 Found 492 hits with Last Name = 'lepist' and Initial = 'ei'
Found 492 hits with Last Name = 'lepist' and Initial = 'ei' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium/nucleoside cotransporter 1
(Homo sapiens (Human)) | BDBM14487

((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H13N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h2-4,6-7,10,16-18H,1H2,(H2,11,12,13)/t4-,6-,7-,10-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human CNT1 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc... |
Drug Metab Dispos 41: 916-22 (2013)
Article DOI: 10.1124/dmd.112.049858
BindingDB Entry DOI: 10.7270/Q21N82VJ |
More data for this
Ligand-Target Pair | |
Solute carrier family 28 member 3
(Homo sapiens (Human)) | BDBM14487

((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H13N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h2-4,6-7,10,16-18H,1H2,(H2,11,12,13)/t4-,6-,7-,10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human CNT3 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 5 mins by sci... |
Drug Metab Dispos 41: 916-22 (2013)
Article DOI: 10.1124/dmd.112.049858
BindingDB Entry DOI: 10.7270/Q21N82VJ |
More data for this
Ligand-Target Pair | |
Solute carrier family 28 member 3
(Homo sapiens (Human)) | BDBM50088517
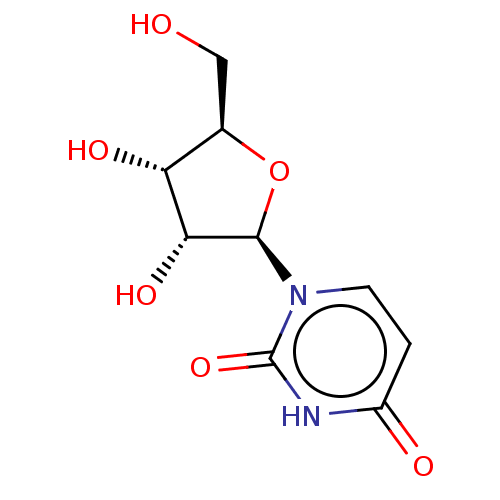
(CHEBI:16704 | Uridine)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C9H12N2O6/c12-3-4-6(14)7(15)8(17-4)11-2-1-5(13)10-9(11)16/h1-2,4,6-8,12,14-15H,3H2,(H,10,13,16)/t4-,6-,7-,8-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human CNT3 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 5 mins by sci... |
Drug Metab Dispos 41: 916-22 (2013)
Article DOI: 10.1124/dmd.112.049858
BindingDB Entry DOI: 10.7270/Q21N82VJ |
More data for this
Ligand-Target Pair | |
Sodium/nucleoside cotransporter 1
(Homo sapiens (Human)) | BDBM50088517

(CHEBI:16704 | Uridine)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C9H12N2O6/c12-3-4-6(14)7(15)8(17-4)11-2-1-5(13)10-9(11)16/h1-2,4,6-8,12,14-15H,3H2,(H,10,13,16)/t4-,6-,7-,8-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human CNT1 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc... |
Drug Metab Dispos 41: 916-22 (2013)
Article DOI: 10.1124/dmd.112.049858
BindingDB Entry DOI: 10.7270/Q21N82VJ |
More data for this
Ligand-Target Pair | |
Sodium/nucleoside cotransporter 2
(Homo sapiens (Human)) | BDBM14487

((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H13N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h2-4,6-7,10,16-18H,1H2,(H2,11,12,13)/t4-,6-,7-,10-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human CNT2 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc... |
Drug Metab Dispos 41: 916-22 (2013)
Article DOI: 10.1124/dmd.112.049858
BindingDB Entry DOI: 10.7270/Q21N82VJ |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM50138530
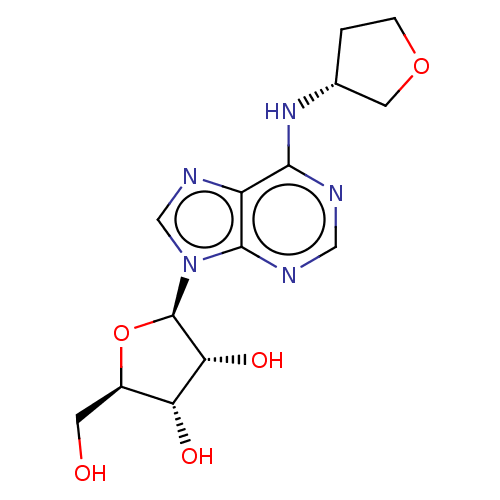
((2R,3R,5R)-2-Hydroxymethyl-5-{6-[(R)-(tetrahydro-f...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N[C@@H]3CCOC3)ncnc12 Show InChI InChI=1S/C14H19N5O5/c20-3-8-10(21)11(22)14(24-8)19-6-17-9-12(15-5-16-13(9)19)18-7-1-2-23-4-7/h5-8,10-11,14,20-22H,1-4H2,(H,15,16,18)/t7?,8-,10-,11-,14?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human ENT1 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc... |
Drug Metab Dispos 41: 916-22 (2013)
Article DOI: 10.1124/dmd.112.049858
BindingDB Entry DOI: 10.7270/Q21N82VJ |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM14487

((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H13N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h2-4,6-7,10,16-18H,1H2,(H2,11,12,13)/t4-,6-,7-,10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human ENT1 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc... |
Drug Metab Dispos 41: 916-22 (2013)
Article DOI: 10.1124/dmd.112.049858
BindingDB Entry DOI: 10.7270/Q21N82VJ |
More data for this
Ligand-Target Pair | |
Sodium/nucleoside cotransporter 2
(Homo sapiens (Human)) | BDBM50088517

(CHEBI:16704 | Uridine)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C9H12N2O6/c12-3-4-6(14)7(15)8(17-4)11-2-1-5(13)10-9(11)16/h1-2,4,6-8,12,14-15H,3H2,(H,10,13,16)/t4-,6-,7-,8-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human CNT2 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc... |
Drug Metab Dispos 41: 916-22 (2013)
Article DOI: 10.1124/dmd.112.049858
BindingDB Entry DOI: 10.7270/Q21N82VJ |
More data for this
Ligand-Target Pair | |
Sodium/nucleoside cotransporter 1
(Homo sapiens (Human)) | BDBM50138530

