 Found 465 hits with Last Name = 'levit' and Initial = 'm'
Found 465 hits with Last Name = 'levit' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518510
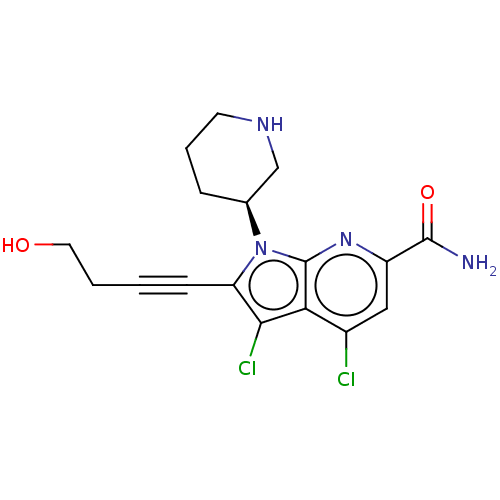
(CHEMBL4448325)Show SMILES NC(=O)c1cc(Cl)c2c(Cl)c(C#CCCO)n([C@H]3CCCNC3)c2n1 |r| Show InChI InChI=1S/C17H18Cl2N4O2/c18-11-8-12(16(20)25)22-17-14(11)15(19)13(5-1-2-7-24)23(17)10-4-3-6-21-9-10/h8,10,21,24H,2-4,6-7,9H2,(H2,20,25)/t10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(MOUSE) | BDBM50240339
((S)-2-((S)-2-((S)-2-((S)-1-((S)-6-amino-2-((S)-5-g...)Show SMILES CN[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=O)N[C@@H](CC(C)C)C(O)=O)C(C)(C)C |r,wU:2.1,43.45,wD:25.26,29.29,47.49,13.12,(-9.76,-17.57,;-8.43,-18.35,;-7.09,-17.6,;-7.09,-16.06,;-8.41,-15.28,;-8.4,-13.73,;-9.73,-12.95,;-9.72,-11.41,;-11.06,-10.63,;-8.38,-10.64,;-5.76,-18.38,;-5.77,-19.91,;-4.42,-17.61,;-3.09,-18.39,;-3.1,-19.93,;-4.44,-20.7,;-4.45,-22.24,;-5.78,-23,;-5.8,-24.54,;-1.74,-17.63,;-1.73,-16.1,;-.42,-18.41,;-0,-19.94,;1.55,-19.94,;2.08,-18.51,;.87,-17.56,;.94,-16,;-.36,-15.18,;2.3,-15.29,;3.6,-16.11,;3.53,-17.65,;4.83,-18.49,;6.26,-17.92,;7.23,-19.11,;6.4,-20.41,;6.81,-21.89,;5.72,-22.98,;4.24,-22.59,;3.84,-21.1,;4.92,-20.02,;4.97,-15.4,;5.05,-13.86,;6.27,-16.23,;7.64,-15.53,;8.93,-16.36,;8.86,-17.9,;10.3,-15.65,;11.59,-16.49,;11.52,-18.02,;12.82,-18.86,;12.82,-20.4,;14.19,-18.15,;12.96,-15.78,;14.25,-16.61,;13.04,-14.25,;7.72,-13.99,;7.71,-12.44,;9.26,-14.02,;6.18,-13.96,)| Show InChI InChI=1S/C41H67N11O7/c1-24(2)21-31(39(58)59)50-37(56)33(41(3,4)5)51-35(54)30(22-25-23-47-27-14-8-7-13-26(25)27)49-36(55)32-17-12-20-52(32)38(57)29(15-9-10-18-42)48-34(53)28(45-6)16-11-19-46-40(43)44/h7-8,13-14,23-24,28-33,45,47H,9-12,15-22,42H2,1-6H3,(H,48,53)(H,49,55)(H,50,56)(H,51,54)(H,58,59)(H4,43,44,46)/t28-,29-,30-,31-,32-,33+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 305-13 (2002)
Article DOI: 10.1124/jpet.300.1.305
BindingDB Entry DOI: 10.7270/Q2X34W09 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(MOUSE) | BDBM82078
(CAS_55508-42-4 | NSC_128644 | Neurotensin)Show SMILES CCC(C)C(NC(=O)C(Cc1ccc(O)cc1)NC(=O)C1CCCN1C(=O)C(CC(N)=O)NC(=O)C(N)CCCCN)C(=O)NC(CC(C)C)C(O)=O Show InChI InChI=1S/C36H58N8O9/c1-5-21(4)30(34(50)42-27(36(52)53)17-20(2)3)43-32(48)25(18-22-11-13-23(45)14-12-22)40-33(49)28-10-8-16-44(28)35(51)26(19-29(39)46)41-31(47)24(38)9-6-7-15-37/h11-14,20-21,24-28,30,45H,5-10,15-19,37-38H2,1-4H3,(H2,39,46)(H,40,49)(H,41,47)(H,42,50)(H,43,48)(H,52,53) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 305-13 (2002)
Article DOI: 10.1124/jpet.300.1.305
BindingDB Entry DOI: 10.7270/Q2X34W09 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518517
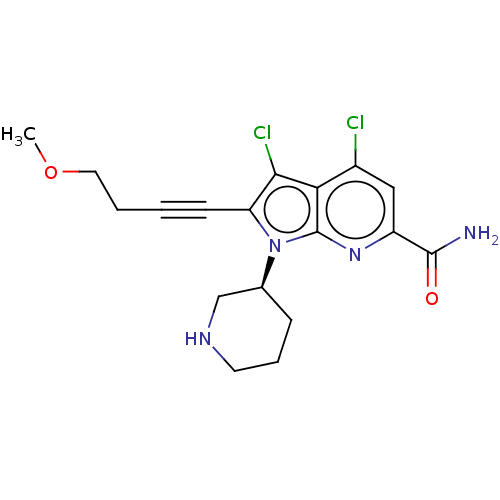
(CHEMBL4540910)Show SMILES COCCC#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C18H20Cl2N4O2/c1-26-8-3-2-6-14-16(20)15-12(19)9-13(17(21)25)23-18(15)24(14)11-5-4-7-22-10-11/h9,11,22H,3-5,7-8,10H2,1H3,(H2,21,25)/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518506

