

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50296704 (3-{4-[2-Amino-6-(4-benzyl-piperazin-1-yl)-pyrimidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse liver microsome 11betaHSD1 reductase activity expressed in HEK293 cells by scintillation proximity assay | Bioorg Med Chem 17: 5722-32 (2009) Article DOI: 10.1016/j.bmc.2009.05.082 BindingDB Entry DOI: 10.7270/Q2571CZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50359991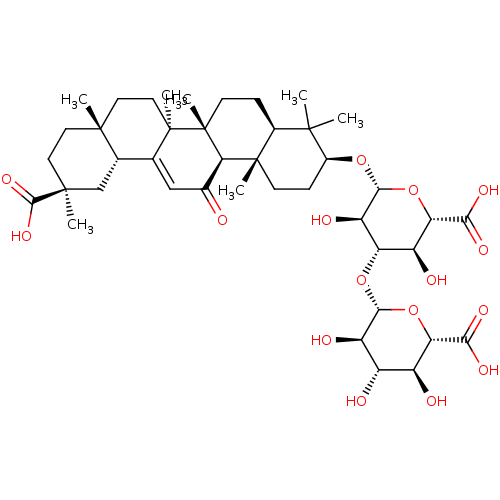 (CHEMBL1927953) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [3H]cortisone into [3H]cortisol by scintillation proximity assay | J Nat Prod 75: 599-604 (2012) Article DOI: 10.1021/np200831c BindingDB Entry DOI: 10.7270/Q27M090Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50359991 (CHEMBL1927953) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Kunming Institute of Botany Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD2 expressed in human HEK293 cells assessed as conversion of [3H]cortisone to [3H]cortisol by scintillation proximity as... | J Nat Prod 74: 2571-5 (2011) Article DOI: 10.1021/np200755t BindingDB Entry DOI: 10.7270/Q2P26ZJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50185127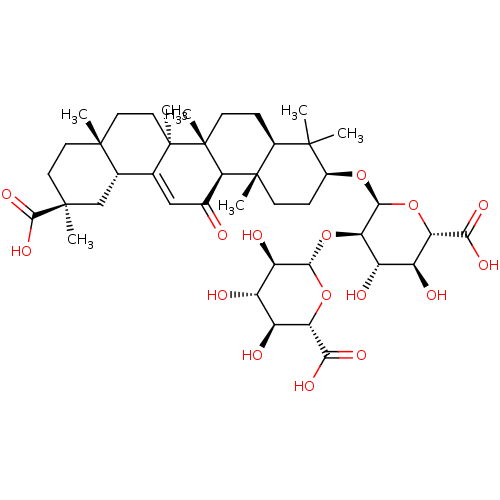 ((3beta,20beta)-20-carboxy-11-oxo-30-norolean-12-en...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human microsomal 11beta-HSD2 expressed in HEK293 cells assessed as conversion of [3H]cortisone to [3H]cortisol by scintillation proximi... | Bioorg Med Chem Lett 19: 4455-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.033 BindingDB Entry DOI: 10.7270/Q2VT1T1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM50509436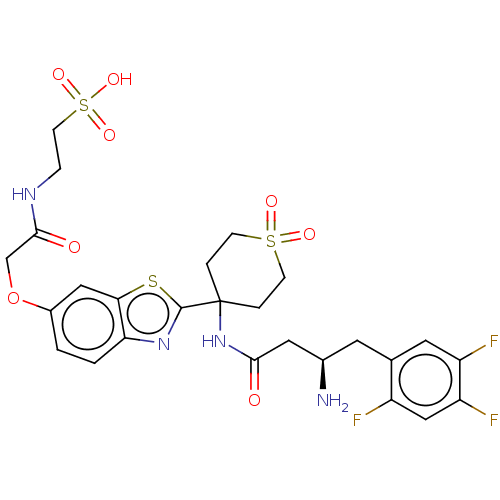 (CHEMBL4463027) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DPP4 in C57BL/6 mouse serum pre-incubated for 5 mins followed by Gly-Pro-7-AMC substrate addition by fluorescence based assay | J Med Chem 62: 10919-10925 (2019) Article DOI: 10.1021/acs.jmedchem.9b01649 BindingDB Entry DOI: 10.7270/Q2PN98X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50233538 (18beta-glycyrrhetic acid | 3beta-hydroxy-11-oxoole...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD2 | Eur J Med Chem 65: 403-14 (2013) Article DOI: 10.1016/j.ejmech.2013.05.010 BindingDB Entry DOI: 10.7270/Q2JS9RV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50233538 (18beta-glycyrrhetic acid | 3beta-hydroxy-11-oxoole...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD2 overexpressed in microsomal fraction of HEK293 cells using [3H]-cortisol as substrate by scintillation proximity assa... | J Nat Prod 78: 330-4 (2015) Article DOI: 10.1021/np500896n BindingDB Entry DOI: 10.7270/Q2930VWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50233538 (18beta-glycyrrhetic acid | 3beta-hydroxy-11-oxoole...