((2R,3R,5R)-2-Hydroxymethyl-5-{6-[(R)-(tetrahydro-f...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N[C@@H]3CCOC3)ncnc12 Show InChI InChI=1S/C14H19N5O5/c20-3-8-10(21)11(22)14(24-8)19-6-17-9-12(15-5-16-13(9)19)18-7-1-2-23-4-7/h5-8,10-11,14,20-22H,1-4H2,(H,15,16,18)/t7?,8-,10-,11-,14?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human CNT1 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc... |
Drug Metab Dispos 41: 916-22 (2013)
Article DOI: 10.1124/dmd.112.049858
BindingDB Entry DOI: 10.7270/Q21N82VJ |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM50088517

(CHEBI:16704 | Uridine)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C9H12N2O6/c12-3-4-6(14)7(15)8(17-4)11-2-1-5(13)10-9(11)16/h1-2,4,6-8,12,14-15H,3H2,(H,10,13,16)/t4-,6-,7-,8-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human ENT1 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc... |
Drug Metab Dispos 41: 916-22 (2013)
Article DOI: 10.1124/dmd.112.049858
BindingDB Entry DOI: 10.7270/Q21N82VJ |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 2
(Homo sapiens (Human)) | BDBM14487

((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H13N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h2-4,6-7,10,16-18H,1H2,(H2,11,12,13)/t4-,6-,7-,10-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human ENT2 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc... |
Drug Metab Dispos 41: 916-22 (2013)
Article DOI: 10.1124/dmd.112.049858
BindingDB Entry DOI: 10.7270/Q21N82VJ |
More data for this
Ligand-Target Pair | |
Solute carrier family 28 member 3
(Homo sapiens (Human)) | BDBM50138530

((2R,3R,5R)-2-Hydroxymethyl-5-{6-[(R)-(tetrahydro-f...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N[C@@H]3CCOC3)ncnc12 Show InChI InChI=1S/C14H19N5O5/c20-3-8-10(21)11(22)14(24-8)19-6-17-9-12(15-5-16-13(9)19)18-7-1-2-23-4-7/h5-8,10-11,14,20-22H,1-4H2,(H,15,16,18)/t7?,8-,10-,11-,14?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.26E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human CNT3 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 5 mins by sci... |
Drug Metab Dispos 41: 916-22 (2013)
Article DOI: 10.1124/dmd.112.049858
BindingDB Entry DOI: 10.7270/Q21N82VJ |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 2
(Homo sapiens (Human)) | BDBM50138530

((2R,3R,5R)-2-Hydroxymethyl-5-{6-[(R)-(tetrahydro-f...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N[C@@H]3CCOC3)ncnc12 Show InChI InChI=1S/C14H19N5O5/c20-3-8-10(21)11(22)14(24-8)19-6-17-9-12(15-5-16-13(9)19)18-7-1-2-23-4-7/h5-8,10-11,14,20-22H,1-4H2,(H,15,16,18)/t7?,8-,10-,11-,14?/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.89E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human ENT2 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc... |
Drug Metab Dispos 41: 916-22 (2013)
Article DOI: 10.1124/dmd.112.049858
BindingDB Entry DOI: 10.7270/Q21N82VJ |
More data for this
Ligand-Target Pair | |
Sodium/nucleoside cotransporter 2
(Homo sapiens (Human)) | BDBM50138530

((2R,3R,5R)-2-Hydroxymethyl-5-{6-[(R)-(tetrahydro-f...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N[C@@H]3CCOC3)ncnc12 Show InChI InChI=1S/C14H19N5O5/c20-3-8-10(21)11(22)14(24-8)19-6-17-9-12(15-5-16-13(9)19)18-7-1-2-23-4-7/h5-8,10-11,14,20-22H,1-4H2,(H,15,16,18)/t7?,8-,10-,11-,14?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.31E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human CNT2 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc... |
Drug Metab Dispos 41: 916-22 (2013)
Article DOI: 10.1124/dmd.112.049858
BindingDB Entry DOI: 10.7270/Q21N82VJ |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 2
(Homo sapiens (Human)) | BDBM50088517

(CHEBI:16704 | Uridine)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C9H12N2O6/c12-3-4-6(14)7(15)8(17-4)11-2-1-5(13)10-9(11)16/h1-2,4,6-8,12,14-15H,3H2,(H,10,13,16)/t4-,6-,7-,8-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.42E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human ENT2 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc... |
Drug Metab Dispos 41: 916-22 (2013)
Article DOI: 10.1124/dmd.112.049858
BindingDB Entry DOI: 10.7270/Q21N82VJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50168293
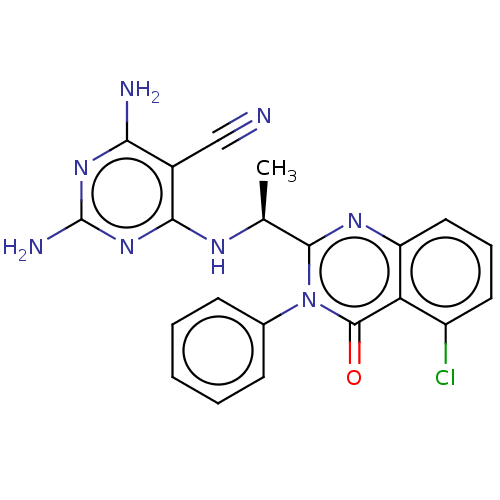
(CHEMBL3805430)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C21H17ClN8O/c1-11(26-18-13(10-23)17(24)28-21(25)29-18)19-27-15-9-5-8-14(22)16(15)20(31)30(19)12-6-3-2-4-7-12/h2-9,11H,1H3,(H5,24,25,26,28,29)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a... |
J Med Chem 59: 3532-48 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00213
BindingDB Entry DOI: 10.7270/Q22Z17F0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50168290
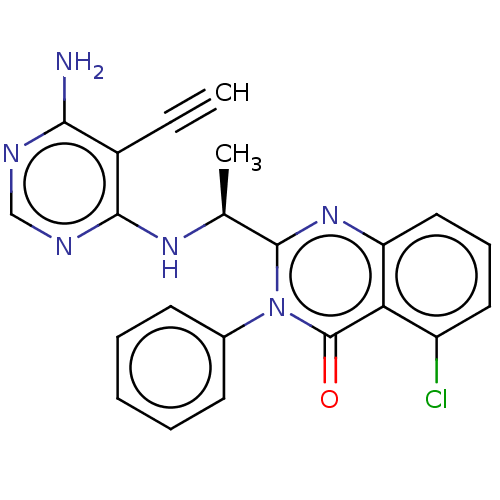
(CHEMBL3805572)Show SMILES C[C@H](Nc1ncnc(N)c1C#C)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C22H17ClN6O/c1-3-15-19(24)25-12-26-20(15)27-13(2)21-28-17-11-7-10-16(23)18(17)22(30)29(21)14-8-5-4-6-9-14/h1,4-13H,2H3,(H3,24,25,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a... |
J Med Chem 59: 3532-48 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00213
BindingDB Entry DOI: 10.7270/Q22Z17F0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50168293