(CHEMBL4588948)Show SMILES CC(O)CC#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C18H20Cl2N4O2/c1-10(25)4-2-6-14-16(20)15-12(19)8-13(17(21)26)23-18(15)24(14)11-5-3-7-22-9-11/h8,10-11,22,25H,3-5,7,9H2,1H3,(H2,21,26)/t10?,11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518520

(CHEMBL4593810)Show SMILES CC(O)C#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C17H18Cl2N4O2/c1-9(24)4-5-13-15(19)14-11(18)7-12(16(20)25)22-17(14)23(13)10-3-2-6-21-8-10/h7,9-10,21,24H,2-3,6,8H2,1H3,(H2,20,25)/t9?,10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518507

(CHEMBL4583118)Show SMILES NC(=O)c1cc(Cl)c2c(Cl)c(C#CC3CNCCO3)n([C@H]3CCCNC3)c2n1 |r| Show InChI InChI=1S/C19H21Cl2N5O2/c20-13-8-14(18(22)27)25-19-16(13)17(21)15(4-3-12-10-24-6-7-28-12)26(19)11-2-1-5-23-9-11/h8,11-12,23-24H,1-2,5-7,9-10H2,(H2,22,27)/t11-,12?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518514
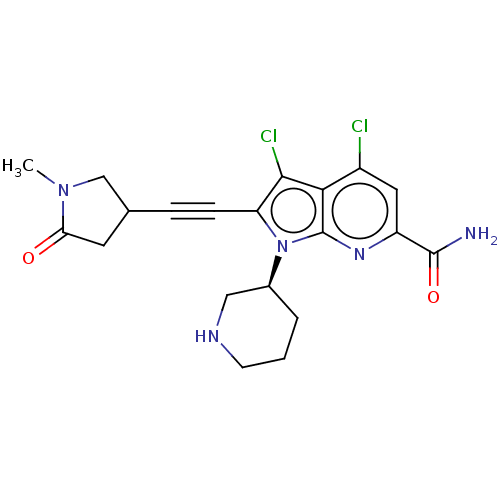
(CHEMBL4563802)Show SMILES CN1CC(CC1=O)C#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C20H21Cl2N5O2/c1-26-10-11(7-16(26)28)4-5-15-18(22)17-13(21)8-14(19(23)29)25-20(17)27(15)12-3-2-6-24-9-12/h8,11-12,24H,2-3,6-7,9-10H2,1H3,(H2,23,29)/t11?,12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518518
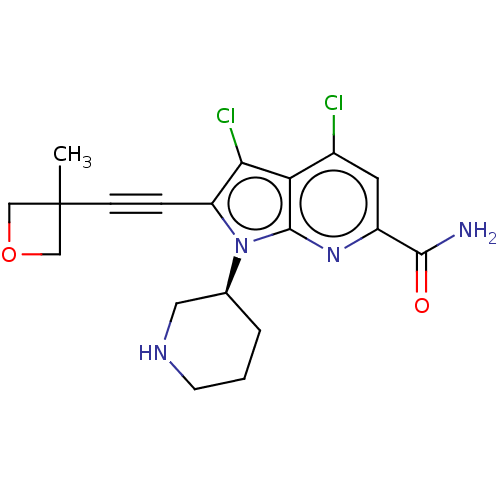
(CHEMBL4470576)Show SMILES CC1(COC1)C#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C19H20Cl2N4O2/c1-19(9-27-10-19)5-4-14-16(21)15-12(20)7-13(17(22)26)24-18(15)25(14)11-3-2-6-23-8-11/h7,11,23H,2-3,6,8-10H2,1H3,(H2,22,26)/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518505
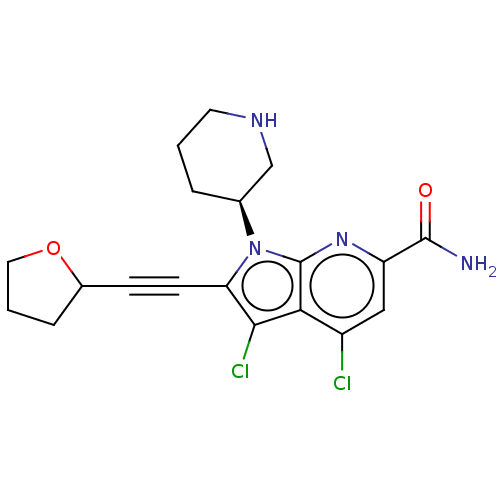
(CHEMBL4518492)Show SMILES NC(=O)c1cc(Cl)c2c(Cl)c(C#CC3CCCO3)n([C@H]3CCCNC3)c2n1 |r| Show InChI InChI=1S/C19H20Cl2N4O2/c20-13-9-14(18(22)26)24-19-16(13)17(21)15(6-5-12-4-2-8-27-12)25(19)11-3-1-7-23-10-11/h9,11-12,23H,1-4,7-8,10H2,(H2,22,26)/t11-,12?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518522

(CHEMBL4466080)Show SMILES NC(=O)c1cc(Cl)c2c(Cl)c(C#CCO)n([C@H]3CCCNC3)c2n1 |r| Show InChI InChI=1S/C16H16Cl2N4O2/c17-10-7-11(15(19)24)21-16-13(10)14(18)12(4-2-6-23)22(16)9-3-1-5-20-8-9/h7,9,20,23H,1,3,5-6,8H2,(H2,19,24)/t9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 2
(MOUSE) | BDBM50240339