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650204, China. Curated by ChEMBL | Assay Description Inhibition of full-length human 11beta-HSD2 expressed in HEK293 microsomal fraction using [3H]cortisone as substrate after 2 hr by scintillation prox... | Eur J Med Chem 135: 324-338 (2017) Article DOI: 10.1016/j.ejmech.2017.04.059 BindingDB Entry DOI: 10.7270/Q2CF9SK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM50509435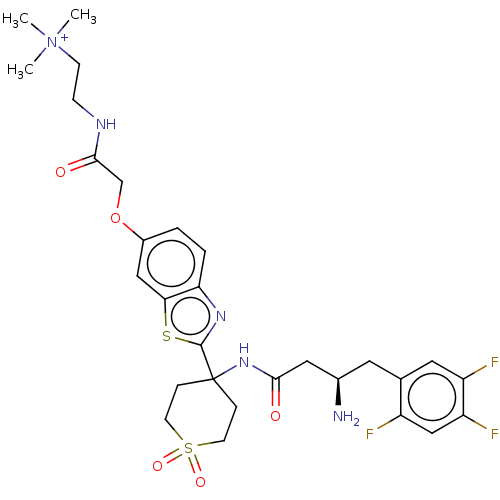 (CHEMBL4593371) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DPP4 in C57BL/6 mouse serum pre-incubated for 5 mins followed by Gly-Pro-7-AMC substrate addition by fluorescence based assay | J Med Chem 62: 10919-10925 (2019) Article DOI: 10.1021/acs.jmedchem.9b01649 BindingDB Entry DOI: 10.7270/Q2PN98X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50233538 (18beta-glycyrrhetic acid | 3beta-hydroxy-11-oxoole...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 expressed in HEK293 cell microsomes assessed as inhibition of [3H]cortisone conversion to [3H]cortisol by scintillati... | Eur J Med Chem 65: 403-14 (2013) Article DOI: 10.1016/j.ejmech.2013.05.010 BindingDB Entry DOI: 10.7270/Q2JS9RV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50297910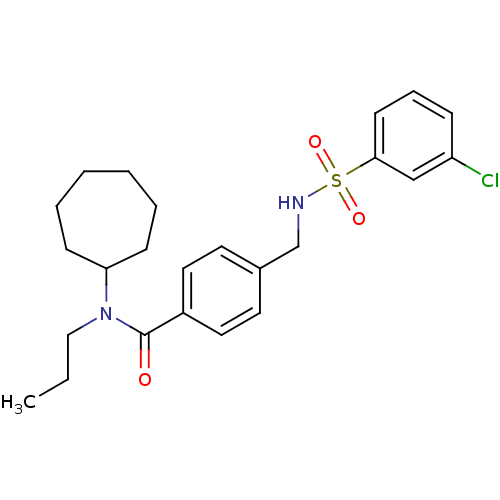 (4-[(3-Chlorophenylsulfonamido)methyl]-N-cyclohepty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human microsomal 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [3H]cortisone to [3H]cortisol by scintillation proximi... | Bioorg Med Chem Lett 19: 4455-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.033 BindingDB Entry DOI: 10.7270/Q2VT1T1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50297909 (4-[(3-Chlorophenylsulfonamido)methyl]-N-cyclohepty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse microsomal 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [3H]cortisone to [3H]cortisol by scintillation proximi... | Bioorg Med Chem Lett 19: 4455-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.033 BindingDB Entry DOI: 10.7270/Q2VT1T1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM50509441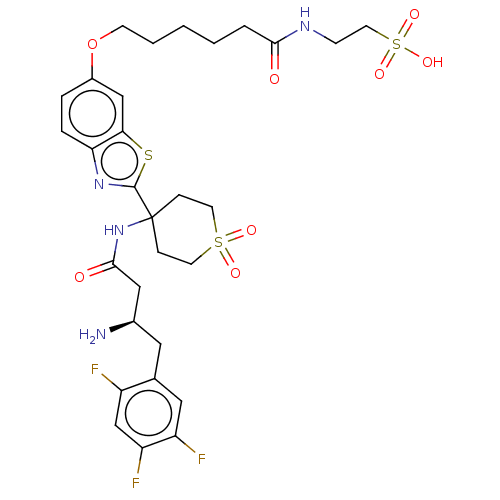 (CHEMBL4452661) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DPP4 in C57BL/6 mouse serum pre-incubated for 5 mins followed by Gly-Pro-7-AMC substrate addition by fluorescence based assay | J Med Chem 62: 10919-10925 (2019) Article DOI: 10.1021/acs.jmedchem.9b01649 BindingDB Entry DOI: 10.7270/Q2PN98X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50297911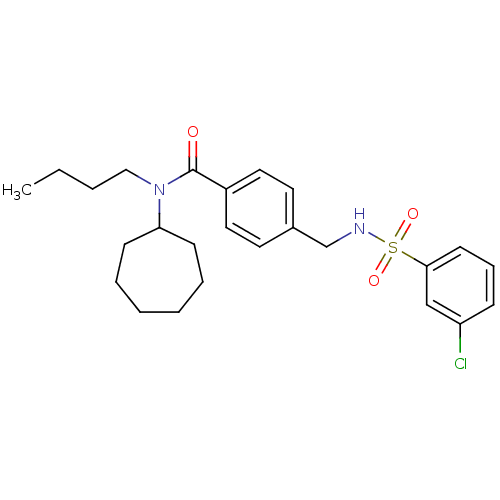 (CHEMBL550065 | N-butyl-4-[(3-chlorophenylsulfonami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse microsomal 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [3H]cortisone to [3H]cortisol by scintillation proximi... | Bioorg Med Chem Lett 19: 4455-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.033 BindingDB Entry DOI: 10.7270/Q2VT1T1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50297910 (4-[(3-Chlorophenylsulfonamido)methyl]-N-cyclohepty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse microsomal 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [3H]cortisone to [3H]cortisol by scintillation proximi... | Bioorg Med Chem Lett 19: 4455-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.033 BindingDB Entry DOI: 10.7270/Q2VT1T1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM50509440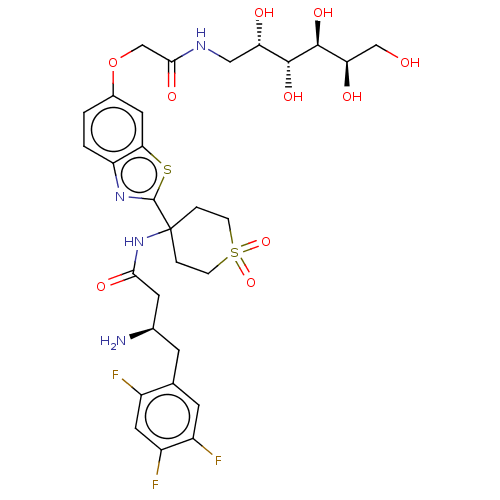 (CHEMBL4533178) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DPP4 in C57BL/6 mouse serum pre-incubated for 5 mins followed by Gly-Pro-7-AMC substrate addition by fluorescence based assay | J Med Chem 62: 10919-10925 (2019) Article DOI: 10.1021/acs.jmedchem.9b01649 BindingDB Entry DOI: 10.7270/Q2PN98X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM50509434 (CHEMBL4443285) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DPP4 in C57BL/6 mouse serum pre-incubated for 5 mins followed by Gly-Pro-7-AMC substrate addition by fluorescence based assay | J Med Chem 62: 10919-10925 (2019) Article DOI: 10.1021/acs.jmedchem.9b01649 BindingDB Entry DOI: 10.7270/Q2PN98X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50359991 (CHEMBL1927953) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [3H]cortisone into [3H]cortisol by scintillation proximity assay | J Nat Prod 75: 599-604 (2012) Article DOI: 10.1021/np200831c BindingDB Entry DOI: 10.7270/Q27M090Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM50509433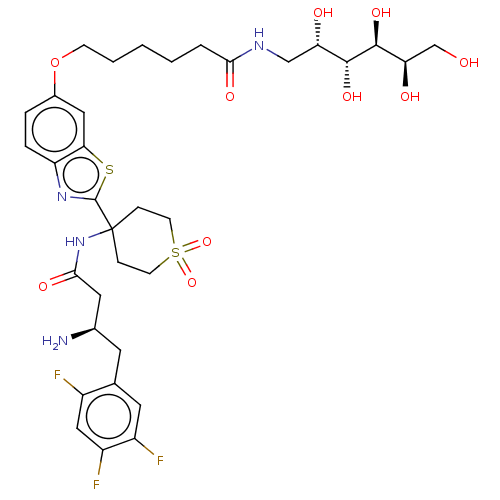 (CHEMBL4532312) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DPP4 in C57BL/6 mouse serum pre-incubated for 5 mins followed by Gly-Pro-7-AMC substrate addition by fluorescence based assay | J Med Chem 62: 10919-10925 (2019) Article DOI: 10.1021/acs.jmedchem.9b01649 BindingDB Entry DOI: 10.7270/Q2PN98X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50233538 (18beta-glycyrrhetic acid | 3beta-hydroxy-11-oxoole...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [3H]cortisone to [3H]cortisol by scintillation proximity assay in... | Eur J Med Chem 44: 1167-71 (2009) Article DOI: 10.1016/j.ejmech.2008.06.005 BindingDB Entry DOI: 10.7270/Q2251J0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50359991 (CHEMBL1927953) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [3H]cortisone into [3H]cortisol by scintillation proximity assay | J Nat Prod 75: 599-604 (2012) Article DOI: 10.1021/np200831c BindingDB Entry DOI: 10.7270/Q27M090Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50233538 (18beta-glycyrrhetic acid | 3beta-hydroxy-11-oxoole...