(CHEMBL3805430)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C21H17ClN8O/c1-11(26-18-13(10-23)17(24)28-21(25)29-18)19-27-15-9-5-8-14(22)16(15)20(31)30(19)12-6-3-2-4-7-12/h2-9,11H,1H3,(H5,24,25,26,28,29)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length PI3K p110delta/p85alpha using phosphatidylinositol 3,4,5-trisphosphate as substrate measured after 30 mins by TR-FRET... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01169
BindingDB Entry DOI: 10.7270/Q20G3PV2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50168297
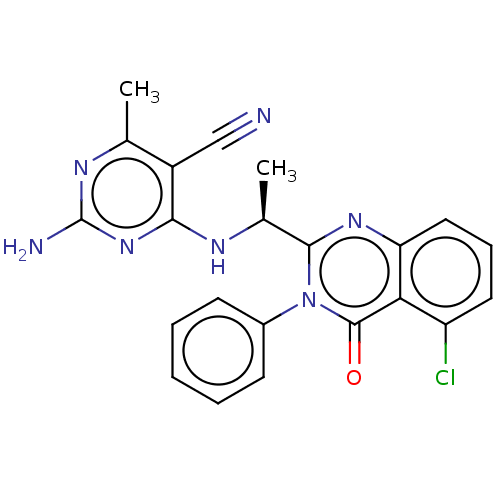
(CHEMBL3805760 | US9765060, Compound 96)Show SMILES C[C@H](Nc1nc(N)nc(C)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C22H18ClN7O/c1-12-15(11-24)19(29-22(25)27-12)26-13(2)20-28-17-10-6-9-16(23)18(17)21(31)30(20)14-7-4-3-5-8-14/h3-10,13H,1-2H3,(H3,25,26,27,29)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a... |
J Med Chem 59: 3532-48 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00213
BindingDB Entry DOI: 10.7270/Q22Z17F0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50168299

(CHEMBL3805664)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1cccc(F)c1 |r| Show InChI InChI=1S/C21H16ClFN8O/c1-10(27-18-13(9-24)17(25)29-21(26)30-18)19-28-15-7-3-6-14(22)16(15)20(32)31(19)12-5-2-4-11(23)8-12/h2-8,10H,1H3,(H5,25,26,27,29,30)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a... |
J Med Chem 59: 3532-48 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00213
BindingDB Entry DOI: 10.7270/Q22Z17F0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50501402

(CHEMBL4071605)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1cc[nH]n1 |r| Show InChI InChI=1S/C18H15ClN10O/c1-8(24-15-9(7-20)14(21)26-18(22)27-15)16-25-11-4-2-3-10(19)13(11)17(30)29(16)12-5-6-23-28-12/h2-6,8H,1H3,(H,23,28)(H5,21,22,24,26,27)/t8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human full length PI3K p110delta/p85 alpha using PIP2/ATP as substrate after 30 mins by TR-FRET assay |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50501399

(CHEMBL4100135)Show SMILES C[C@H](Nc1ncnc(N)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1ccc(O)cc1 |r| Show InChI InChI=1S/C21H16ClN7O2/c1-11(27-19-14(9-23)18(24)25-10-26-19)20-28-16-4-2-3-15(22)17(16)21(31)29(20)12-5-7-13(30)8-6-12/h2-8,10-11,30H,1H3,(H3,24,25,26,27)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human full length PI3K p110delta/p85 alpha using PIP2/ATP as substrate after 30 mins by TR-FRET assay |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM198050

(BDBM198051 | US9221795, 46)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1cncc(F)c1C |r,wD:1.0,(2,.38,;.67,-.39,;.67,-1.93,;2,-2.7,;3.33,-1.93,;4.67,-2.7,;6,-1.93,;4.67,-4.24,;3.33,-5,;3.33,-6.54,;2,-4.24,;.67,-5.01,;-.67,-5.78,;-.67,.38,;-2,-.39,;-3.33,.38,;-4.67,-.39,;-6,.38,;-6,1.92,;-4.67,2.69,;-4.67,4.23,;-3.33,1.92,;-2,2.69,;-2,4.23,;-.67,1.92,;.67,2.69,;2,1.92,;3.33,2.69,;3.33,4.23,;2,5,;2,6.54,;.67,4.23,;-.67,5,)| Show InChI InChI=1S/C21H17ClFN9O/c1-9-13(23)7-27-8-15(9)32-19(29-14-5-3-4-12(22)16(14)20(32)33)10(2)28-18-11(6-24)17(25)30-21(26)31-18/h3-5,7-8,10H,1-2H3,(H5,25,26,28,30,31)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length PI3K p110delta/p85alpha using phosphatidylinositol 3,4,5-trisphosphate as substrate measured after 30 mins by TR-FRET... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01169
BindingDB Entry DOI: 10.7270/Q20G3PV2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM208824
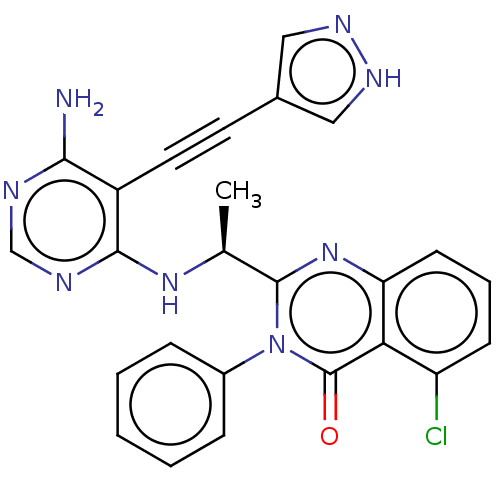
(US9266878, 49a)Show SMILES C[C@H](Nc1ncnc(N)c1C#Cc1cn[nH]c1)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a... |
J Med Chem 59: 3532-48 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00213
BindingDB Entry DOI: 10.7270/Q22Z17F0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM198050