((S)-2-((S)-2-((S)-2-((S)-1-((S)-6-amino-2-((S)-5-g...)Show SMILES CN[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=O)N[C@@H](CC(C)C)C(O)=O)C(C)(C)C |r,wU:2.1,43.45,wD:25.26,29.29,47.49,13.12,(-9.76,-17.57,;-8.43,-18.35,;-7.09,-17.6,;-7.09,-16.06,;-8.41,-15.28,;-8.4,-13.73,;-9.73,-12.95,;-9.72,-11.41,;-11.06,-10.63,;-8.38,-10.64,;-5.76,-18.38,;-5.77,-19.91,;-4.42,-17.61,;-3.09,-18.39,;-3.1,-19.93,;-4.44,-20.7,;-4.45,-22.24,;-5.78,-23,;-5.8,-24.54,;-1.74,-17.63,;-1.73,-16.1,;-.42,-18.41,;-0,-19.94,;1.55,-19.94,;2.08,-18.51,;.87,-17.56,;.94,-16,;-.36,-15.18,;2.3,-15.29,;3.6,-16.11,;3.53,-17.65,;4.83,-18.49,;6.26,-17.92,;7.23,-19.11,;6.4,-20.41,;6.81,-21.89,;5.72,-22.98,;4.24,-22.59,;3.84,-21.1,;4.92,-20.02,;4.97,-15.4,;5.05,-13.86,;6.27,-16.23,;7.64,-15.53,;8.93,-16.36,;8.86,-17.9,;10.3,-15.65,;11.59,-16.49,;11.52,-18.02,;12.82,-18.86,;12.82,-20.4,;14.19,-18.15,;12.96,-15.78,;14.25,-16.61,;13.04,-14.25,;7.72,-13.99,;7.71,-12.44,;9.26,-14.02,;6.18,-13.96,)| Show InChI InChI=1S/C41H67N11O7/c1-24(2)21-31(39(58)59)50-37(56)33(41(3,4)5)51-35(54)30(22-25-23-47-27-14-8-7-13-26(25)27)49-36(55)32-17-12-20-52(32)38(57)29(15-9-10-18-42)48-34(53)28(45-6)16-11-19-46-40(43)44/h7-8,13-14,23-24,28-33,45,47H,9-12,15-22,42H2,1-6H3,(H,48,53)(H,49,55)(H,50,56)(H,51,54)(H,58,59)(H4,43,44,46)/t28-,29-,30-,31-,32-,33+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 305-13 (2002)
Article DOI: 10.1124/jpet.300.1.305
BindingDB Entry DOI: 10.7270/Q2X34W09 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 2
(MOUSE) | BDBM82078

(CAS_55508-42-4 | NSC_128644 | Neurotensin)Show SMILES CCC(C)C(NC(=O)C(Cc1ccc(O)cc1)NC(=O)C1CCCN1C(=O)C(CC(N)=O)NC(=O)C(N)CCCCN)C(=O)NC(CC(C)C)C(O)=O Show InChI InChI=1S/C36H58N8O9/c1-5-21(4)30(34(50)42-27(36(52)53)17-20(2)3)43-32(48)25(18-22-11-13-23(45)14-12-22)40-33(49)28-10-8-16-44(28)35(51)26(19-29(39)46)41-31(47)24(38)9-6-7-15-37/h11-14,20-21,24-28,30,45H,5-10,15-19,37-38H2,1-4H3,(H2,39,46)(H,40,49)(H,41,47)(H,42,50)(H,43,48)(H,52,53) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 305-13 (2002)
Article DOI: 10.1124/jpet.300.1.305
BindingDB Entry DOI: 10.7270/Q2X34W09 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50129806
(CHEMBL3627987)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCNC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CCCCN)NC1=O)C(=O)N[C@@H](CC(C)C)C(N)=O)[C@H](C)O |r| Show InChI InChI=1S/C43H71N15O9S2/c1-22(2)17-31(35(47)60)55-41(66)33-21-69-68-20-27(46)36(61)50-16-13-26(45)37(62)53-30(12-8-15-51-43(48)49)38(63)56-32(19-25-18-24-9-4-5-10-28(24)52-25)40(65)58-34(23(3)59)42(67)54-29(39(64)57-33)11-6-7-14-44/h4-5,9-10,18,22-23,26-27,29-34,52,59H,6-8,11-17,19-21,44-46H2,1-3H3,(H2,47,60)(H,50,61)(H,53,62)(H,54,67)(H,55,66)(H,56,63)(H,57,64)(H,58,65)(H4,48,49,51)/t23-,26-,27-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brookhaven National Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain 448 residue preincubated for 30 mins by FRET assay |
Bioorg Med Chem 23: 7264-73 (2015)
Article DOI: 10.1016/j.bmc.2015.10.024
BindingDB Entry DOI: 10.7270/Q2MG7RB8 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 2
(MOUSE) | BDBM85844
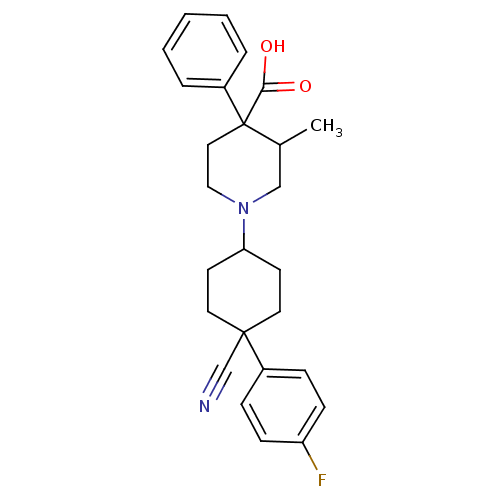
(CAS_54385 | Levocabastine | NSC_54385)Show SMILES CC1CN(CCC1(C(O)=O)c1ccccc1)C1CCC(CC1)(C#N)c1ccc(F)cc1 |(1.11,-6.21,;-.22,-6.98,;-.22,-8.52,;-1.56,-9.29,;-2.89,-8.52,;-2.89,-6.98,;-1.56,-6.21,;-.84,-4.85,;-.73,-3.31,;.7,-4.8,;-2.28,-4.85,;-3.82,-4.8,;-4.54,-3.44,;-3.73,-2.13,;-2.19,-2.18,;-1.47,-3.54,;-1.56,-10.83,;-2.89,-11.6,;-2.89,-13.14,;-1.56,-13.91,;-.22,-13.14,;-.22,-11.6,;-2.28,-15.27,;-3,-16.63,;-.84,-15.27,;.7,-15.32,;1.43,-16.68,;.61,-17.99,;1.33,-19.35,;-.93,-17.93,;-1.65,-16.57,)| Show InChI InChI=1S/C26H29FN2O2/c1-19-17-29(16-15-26(19,24(30)31)21-5-3-2-4-6-21)23-11-13-25(18-28,14-12-23)20-7-9-22(27)10-8-20/h2-10,19,23H,11-17H2,1H3,(H,30,31) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 305-13 (2002)
Article DOI: 10.1124/jpet.300.1.305
BindingDB Entry DOI: 10.7270/Q2X34W09 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50129806