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650204, China. Curated by ChEMBL | Assay Description Inhibition of full-length mouse 11beta-HSD1 expressed in HEK293 microsomal fraction using [3H]cortisone as substrate after 1 hr by scintillation prox... | Eur J Med Chem 135: 324-338 (2017) Article DOI: 10.1016/j.ejmech.2017.04.059 BindingDB Entry DOI: 10.7270/Q2CF9SK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50239401 (3-Adamantan-1-yl-6,7,8,9-tetrahydro-5H-[1,2,4]tria...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 | Eur J Med Chem 65: 403-14 (2013) Article DOI: 10.1016/j.ejmech.2013.05.010 BindingDB Entry DOI: 10.7270/Q2JS9RV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50185127 ((3beta,20beta)-20-carboxy-11-oxo-30-norolean-12-en...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse microsomal 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [3H]cortisone to [3H]cortisol by scintillation proximi... | Bioorg Med Chem Lett 19: 4455-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.033 BindingDB Entry DOI: 10.7270/Q2VT1T1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50233538 (18beta-glycyrrhetic acid | 3beta-hydroxy-11-oxoole...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human microsomal 11beta-HSD1 overexpressed in HEK293 cells using [3H]-cortisone as substrate assessed as formation of [3H]-cortisol usi... | J Nat Prod 79: 899-906 (2016) Article DOI: 10.1021/acs.jnatprod.5b00952 BindingDB Entry DOI: 10.7270/Q23200DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50436985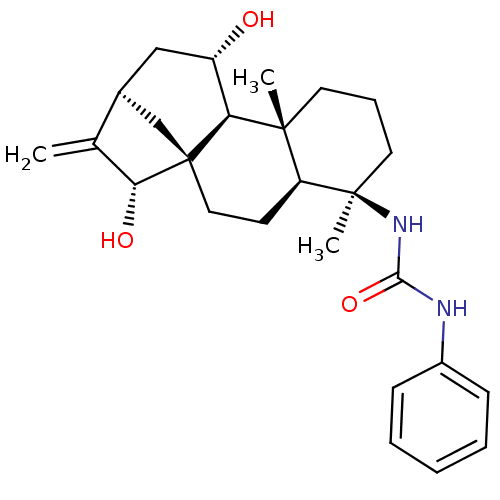 (CHEMBL2402457) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in HEK293 cell microsomes assessed as inhibition of [3H]cortisone conversion to [3H]cortisol by scintillati... | Eur J Med Chem 65: 403-14 (2013) Article DOI: 10.1016/j.ejmech.2013.05.010 BindingDB Entry DOI: 10.7270/Q2JS9RV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50233538 (18beta-glycyrrhetic acid | 3beta-hydroxy-11-oxoole...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse microsomal 11beta-HSD1 overexpressed in HEK293 cells using [3H]-cortisone as substrate assessed as formation of [3H]-cortisol usi... | J Nat Prod 79: 899-906 (2016) Article DOI: 10.1021/acs.jnatprod.5b00952 BindingDB Entry DOI: 10.7270/Q23200DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50185127 ((3beta,20beta)-20-carboxy-11-oxo-30-norolean-12-en...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human microsomal 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [3H]cortisone to [3H]cortisol by scintillation proximi... | Bioorg Med Chem Lett 19: 4455-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.033 BindingDB Entry DOI: 10.7270/Q2VT1T1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50297912 (4-[(3-Chlorophenylsulfonamido)methyl]-N-(1-adamant...