(BDBM198051 | US9221795, 46)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1cncc(F)c1C |r,wD:1.0,(2,.38,;.67,-.39,;.67,-1.93,;2,-2.7,;3.33,-1.93,;4.67,-2.7,;6,-1.93,;4.67,-4.24,;3.33,-5,;3.33,-6.54,;2,-4.24,;.67,-5.01,;-.67,-5.78,;-.67,.38,;-2,-.39,;-3.33,.38,;-4.67,-.39,;-6,.38,;-6,1.92,;-4.67,2.69,;-4.67,4.23,;-3.33,1.92,;-2,2.69,;-2,4.23,;-.67,1.92,;.67,2.69,;2,1.92,;3.33,2.69,;3.33,4.23,;2,5,;2,6.54,;.67,4.23,;-.67,5,)| Show InChI InChI=1S/C21H17ClFN9O/c1-9-13(23)7-27-8-15(9)32-19(29-14-5-3-4-12(22)16(14)20(32)33)10(2)28-18-11(6-24)17(25)30-21(26)31-18/h3-5,7-8,10H,1-2H3,(H5,25,26,28,30,31)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length PI3K p110delta/p85alpha using phosphatidylinositol 3,4,5-trisphosphate as substrate measured after 30 mins by TR-FRET... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01169
BindingDB Entry DOI: 10.7270/Q20G3PV2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM208826
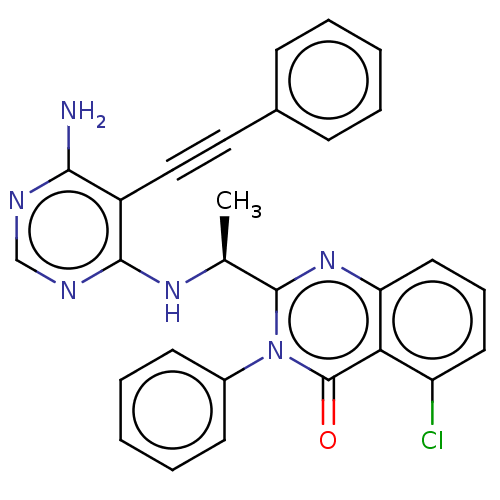
(US9266878, 51a)Show SMILES C[C@H](Nc1ncnc(N)c1C#Cc1ccccc1)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C28H21ClN6O/c1-18(33-26-21(25(30)31-17-32-26)16-15-19-9-4-2-5-10-19)27-34-23-14-8-13-22(29)24(23)28(36)35(27)20-11-6-3-7-12-20/h2-14,17-18H,1H3,(H3,30,31,32,33)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a... |
J Med Chem 59: 3532-48 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00213
BindingDB Entry DOI: 10.7270/Q22Z17F0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50168484

(CHEMBL3805137 | US9765060, Compound Y)Show SMILES C[C@H](Nc1ncnc(N)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C21H16ClN7O/c1-12(27-19-14(10-23)18(24)25-11-26-19)20-28-16-9-5-8-15(22)17(16)21(30)29(20)13-6-3-2-4-7-13/h2-9,11-12H,1H3,(H3,24,25,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human full length PI3K p110delta/p85 alpha using PIP2/ATP as substrate after 30 mins by TR-FRET assay |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM198028