(CHEMBL3627987)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCNC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CCCCN)NC1=O)C(=O)N[C@@H](CC(C)C)C(N)=O)[C@H](C)O |r| Show InChI InChI=1S/C43H71N15O9S2/c1-22(2)17-31(35(47)60)55-41(66)33-21-69-68-20-27(46)36(61)50-16-13-26(45)37(62)53-30(12-8-15-51-43(48)49)38(63)56-32(19-25-18-24-9-4-5-10-28(24)52-25)40(65)58-34(23(3)59)42(67)54-29(39(64)57-33)11-6-7-14-44/h4-5,9-10,18,22-23,26-27,29-34,52,59H,6-8,11-17,19-21,44-46H2,1-3H3,(H2,47,60)(H,50,61)(H,53,62)(H,54,67)(H,55,66)(H,56,63)(H,57,64)(H,58,65)(H4,48,49,51)/t23-,26-,27-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brookhaven National Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain 448 residue coincubated with TCEP for 30 mins and TCEP absent during reaction by FRET assay |
Bioorg Med Chem 23: 7264-73 (2015)
Article DOI: 10.1016/j.bmc.2015.10.024
BindingDB Entry DOI: 10.7270/Q2MG7RB8 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50129806

(CHEMBL3627987)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCNC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CCCCN)NC1=O)C(=O)N[C@@H](CC(C)C)C(N)=O)[C@H](C)O |r| Show InChI InChI=1S/C43H71N15O9S2/c1-22(2)17-31(35(47)60)55-41(66)33-21-69-68-20-27(46)36(61)50-16-13-26(45)37(62)53-30(12-8-15-51-43(48)49)38(63)56-32(19-25-18-24-9-4-5-10-28(24)52-25)40(65)58-34(23(3)59)42(67)54-29(39(64)57-33)11-6-7-14-44/h4-5,9-10,18,22-23,26-27,29-34,52,59H,6-8,11-17,19-21,44-46H2,1-3H3,(H2,47,60)(H,50,61)(H,53,62)(H,54,67)(H,55,66)(H,56,63)(H,57,64)(H,58,65)(H4,48,49,51)/t23-,26-,27-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brookhaven National Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum truncated-BoNT/A light chain 424 residue preincubated for 30 mins by FRET assay |
Bioorg Med Chem 23: 7264-73 (2015)
Article DOI: 10.1016/j.bmc.2015.10.024
BindingDB Entry DOI: 10.7270/Q2MG7RB8 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50129806

(CHEMBL3627987)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCNC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CCCCN)NC1=O)C(=O)N[C@@H](CC(C)C)C(N)=O)[C@H](C)O |r| Show InChI InChI=1S/C43H71N15O9S2/c1-22(2)17-31(35(47)60)55-41(66)33-21-69-68-20-27(46)36(61)50-16-13-26(45)37(62)53-30(12-8-15-51-43(48)49)38(63)56-32(19-25-18-24-9-4-5-10-28(24)52-25)40(65)58-34(23(3)59)42(67)54-29(39(64)57-33)11-6-7-14-44/h4-5,9-10,18,22-23,26-27,29-34,52,59H,6-8,11-17,19-21,44-46H2,1-3H3,(H2,47,60)(H,50,61)(H,53,62)(H,54,67)(H,55,66)(H,56,63)(H,57,64)(H,58,65)(H4,48,49,51)/t23-,26-,27-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brookhaven National Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain 448 residue coincubated with TCEP for 60 mins and TCEP absent during reaction by FRET assay |
Bioorg Med Chem 23: 7264-73 (2015)
Article DOI: 10.1016/j.bmc.2015.10.024
BindingDB Entry DOI: 10.7270/Q2MG7RB8 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50129806

(CHEMBL3627987)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCNC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CCCCN)NC1=O)C(=O)N[C@@H](CC(C)C)C(N)=O)[C@H](C)O |r| Show InChI InChI=1S/C43H71N15O9S2/c1-22(2)17-31(35(47)60)55-41(66)33-21-69-68-20-27(46)36(61)50-16-13-26(45)37(62)53-30(12-8-15-51-43(48)49)38(63)56-32(19-25-18-24-9-4-5-10-28(24)52-25)40(65)58-34(23(3)59)42(67)54-29(39(64)57-33)11-6-7-14-44/h4-5,9-10,18,22-23,26-27,29-34,52,59H,6-8,11-17,19-21,44-46H2,1-3H3,(H2,47,60)(H,50,61)(H,53,62)(H,54,67)(H,55,66)(H,56,63)(H,57,64)(H,58,65)(H4,48,49,51)/t23-,26-,27-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brookhaven National Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain 448 residue coincubated with TCEP for 30 mins and TCEP present during reaction by FRET assay |
Bioorg Med Chem 23: 7264-73 (2015)
Article DOI: 10.1016/j.bmc.2015.10.024
BindingDB Entry DOI: 10.7270/Q2MG7RB8 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50129806