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse microsomal 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [3H]cortisone to [3H]cortisol by scintillation proximi... | Bioorg Med Chem Lett 19: 4455-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.033 BindingDB Entry DOI: 10.7270/Q2VT1T1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50233538 (18beta-glycyrrhetic acid | 3beta-hydroxy-11-oxoole...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 | Bioorg Med Chem Lett 18: 1340-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.020 BindingDB Entry DOI: 10.7270/Q2474BQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50233538 (18beta-glycyrrhetic acid | 3beta-hydroxy-11-oxoole...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650204, China. Curated by ChEMBL | Assay Description Inhibition of full-length human 11beta-HSD1 expressed in HEK293 microsomal fraction using [3H]cortisone as substrate after 2 hr by scintillation prox... | Eur J Med Chem 135: 324-338 (2017) Article DOI: 10.1016/j.ejmech.2017.04.059 BindingDB Entry DOI: 10.7270/Q2CF9SK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50233538 (18beta-glycyrrhetic acid | 3beta-hydroxy-11-oxoole...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 11.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 | Bioorg Med Chem Lett 18: 1340-5 (2008) Article DOI: 10.1016/j.bmcl.2008.01.020 BindingDB Entry DOI: 10.7270/Q2474BQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50384887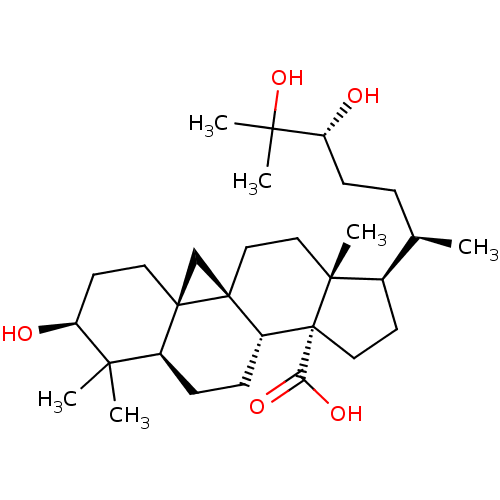 (CHEMBL2035569) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [3H]cortisone into [3H]cortisol by scintillation proximity assay | J Nat Prod 75: 599-604 (2012) Article DOI: 10.1021/np200831c BindingDB Entry DOI: 10.7270/Q27M090Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50267570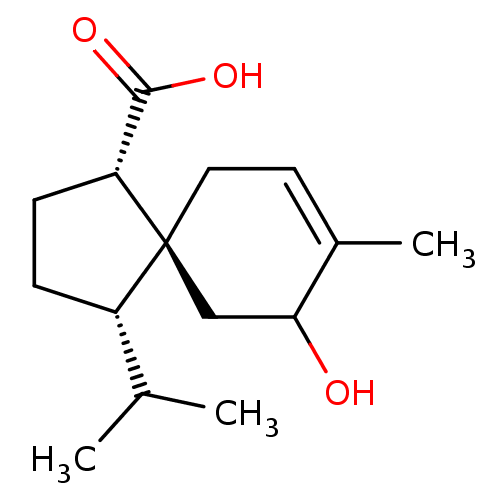 (CHEMBL4081242) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650204, China. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His-tagged 11beta-HSD1 expressed in Escherichia coli using cortisol as substrate by fluorescence-based assay | Eur J Med Chem 135: 324-338 (2017) Article DOI: 10.1016/j.ejmech.2017.04.059 BindingDB Entry DOI: 10.7270/Q2CF9SK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50233538 (18beta-glycyrrhetic acid | 3beta-hydroxy-11-oxoole...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [3H]cortisone to [3H]cortisol by scintillation proximity assay in... | Eur J Med Chem 44: 1167-71 (2009) Article DOI: 10.1016/j.ejmech.2008.06.005 BindingDB Entry DOI: 10.7270/Q2251J0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50297912 (4-[(3-Chlorophenylsulfonamido)methyl]-N-(1-adamant...