(US9221795, 24)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2c(F)ccc(Cl)c2c(=O)n1-c1cccnc1 |r| Show InChI InChI=1S/C20H15ClFN9O/c1-9(27-17-11(7-23)16(24)29-20(25)30-17)18-28-15-13(22)5-4-12(21)14(15)19(32)31(18)10-3-2-6-26-8-10/h2-6,8-9H,1H3,(H5,24,25,27,29,30)/t9-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length PI3K p110delta/p85alpha using phosphatidylinositol 3,4,5-trisphosphate as substrate measured after 30 mins by TR-FRET... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01169
BindingDB Entry DOI: 10.7270/Q20G3PV2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM208793
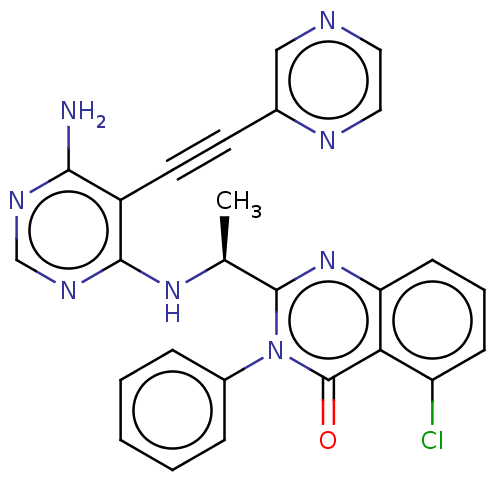
(US9266878, 16a)Show SMILES C[C@H](Nc1ncnc(N)c1C#Cc1cnccn1)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C26H19ClN8O/c1-16(33-24-19(23(28)31-15-32-24)11-10-17-14-29-12-13-30-17)25-34-21-9-5-8-20(27)22(21)26(36)35(25)18-6-3-2-4-7-18/h2-9,12-16H,1H3,(H3,28,31,32,33)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a... |
J Med Chem 59: 3532-48 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00213
BindingDB Entry DOI: 10.7270/Q22Z17F0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM198044
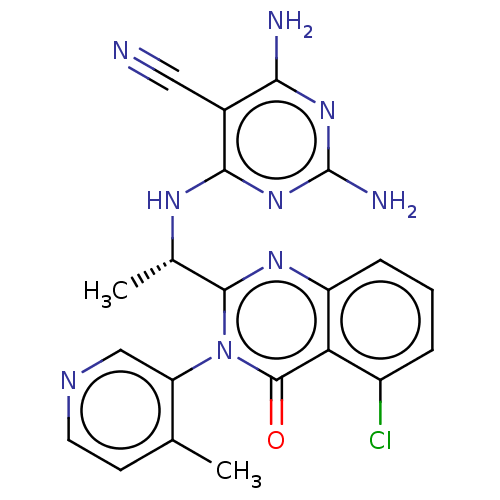
(BDBM198045 | US9221795, 40)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1cnccc1C |r,wD:1.0,(2,1.15,;.67,.38,;.67,-1.16,;2,-1.93,;3.33,-1.16,;4.67,-1.93,;6,-1.15,;4.67,-3.47,;3.33,-4.23,;3.33,-5.78,;2,-3.47,;.67,-4.24,;-.67,-5.01,;-.67,1.15,;-2,.38,;-3.33,1.15,;-4.67,.38,;-6,1.15,;-6,2.69,;-4.67,3.46,;-4.67,5,;-3.33,2.69,;-2,3.46,;-2,5,;-.67,2.69,;.67,3.46,;2,2.69,;3.33,3.46,;3.33,5,;2,5.78,;.67,5,;-.67,5.77,)| Show InChI InChI=1S/C21H18ClN9O/c1-10-6-7-26-9-15(10)31-19(28-14-5-3-4-13(22)16(14)20(31)32)11(2)27-18-12(8-23)17(24)29-21(25)30-18/h3-7,9,11H,1-2H3,(H5,24,25,27,29,30)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length PI3K p110delta/p85alpha using phosphatidylinositol 3,4,5-trisphosphate as substrate measured after 30 mins by TR-FRET... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01169
BindingDB Entry DOI: 10.7270/Q20G3PV2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50168484

(CHEMBL3805137 | US9765060, Compound Y)Show SMILES C[C@H](Nc1ncnc(N)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C21H16ClN7O/c1-12(27-19-14(10-23)18(24)25-11-26-19)20-28-16-9-5-8-15(22)17(16)21(30)29(20)13-6-3-2-4-7-13/h2-9,11-12H,1H3,(H3,24,25,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a... |
J Med Chem 59: 3532-48 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00213
BindingDB Entry DOI: 10.7270/Q22Z17F0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM198044

(BDBM198045 | US9221795, 40)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1cnccc1C |r,wD:1.0,(2,1.15,;.67,.38,;.67,-1.16,;2,-1.93,;3.33,-1.16,;4.67,-1.93,;6,-1.15,;4.67,-3.47,;3.33,-4.23,;3.33,-5.78,;2,-3.47,;.67,-4.24,;-.67,-5.01,;-.67,1.15,;-2,.38,;-3.33,1.15,;-4.67,.38,;-6,1.15,;-6,2.69,;-4.67,3.46,;-4.67,5,;-3.33,2.69,;-2,3.46,;-2,5,;-.67,2.69,;.67,3.46,;2,2.69,;3.33,3.46,;3.33,5,;2,5.78,;.67,5,;-.67,5.77,)| Show InChI InChI=1S/C21H18ClN9O/c1-10-6-7-26-9-15(10)31-19(28-14-5-3-4-13(22)16(14)20(31)32)11(2)27-18-12(8-23)17(24)29-21(25)30-18/h3-7,9,11H,1-2H3,(H5,24,25,27,29,30)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length PI3K p110delta/p85alpha using phosphatidylinositol 3,4,5-trisphosphate as substrate measured after 30 mins by TR-FRET... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01169
BindingDB Entry DOI: 10.7270/Q20G3PV2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
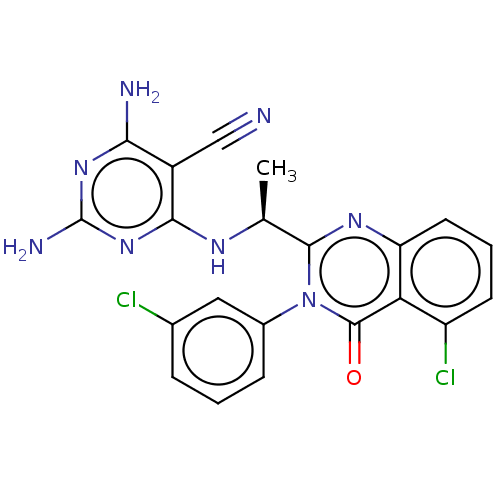
(Homo sapiens (Human)) | BDBM50168300
(CHEMBL3804870)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1cccc(Cl)c1 |r| Show InChI InChI=1S/C21H16Cl2N8O/c1-10(27-18-13(9-24)17(25)29-21(26)30-18)19-28-15-7-3-6-14(23)16(15)20(32)31(19)12-5-2-4-11(22)8-12/h2-8,10H,1H3,(H5,25,26,27,29,30)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a... |
J Med Chem 59: 3532-48 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00213
BindingDB Entry DOI: 10.7270/Q22Z17F0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
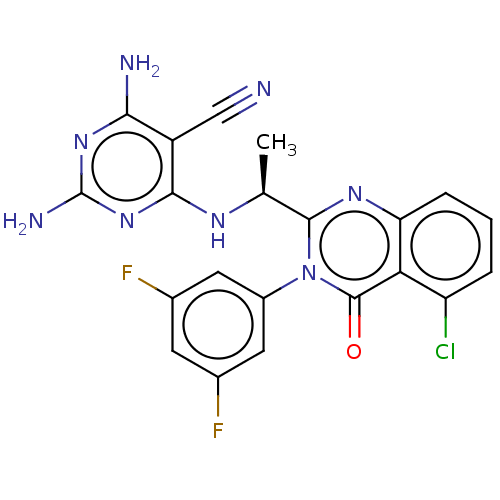
(Homo sapiens (Human)) | BDBM50168431
(CHEMBL3805913)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C21H15ClF2N8O/c1-9(28-18-13(8-25)17(26)30-21(27)31-18)19-29-15-4-2-3-14(22)16(15)20(33)32(19)12-6-10(23)5-11(24)7-12/h2-7,9H,1H3,(H5,26,27,28,30,31)/t9-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a... |
J Med Chem 59: 3532-48 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00213
BindingDB Entry DOI: 10.7270/Q22Z17F0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
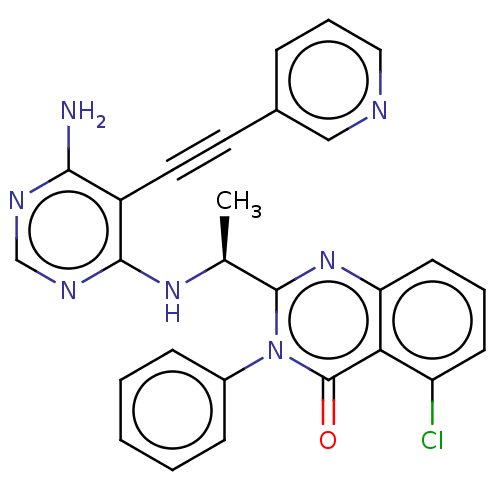
(Homo sapiens (Human)) | BDBM208819
(US9266878, 44a)Show SMILES C[C@H](Nc1ncnc(N)c1C#Cc1cccnc1)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C27H20ClN7O/c1-17(33-25-20(24(29)31-16-32-25)13-12-18-7-6-14-30-15-18)26-34-22-11-5-10-21(28)23(22)27(36)35(26)19-8-3-2-4-9-19/h2-11,14-17H,1H3,(H3,29,31,32,33)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a... |
J Med Chem 59: 3532-48 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00213
BindingDB Entry DOI: 10.7270/Q22Z17F0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50168427

