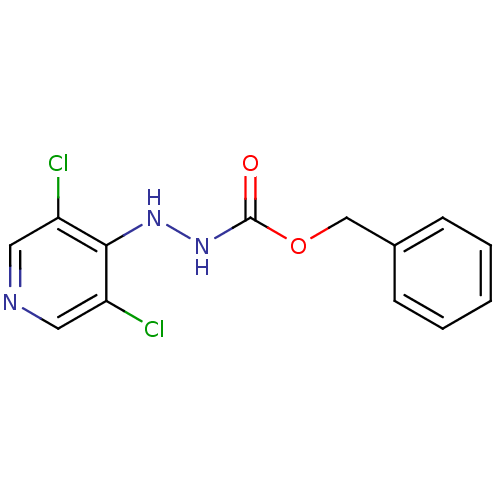 (CHEMBL2048274) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced ethidium bromide uptake after 2 hrs by f... | J Med Chem 55: 3687-98 (2012) Article DOI: 10.1021/jm2012326 BindingDB Entry DOI: 10.7270/Q21G0NB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50386574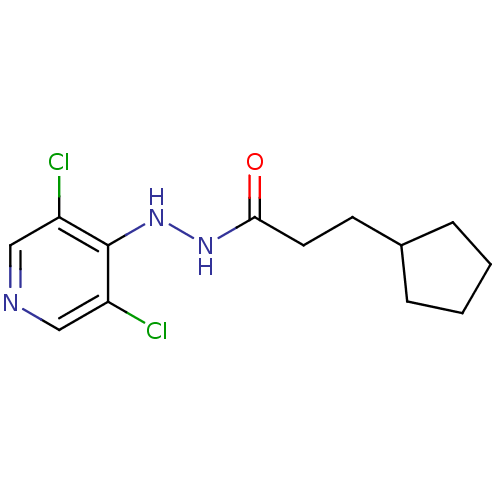 (CHEMBL2048284) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced ethidium bromide uptake after 2 hrs by f... | J Med Chem 55: 3687-98 (2012) Article DOI: 10.1021/jm2012326 BindingDB Entry DOI: 10.7270/Q21G0NB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50485820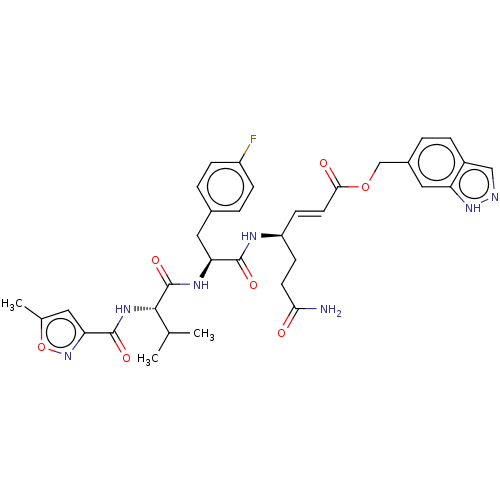 (CHEMBL2165220) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of School of Life Sciences Curated by ChEMBL | Assay Description Inhibition of Coxsackievirus B3 protease 3c using NMA-EALFQGPPVK-DNP as substrate incubated for 5 mins prior to substrate addition measured after 1 h... | Bioorg Med Chem Lett 22: 6952-6 (2012) Article DOI: 10.1016/j.bmcl.2012.08.120 BindingDB Entry DOI: 10.7270/Q2MS3WM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50485812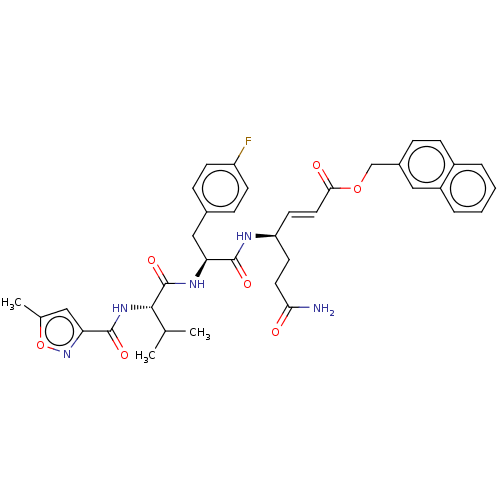 (CHEMBL2164931) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of School of Life Sciences Curated by ChEMBL | Assay Description Inhibition of Coxsackievirus B3 protease 3c using NMA-EALFQGPPVK-DNP as substrate incubated for 5 mins prior to substrate addition measured after 1 h... | Bioorg Med Chem Lett 22: 6952-6 (2012) Article DOI: 10.1016/j.bmcl.2012.08.120 BindingDB Entry DOI: 10.7270/Q2MS3WM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50485818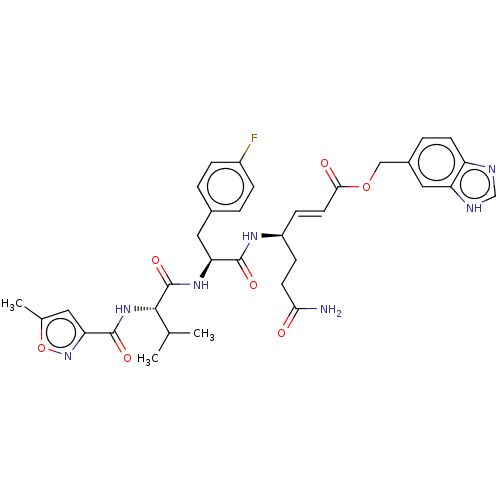 (CHEMBL2165219) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of School of Life Sciences Curated by ChEMBL | Assay Description Inhibition of Coxsackievirus B3 protease 3c using NMA-EALFQGPPVK-DNP as substrate incubated for 5 mins prior to substrate addition measured after 1 h... | Bioorg Med Chem Lett 22: 6952-6 (2012) Article DOI: 10.1016/j.bmcl.2012.08.120 BindingDB Entry DOI: 10.7270/Q2MS3WM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50386585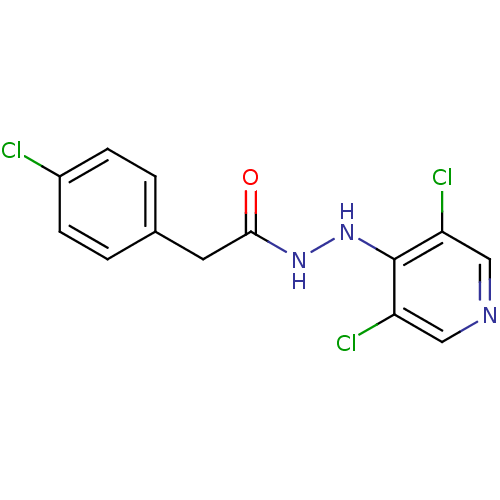 (CHEMBL2048265) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced ethidium bromide uptake after 2 hrs by f... | J Med Chem 55: 3687-98 (2012) Article DOI: 10.1021/jm2012326 BindingDB Entry DOI: 10.7270/Q21G0NB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50485816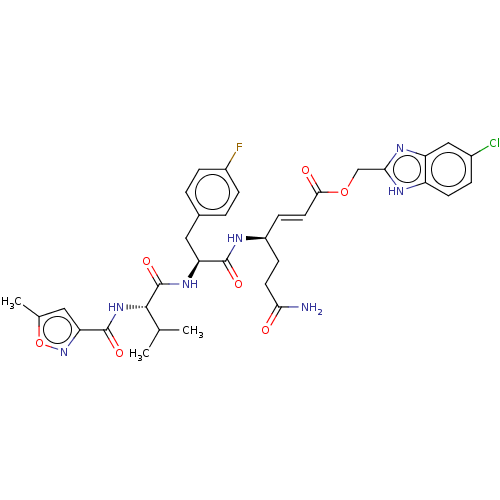 (CHEMBL2165218) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of School of Life Sciences Curated by ChEMBL | Assay Description Inhibition of Coxsackievirus B3 protease 3c using NMA-EALFQGPPVK-DNP as substrate incubated for 5 mins prior to substrate addition measured after 1 h... | Bioorg Med Chem Lett 22: 6952-6 (2012) Article DOI: 10.1016/j.bmcl.2012.08.120 BindingDB Entry DOI: 10.7270/Q2MS3WM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50386592 (CHEMBL2048251) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced ethidium bromide uptake after 2 hrs by f... | J Med Chem 55: 3687-98 (2012) Article DOI: 10.1021/jm2012326 BindingDB Entry DOI: 10.7270/Q21G0NB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM50485813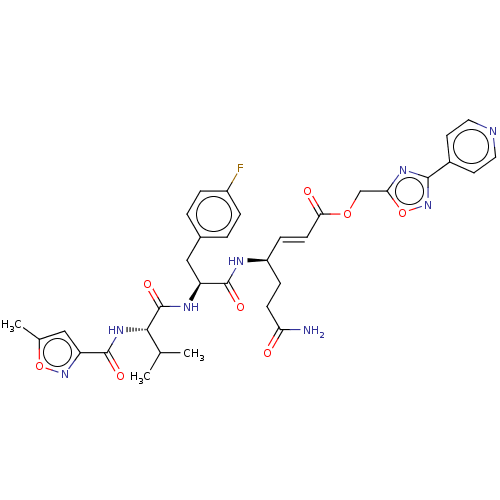 (CHEMBL2164930) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of