(CHEMBL3627987)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCNC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CCCCN)NC1=O)C(=O)N[C@@H](CC(C)C)C(N)=O)[C@H](C)O |r| Show InChI InChI=1S/C43H71N15O9S2/c1-22(2)17-31(35(47)60)55-41(66)33-21-69-68-20-27(46)36(61)50-16-13-26(45)37(62)53-30(12-8-15-51-43(48)49)38(63)56-32(19-25-18-24-9-4-5-10-28(24)52-25)40(65)58-34(23(3)59)42(67)54-29(39(64)57-33)11-6-7-14-44/h4-5,9-10,18,22-23,26-27,29-34,52,59H,6-8,11-17,19-21,44-46H2,1-3H3,(H2,47,60)(H,50,61)(H,53,62)(H,54,67)(H,55,66)(H,56,63)(H,57,64)(H,58,65)(H4,48,49,51)/t23-,26-,27-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brookhaven National Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain 448 residue coincubated with TCEP for 60 mins and TCEP present during reaction by FRET assay |
Bioorg Med Chem 23: 7264-73 (2015)
Article DOI: 10.1016/j.bmc.2015.10.024
BindingDB Entry DOI: 10.7270/Q2MG7RB8 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50129807

(CHEMBL3627988)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCNC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CCCCN)NC1=O)C(N)=O)[C@H](C)O |r| Show InChI InChI=1S/C37H60N14O8S2/c1-19(52)29-36(59)48-25(9-4-5-12-38)34(57)50-28(30(41)53)18-61-60-17-23(40)31(54)44-14-11-22(39)32(55)47-26(10-6-13-45-37(42)43)33(56)49-27(35(58)51-29)16-21-15-20-7-2-3-8-24(20)46-21/h2-3,7-8,15,19,22-23,25-29,46,52H,4-6,9-14,16-18,38-40H2,1H3,(H2,41,53)(H,44,54)(H,47,55)(H,48,59)(H,49,56)(H,50,57)(H,51,58)(H4,42,43,45)/t19-,22-,23-,25-,26-,27-,28-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 468 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brookhaven National Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain 448 residue preincubated for 30 mins by FRET assay |
Bioorg Med Chem 23: 7264-73 (2015)
Article DOI: 10.1016/j.bmc.2015.10.024
BindingDB Entry DOI: 10.7270/Q2MG7RB8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50402386
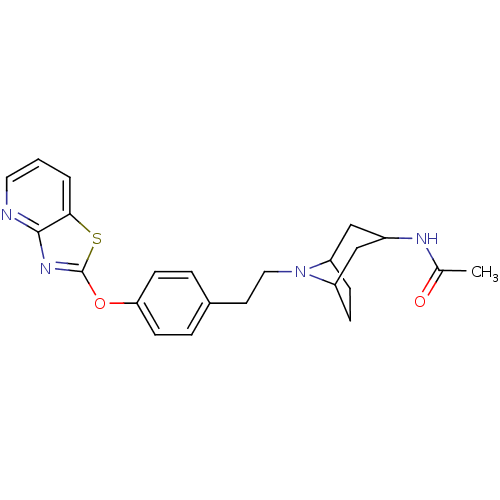
(CHEMBL2207747)Show SMILES CC(=O)NC1CC2CCC(C1)N2CCc1ccc(Oc2nc3ncccc3s2)cc1 |TLB:3:4:11:7.8,THB:12:11:4.5.10:7.8| Show InChI InChI=1S/C23H26N4O2S/c1-15(28)25-17-13-18-6-7-19(14-17)27(18)12-10-16-4-8-20(9-5-16)29-23-26-22-21(30-23)3-2-11-24-22/h2-5,8-9,11,17-19H,6-7,10,12-14H2,1H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... |
Bioorg Med Chem Lett 22: 7504-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.036
BindingDB Entry DOI: 10.7270/Q2ZG6TDQ |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50377765
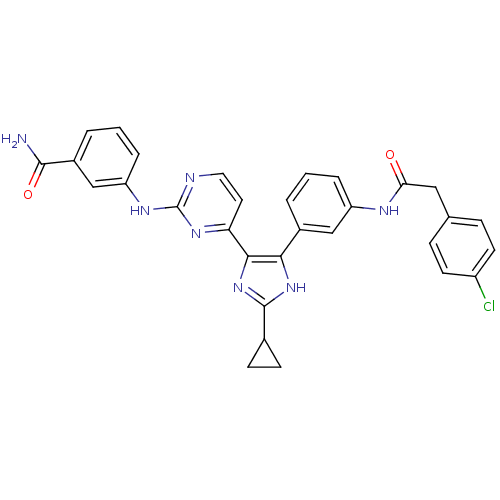
(CHEMBL254966)Show SMILES NC(=O)c1cccc(Nc2nccc(n2)-c2nc([nH]c2-c2cccc(NC(=O)Cc3ccc(Cl)cc3)c2)C2CC2)c1 Show InChI InChI=1S/C31H26ClN7O2/c32-22-11-7-18(8-12-22)15-26(40)35-23-5-1-3-20(16-23)27-28(39-30(38-27)19-9-10-19)25-13-14-34-31(37-25)36-24-6-2-4-21(17-24)29(33)41/h1-8,11-14,16-17,19H,9-10,15H2,(H2,33,41)(H,35,40)(H,38,39)(H,34,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of C-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518510