(CHEMBL3805887)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1cccc(c1)C(F)F |r| Show InChI InChI=1S/C22H17ClF2N8O/c1-10(29-19-13(9-26)18(27)31-22(28)32-19)20-30-15-7-3-6-14(23)16(15)21(34)33(20)12-5-2-4-11(8-12)17(24)25/h2-8,10,17H,1H3,(H5,27,28,29,31,32)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a... |
J Med Chem 59: 3532-48 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00213
BindingDB Entry DOI: 10.7270/Q22Z17F0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50501408
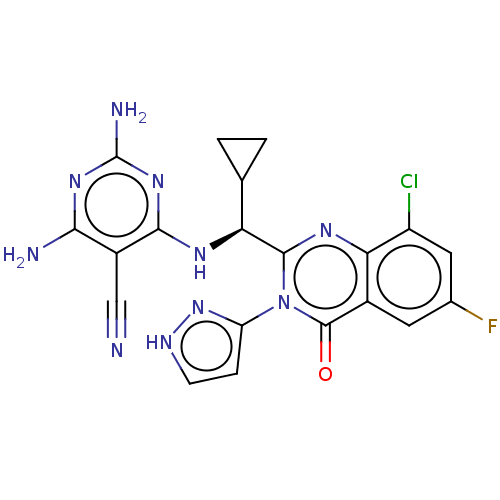
(CHEMBL4081433)Show SMILES Nc1nc(N)c(C#N)c(N[C@@H](C2CC2)c2nc3c(Cl)cc(F)cc3c(=O)n2-c2cc[nH]n2)n1 |r| Show InChI InChI=1S/C20H16ClFN10O/c21-12-6-9(22)5-10-15(12)28-18(32(19(10)33)13-3-4-26-31-13)14(8-1-2-8)27-17-11(7-23)16(24)29-20(25)30-17/h3-6,8,14H,1-2H2,(H,26,31)(H5,24,25,27,29,30)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human full length PI3K p110delta/p85 alpha using PIP2/ATP as substrate after 30 mins by TR-FRET assay |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50501403
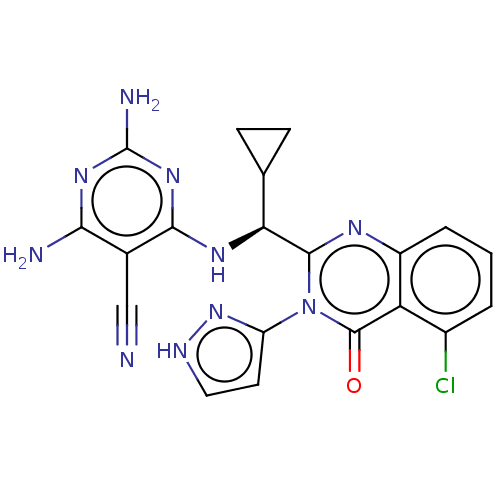
(CHEMBL4084645)Show SMILES Nc1nc(N)c(C#N)c(N[C@@H](C2CC2)c2nc3cccc(Cl)c3c(=O)n2-c2cc[nH]n2)n1 |r| Show InChI InChI=1S/C20H17ClN10O/c21-11-2-1-3-12-14(11)19(32)31(13-6-7-25-30-13)18(26-12)15(9-4-5-9)27-17-10(8-22)16(23)28-20(24)29-17/h1-3,6-7,9,15H,4-5H2,(H,25,30)(H5,23,24,27,28,29)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human full length PI3K p110delta/p85 alpha using PIP2/ATP as substrate after 30 mins by TR-FRET assay |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50501400
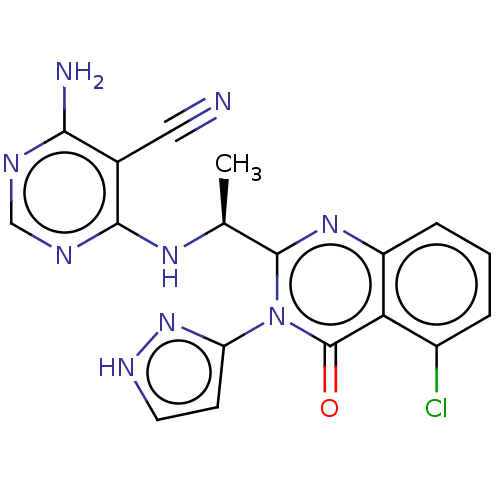
(CHEMBL4082130)Show SMILES C[C@H](Nc1ncnc(N)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1cc[nH]n1 |r| Show InChI InChI=1S/C18H14ClN9O/c1-9(25-16-10(7-20)15(21)22-8-23-16)17-26-12-4-2-3-11(19)14(12)18(29)28(17)13-5-6-24-27-13/h2-6,8-9H,1H3,(H,24,27)(H3,21,22,23,25)/t9-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human full length PI3K p110delta/p85 alpha using PIP2/ATP as substrate after 30 mins by TR-FRET assay |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM198006
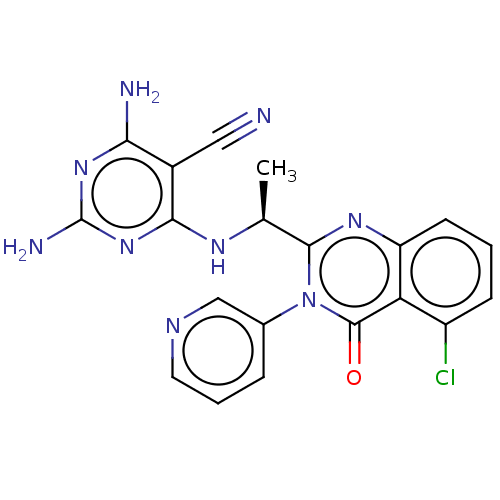
(US9221795, 1)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1cccnc1 |r| Show InChI InChI=1S/C20H16ClN9O/c1-10(26-17-12(8-22)16(23)28-20(24)29-17)18-27-14-6-2-5-13(21)15(14)19(31)30(18)11-4-3-7-25-9-11/h2-7,9-10H,1H3,(H5,23,24,26,28,29)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length PI3K p110delta/p85alpha using phosphatidylinositol 3,4,5-trisphosphate as substrate measured after 30 mins by TR-FRET... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01169
BindingDB Entry DOI: 10.7270/Q20G3PV2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM198027
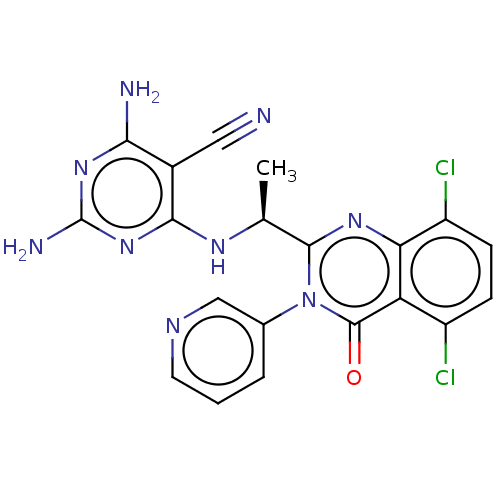
(US9221795, 23)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2c(Cl)ccc(Cl)c2c(=O)n1-c1cccnc1 |r| Show InChI InChI=1S/C20H15Cl2N9O/c1-9(27-17-11(7-23)16(24)29-20(25)30-17)18-28-15-13(22)5-4-12(21)14(15)19(32)31(18)10-3-2-6-26-8-10/h2-6,8-9H,1H3,(H5,24,25,27,29,30)/t9-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length PI3K p110delta/p85alpha using phosphatidylinositol 3,4,5-trisphosphate as substrate measured after 30 mins by TR-FRET... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01169
BindingDB Entry DOI: 10.7270/Q20G3PV2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50501408