School of Life Sciences Curated by ChEMBL | Assay Description Inhibition of Coxsackievirus B3 protease 3c using NMA-EALFQGPPVK-DNP as substrate incubated for 5 mins prior to substrate addition measured after 1 h... | Bioorg Med Chem Lett 22: 6952-6 (2012) Article DOI: 10.1016/j.bmcl.2012.08.120 BindingDB Entry DOI: 10.7270/Q2MS3WM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM152605 (3,5-Dimethyl-N'-(quinolin-4-yl)adamantane-1-ca...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 726 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Gwangju Institute of Science and Technology | Assay Description hP2X7-expressing HEK 293 cells were re-suspended at 2.5 × 10^6 cells/mL in assay buffer composed of 10 mM HEPES, 5 mM N-methyl-D-glutamine, 5.6 mM KC... | Bioorg Chem 61: 58-65 (2015) Article DOI: 10.1016/j.bioorg.2015.06.003 BindingDB Entry DOI: 10.7270/Q2833QRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50386571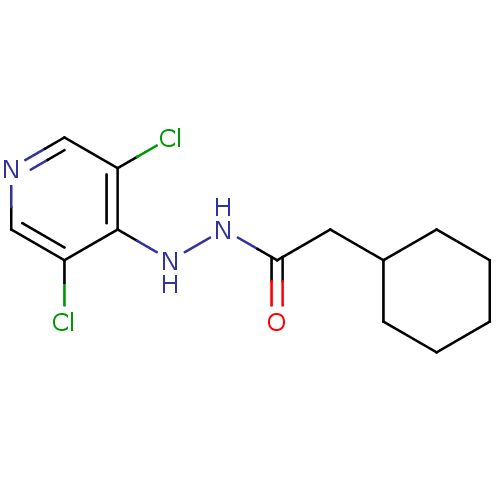 (CHEMBL2048433) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced ethidium bromide uptake after 2 hrs by f... | J Med Chem 55: 3687-98 (2012) Article DOI: 10.1021/jm2012326 BindingDB Entry DOI: 10.7270/Q21G0NB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50386582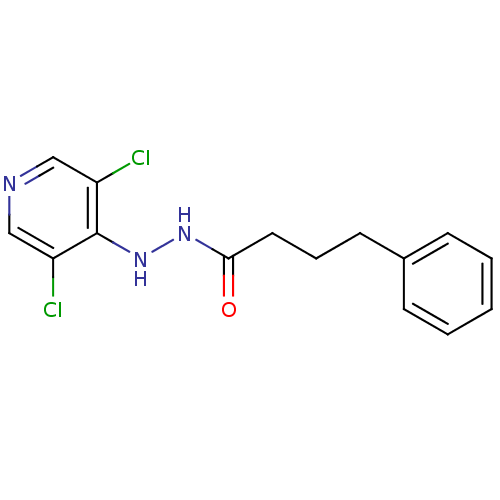 (CHEMBL2048270) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology (GIST) Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced ethidium bromide uptake after 2 hrs by f... | J Med Chem 55: 3687-98 (2012) Article DOI: 10.1021/jm2012326 BindingDB Entry DOI: 10.7270/Q21G0NB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 130 total ) | Next | Last >> |