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human microsomal 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [3H]cortisone to [3H]cortisol by scintillation proximi... | Bioorg Med Chem Lett 19: 4455-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.033 BindingDB Entry DOI: 10.7270/Q2VT1T1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50297908 (4-[(3-Chlorophenylsulfonamido)methyl]-N-cyclohepty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse microsomal 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [3H]cortisone to [3H]cortisol by scintillation proximi... | Bioorg Med Chem Lett 19: 4455-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.033 BindingDB Entry DOI: 10.7270/Q2VT1T1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50185127 ((3beta,20beta)-20-carboxy-11-oxo-30-norolean-12-en...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse liver microsome 11betaHSD1 expressed in HEK293 cells assessed as conversion of [3H]cortisone into [3H]cortisol by scintillation p... | Bioorg Med Chem 17: 5722-32 (2009) Article DOI: 10.1016/j.bmc.2009.05.082 BindingDB Entry DOI: 10.7270/Q2571CZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Mus musculus) | BDBM11162 ((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of DPP4 in C57BL/6 mouse serum pre-incubated for 5 mins followed by Gly-Pro-7-AMC substrate addition by fluorescence based assay | J Med Chem 62: 10919-10925 (2019) Article DOI: 10.1021/acs.jmedchem.9b01649 BindingDB Entry DOI: 10.7270/Q2PN98X6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50080413 (CHEMBL3422265) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 overexpressed in microsomal fraction of HEK293 cells assessed as formation of [3H]-cortisol from [3H]-cortisone by sc... | J Nat Prod 78: 330-4 (2015) Article DOI: 10.1021/np500896n BindingDB Entry DOI: 10.7270/Q2930VWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50359991 (CHEMBL1927953) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Kunming Institute of Botany Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in human HEK293 cells assessed as conversion of [3H]cortisone to [3H]cortisol by scintillation proximity as... | J Nat Prod 74: 2571-5 (2011) Article DOI: 10.1021/np200755t BindingDB Entry DOI: 10.7270/Q2P26ZJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50436987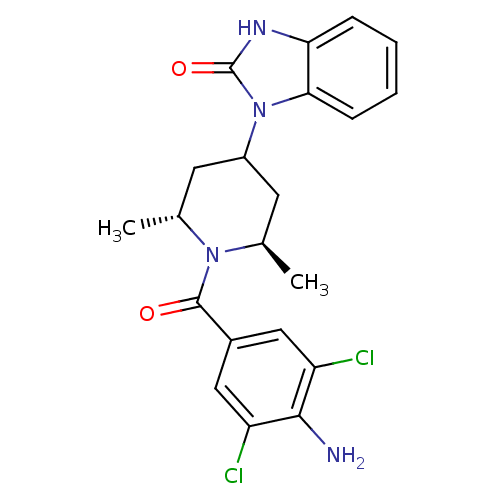 (CHEMBL2402470) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 | Eur J Med Chem 65: 403-14 (2013) Article DOI: 10.1016/j.ejmech.2013.05.010 BindingDB Entry DOI: 10.7270/Q2JS9RV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50233538 (18beta-glycyrrhetic acid | 3beta-hydroxy-11-oxoole...