(CHEMBL4448325)Show SMILES NC(=O)c1cc(Cl)c2c(Cl)c(C#CCCO)n([C@H]3CCCNC3)c2n1 |r| Show InChI InChI=1S/C17H18Cl2N4O2/c18-11-8-12(16(20)25)22-17-14(11)15(19)13(5-1-2-7-24)23(17)10-4-3-6-21-9-10/h8,10,21,24H,2-4,6-7,9H2,(H2,20,25)/t10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50261864
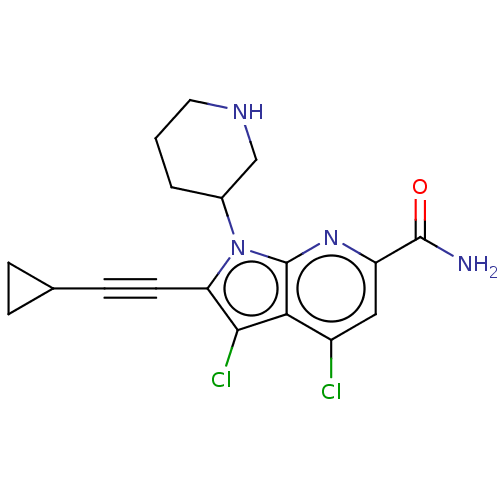
(CHEMBL4100435)Show SMILES NC(=O)c1cc(Cl)c2c(Cl)c(C#CC3CC3)n(C3CCCNC3)c2n1 Show InChI InChI=1S/C18H18Cl2N4O/c19-12-8-13(17(21)25)23-18-15(12)16(20)14(6-5-10-3-4-10)24(18)11-2-1-7-22-9-11/h8,10-11,22H,1-4,7,9H2,(H2,21,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com.
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay |
Bioorg Med Chem Lett 27: 4735-4740 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.068
BindingDB Entry DOI: 10.7270/Q2NG4T34 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50554405
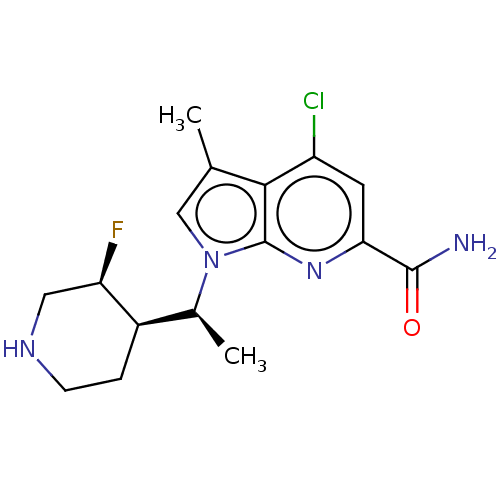
(CHEMBL4759685)Show SMILES [H][C@@]1(CCNC[C@H]1F)[C@H](C)n1cc(C)c2c(Cl)cc(nc12)C(N)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM1 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127625
BindingDB Entry DOI: 10.7270/Q2Z3239C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518521

(CHEMBL4564586)Show SMILES NC(=O)c1cc(Cl)c2c(Cl)c(C#CC3CC3)n([C@H]3CCCNC3)c2n1 |r| Show InChI InChI=1S/C18H18Cl2N4O/c19-12-8-13(17(21)25)23-18-15(12)16(20)14(6-5-10-3-4-10)24(18)11-2-1-7-22-9-11/h8,10-11,22H,1-4,7,9H2,(H2,21,25)/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518517

(CHEMBL4540910)Show SMILES COCCC#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C18H20Cl2N4O2/c1-26-8-3-2-6-14-16(20)15-12(19)9-13(17(21)25)23-18(15)24(14)11-5-4-7-22-10-11/h9,11,22H,3-5,7-8,10H2,1H3,(H2,21,25)/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50377766
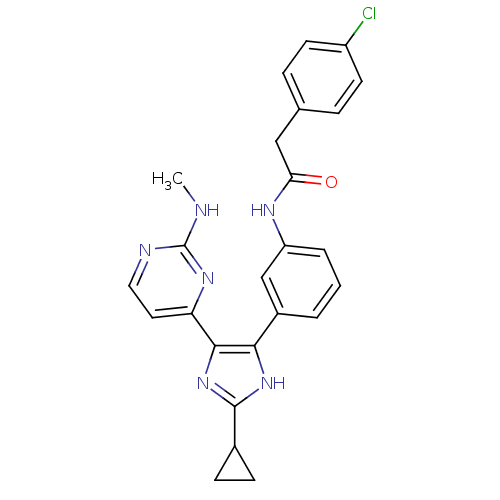
(CHEMBL403854)Show SMILES CNc1nccc(n1)-c1nc([nH]c1-c1cccc(NC(=O)Cc2ccc(Cl)cc2)c1)C1CC1 Show InChI InChI=1S/C25H23ClN6O/c1-27-25-28-12-11-20(30-25)23-22(31-24(32-23)16-7-8-16)17-3-2-4-19(14-17)29-21(33)13-15-5-9-18(26)10-6-15/h2-6,9-12,14,16H,7-8,13H2,1H3,(H,29,33)(H,31,32)(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of C-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518506

(CHEMBL4588948)Show SMILES CC(O)CC#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C18H20Cl2N4O2/c1-10(25)4-2-6-14-16(20)15-12(19)8-13(17(21)26)23-18(15)24(14)11-5-3-7-22-9-11/h8,10-11,22,25H,3-5,7,9H2,1H3,(H2,21,26)/t10?,11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50261857