(CHEMBL4081433)Show SMILES Nc1nc(N)c(C#N)c(N[C@@H](C2CC2)c2nc3c(Cl)cc(F)cc3c(=O)n2-c2cc[nH]n2)n1 |r| Show InChI InChI=1S/C20H16ClFN10O/c21-12-6-9(22)5-10-15(12)28-18(32(19(10)33)13-3-4-26-31-13)14(8-1-2-8)27-17-11(7-23)16(24)29-20(25)30-17/h3-6,8,14H,1-2H2,(H,26,31)(H5,24,25,27,29,30)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged recombinant full-length human PI3K p110beta/untagged recombinant full length human p85alpha expressed in baculov... |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50501402

(CHEMBL4071605)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1cc[nH]n1 |r| Show InChI InChI=1S/C18H15ClN10O/c1-8(24-15-9(7-20)14(21)26-18(22)27-15)16-25-11-4-2-3-10(19)13(11)17(30)29(16)12-5-6-23-28-12/h2-6,8H,1H3,(H,23,28)(H5,21,22,24,26,27)/t8-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged recombinant full-length human PI3K p110beta/untagged recombinant full length human p85alpha expressed in baculov... |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50501399

(CHEMBL4100135)Show SMILES C[C@H](Nc1ncnc(N)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1ccc(O)cc1 |r| Show InChI InChI=1S/C21H16ClN7O2/c1-11(27-19-14(9-23)18(24)25-10-26-19)20-28-16-4-2-3-15(22)17(16)21(31)29(20)12-5-7-13(30)8-6-12/h2-8,10-11,30H,1H3,(H3,24,25,26,27)/t11-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged recombinant full-length human PI3K p110beta/untagged recombinant full length human p85alpha expressed in baculov... |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50501411