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in HEK293 cell microsomes assessed as inhibition of [3H]cortisone conversion to [3H]cortisol by scintillati... | Eur J Med Chem 65: 403-14 (2013) Article DOI: 10.1016/j.ejmech.2013.05.010 BindingDB Entry DOI: 10.7270/Q2JS9RV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50233538 (18beta-glycyrrhetic acid | 3beta-hydroxy-11-oxoole...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 overexpressed in microsomal fraction of HEK293 cells assessed as formation of [3H]-cortisol from [3H]-cortisone by sc... | J Nat Prod 78: 330-4 (2015) Article DOI: 10.1021/np500896n BindingDB Entry DOI: 10.7270/Q2930VWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50359991 (CHEMBL1927953) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kunming Institute of Botany Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 expressed in human HEK293 cells assessed as conversion of [3H]cortisone to [3H]cortisol by scintillation proximity as... | J Nat Prod 74: 2571-5 (2011) Article DOI: 10.1021/np200755t BindingDB Entry DOI: 10.7270/Q2P26ZJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50080410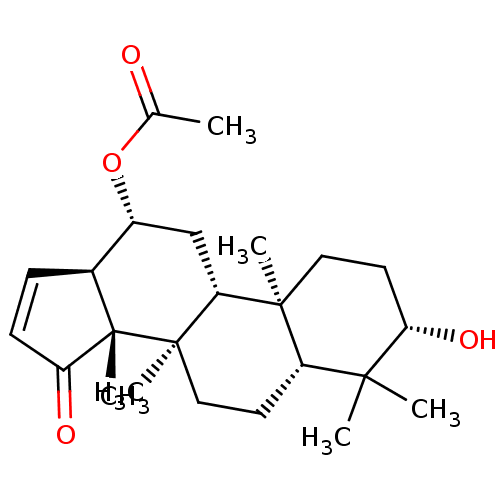 (CHEMBL3422416) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 overexpressed in microsomal fraction of HEK293 cells assessed as formation of [3H]-cortisol from [3H]-cortisone by sc... | J Nat Prod 78: 330-4 (2015) Article DOI: 10.1021/np500896n BindingDB Entry DOI: 10.7270/Q2930VWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Rattus norvegicus (rat)) | BDBM50436987 (CHEMBL2402470) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of rat 11beta-HSD1 | Eur J Med Chem 65: 403-14 (2013) Article DOI: 10.1016/j.ejmech.2013.05.010 BindingDB Entry DOI: 10.7270/Q2JS9RV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50233538 (18beta-glycyrrhetic acid | 3beta-hydroxy-11-oxoole...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 overexpressed in microsomal fraction of HEK293 cells assessed as formation of [3H]-cortisol from [3H]-cortisone by sc... | J Nat Prod 78: 330-4 (2015) Article DOI: 10.1021/np500896n BindingDB Entry DOI: 10.7270/Q2930VWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM32530 (arylsulfonylpiperazine, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human 11betaHSD1 | Bioorg Med Chem 17: 5722-32 (2009) Article DOI: 10.1016/j.bmc.2009.05.082 BindingDB Entry DOI: 10.7270/Q2571CZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50297911 (CHEMBL550065 | N-butyl-4-[(3-chlorophenylsulfonami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human microsomal 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [3H]cortisone to [3H]cortisol by scintillation proximi... | Bioorg Med Chem Lett 19: 4455-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.033 BindingDB Entry DOI: 10.7270/Q2VT1T1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 958 total ) | Next | Last >> |