(CHEMBL4082422)Show SMILES NC(=O)c1cc(Cl)c2c(Cl)c(C3CC3)n(C3CCCNC3)c2n1 Show InChI InChI=1S/C16H18Cl2N4O/c17-10-6-11(15(19)23)21-16-12(10)13(18)14(8-3-4-8)22(16)9-2-1-5-20-7-9/h6,8-9,20H,1-5,7H2,(H2,19,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com.
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay |
Bioorg Med Chem Lett 27: 4735-4740 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.068
BindingDB Entry DOI: 10.7270/Q2NG4T34 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50261808
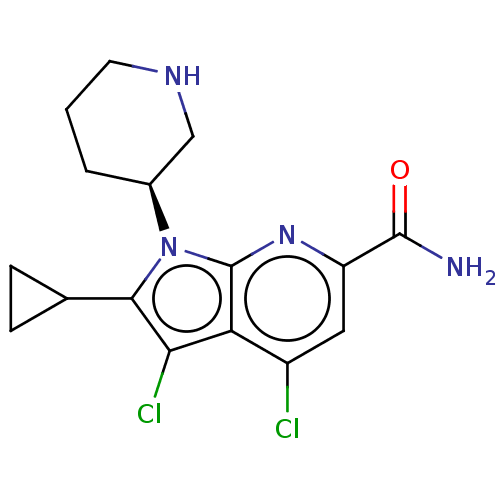
(CHEMBL4104355)Show SMILES NC(=O)c1cc(Cl)c2c(Cl)c(C3CC3)n([C@H]3CCCNC3)c2n1 |r| Show InChI InChI=1S/C16H18Cl2N4O/c17-10-6-11(15(19)23)21-16-12(10)13(18)14(8-3-4-8)22(16)9-2-1-5-20-7-9/h6,8-9,20H,1-5,7H2,(H2,19,23)/t9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com.
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay |
Bioorg Med Chem Lett 27: 4735-4740 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.068
BindingDB Entry DOI: 10.7270/Q2NG4T34 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50554404
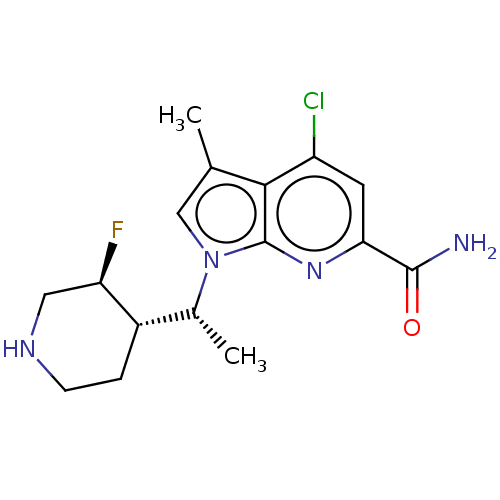
(CHEMBL4745141)Show SMILES [H][C@]1(CCNC[C@H]1F)[C@@H](C)n1cc(C)c2c(Cl)cc(nc12)C(N)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM1 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127625
BindingDB Entry DOI: 10.7270/Q2Z3239C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50554403
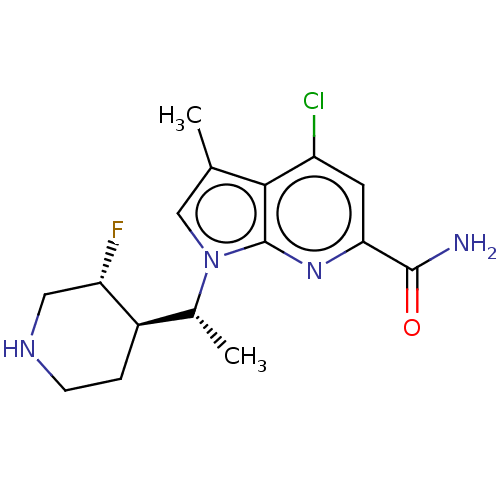
(CHEMBL4750046)Show SMILES [H][C@@]1(CCNC[C@@H]1F)[C@@H](C)n1cc(C)c2c(Cl)cc(nc12)C(N)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM1 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127625
BindingDB Entry DOI: 10.7270/Q2Z3239C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50554401
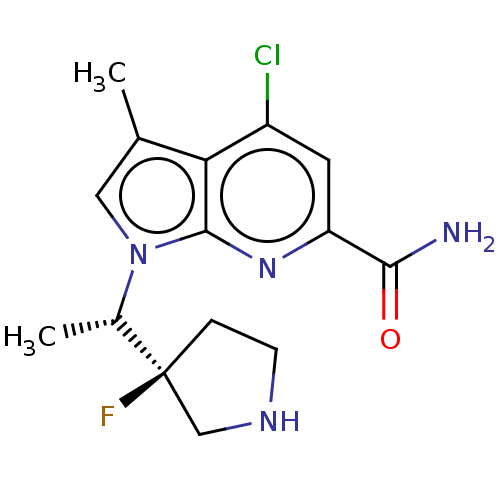
(CHEMBL4778578)Show SMILES C[C@H](n1cc(C)c2c(Cl)cc(nc12)C(N)=O)[C@]1(F)CCNC1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM1 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127625
BindingDB Entry DOI: 10.7270/Q2Z3239C |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50402382
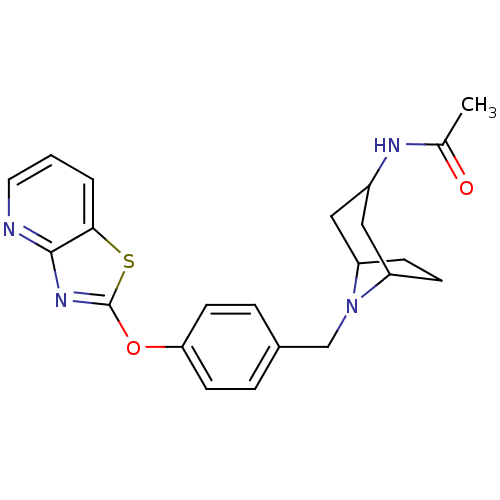
(CHEMBL2207751)Show SMILES CC(=O)NC1CC2CCC(C1)N2Cc1ccc(Oc2nc3ncccc3s2)cc1 |TLB:3:4:11:7.8| Show InChI InChI=1S/C22H24N4O2S/c1-14(27)24-16-11-17-6-7-18(12-16)26(17)13-15-4-8-19(9-5-15)28-22-25-21-20(29-22)3-2-10-23-21/h2-5,8-10,16-18H,6-7,11-13H2,1H3,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LTA4 hydrolase expressed in SF9 cells using LTA4 as substrate assessed as LTB4 production incubated for 10 mins prior... |
Bioorg Med Chem Lett 22: 7504-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.036
BindingDB Entry DOI: 10.7270/Q2ZG6TDQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50261822