(CHEMBL4068742)Show SMILES Cc1nc(N)nc(N[C@@H](C2CC2)c2nc3cccc(Cl)c3c(=O)n2-c2cc[nH]n2)c1C#N |r| Show InChI InChI=1S/C21H18ClN9O/c1-10-12(9-23)18(29-21(24)26-10)28-17(11-5-6-11)19-27-14-4-2-3-13(22)16(14)20(32)31(19)15-7-8-25-30-15/h2-4,7-8,11,17H,5-6H2,1H3,(H,25,30)(H3,24,26,28,29)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human full length PI3K p110delta/p85 alpha using PIP2/ATP as substrate after 30 mins by TR-FRET assay |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50168295

(CHEMBL3805769)Show SMILES C[C@H](Nc1nc(N)nc(N)c1Cl)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C20H17Cl2N7O/c1-10(25-17-15(22)16(23)27-20(24)28-17)18-26-13-9-5-8-12(21)14(13)19(30)29(18)11-6-3-2-4-7-11/h2-10H,1H3,(H5,23,24,25,27,28)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a... |
J Med Chem 59: 3532-48 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00213
BindingDB Entry DOI: 10.7270/Q22Z17F0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50168311
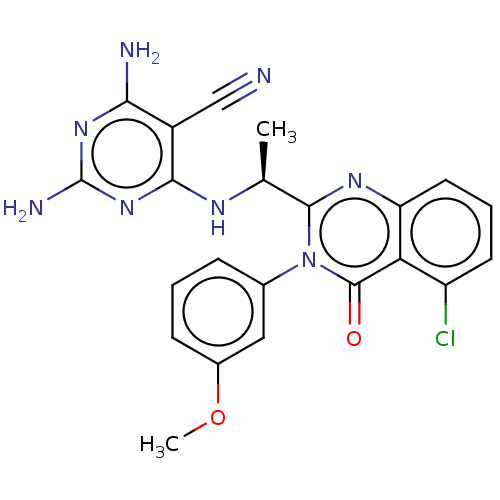
(CHEMBL3805522)Show SMILES COc1cccc(c1)-n1c(nc2cccc(Cl)c2c1=O)[C@H](C)Nc1nc(N)nc(N)c1C#N |r| Show InChI InChI=1S/C22H19ClN8O2/c1-11(27-19-14(10-24)18(25)29-22(26)30-19)20-28-16-8-4-7-15(23)17(16)21(32)31(20)12-5-3-6-13(9-12)33-2/h3-9,11H,1-2H3,(H5,25,26,27,29,30)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a... |
J Med Chem 59: 3532-48 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00213
BindingDB Entry DOI: 10.7270/Q22Z17F0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM198035

(BDBM198052 | US9221795, 31)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2c(F)ccc(Cl)c2c(=O)n1-c1cncc(F)c1 |r| Show InChI InChI=1S/C20H14ClF2N9O/c1-8(28-17-11(5-24)16(25)30-20(26)31-17)18-29-15-13(23)3-2-12(21)14(15)19(33)32(18)10-4-9(22)6-27-7-10/h2-4,6-8H,1H3,(H5,25,26,28,30,31)/t8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length PI3K p110delta/p85alpha using phosphatidylinositol 3,4,5-trisphosphate as substrate measured after 30 mins by TR-FRET... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01169
BindingDB Entry DOI: 10.7270/Q20G3PV2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50168296
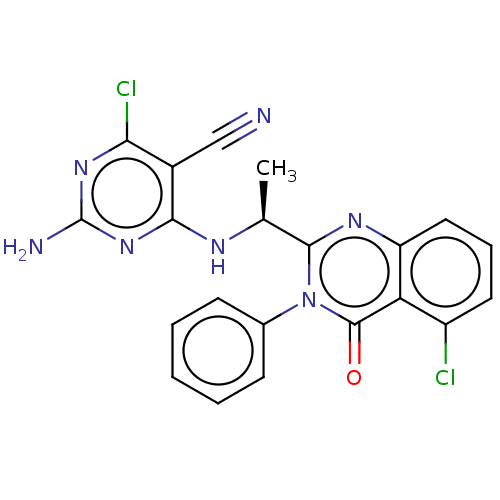
(CHEMBL3805180 | US9765060, Compound 101)Show SMILES C[C@H](Nc1nc(N)nc(Cl)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C21H15Cl2N7O/c1-11(26-18-13(10-24)17(23)28-21(25)29-18)19-27-15-9-5-8-14(22)16(15)20(31)30(19)12-6-3-2-4-7-12/h2-9,11H,1H3,(H3,25,26,28,29)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a... |
J Med Chem 59: 3532-48 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00213
BindingDB Entry DOI: 10.7270/Q22Z17F0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50168472
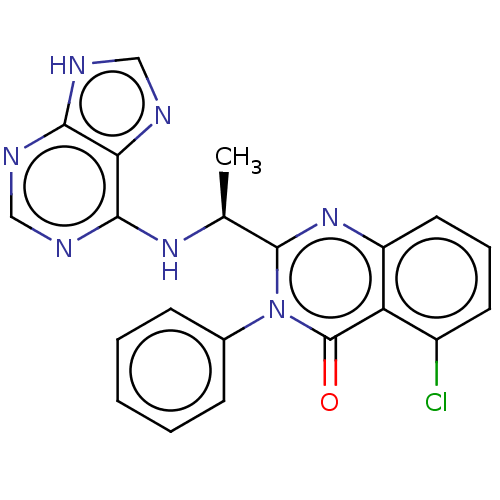
(CHEMBL3805348 | US9765060, Compound X)Show SMILES C[C@H](Nc1ncnc2[nH]cnc12)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C21H16ClN7O/c1-12(27-19-17-18(24-10-23-17)25-11-26-19)20-28-15-9-5-8-14(22)16(15)21(30)29(20)13-6-3-2-4-7-13/h2-12H,1H3,(H2,23,24,25,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a... |
J Med Chem 59: 3532-48 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00213
BindingDB Entry DOI: 10.7270/Q22Z17F0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data