(CHEMBL4093592)Show InChI InChI=1S/C13H13Cl2IN4O/c14-7-3-8(12(17)21)19-13-9(7)10(15)11(16)20(13)5-6-1-2-18-4-6/h3,6,18H,1-2,4-5H2,(H2,17,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com.
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay |
Bioorg Med Chem Lett 27: 4735-4740 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.068
BindingDB Entry DOI: 10.7270/Q2NG4T34 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50261827

(CHEMBL4070441)Show SMILES NC1CCC(CC1)n1c(I)c(Cl)c2c(Cl)cc(nc12)C(N)=O |(71.97,-24.68,;71.49,-23.21,;69.98,-22.89,;69.5,-21.44,;70.53,-20.3,;72.04,-20.6,;72.52,-22.06,;70.05,-18.83,;70.95,-17.58,;72.49,-17.57,;70.03,-16.34,;70.5,-14.87,;68.57,-16.82,;67.24,-16.06,;67.23,-14.52,;65.91,-16.83,;65.91,-18.37,;67.24,-19.15,;68.58,-18.37,;64.57,-19.14,;64.57,-20.68,;63.24,-18.37,)| Show InChI InChI=1S/C14H15Cl2IN4O/c15-8-5-9(13(19)22)20-14-10(8)11(16)12(17)21(14)7-3-1-6(18)2-4-7/h5-7H,1-4,18H2,(H2,19,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com.
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay |
Bioorg Med Chem Lett 27: 4735-4740 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.068
BindingDB Entry DOI: 10.7270/Q2NG4T34 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50261822

(CHEMBL4093592)Show InChI InChI=1S/C13H13Cl2IN4O/c14-7-3-8(12(17)21)19-13-9(7)10(15)11(16)20(13)5-6-1-2-18-4-6/h3,6,18H,1-2,4-5H2,(H2,17,21) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com.
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis H37RV InhA |
Bioorg Med Chem Lett 27: 4735-4740 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.068
BindingDB Entry DOI: 10.7270/Q2NG4T34 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50261826
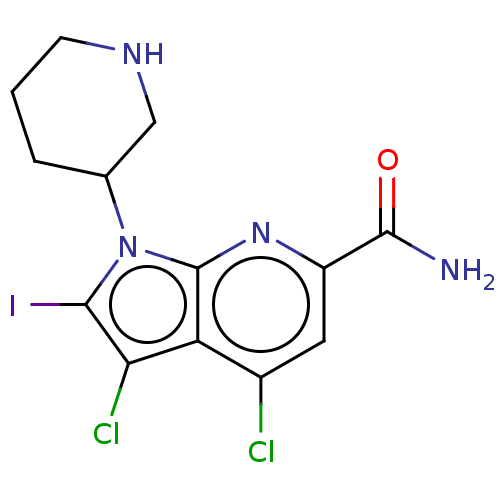
(CHEMBL4084988)Show InChI InChI=1S/C13H13Cl2IN4O/c14-7-4-8(12(17)21)19-13-9(7)10(15)11(16)20(13)6-2-1-3-18-5-6/h4,6,18H,1-3,5H2,(H2,17,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com.
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay |
Bioorg Med Chem Lett 27: 4735-4740 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.068
BindingDB Entry DOI: 10.7270/Q2NG4T34 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50261828
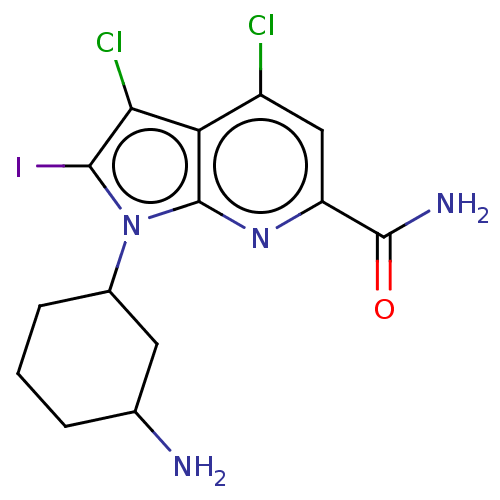
(CHEMBL4095220)Show SMILES NC1CCCC(C1)n1c(I)c(Cl)c2c(Cl)cc(nc12)C(N)=O Show InChI InChI=1S/C14H15Cl2IN4O/c15-8-5-9(13(19)22)20-14-10(8)11(16)12(17)21(14)7-3-1-2-6(18)4-7/h5-7H,1-4,18H2,(H2,19,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com.
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay |
Bioorg Med Chem Lett 27: 4735-4740 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.068
BindingDB Entry DOI: 10.7270/Q2NG4T34 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50261829
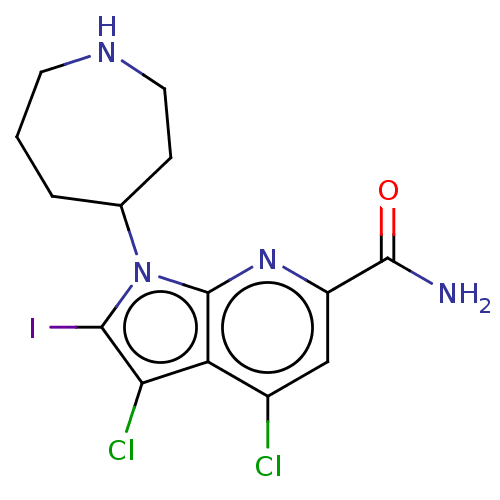
(CHEMBL4067797)Show InChI InChI=1S/C14H15Cl2IN4O/c15-8-6-9(13(18)22)20-14-10(8)11(16)12(17)21(14)7-2-1-4-19-5-3-7/h6-7,19H,1-5H2,(H2,18,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com.
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay |
Bioorg Med Chem Lett 27: 4735-4740 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.068
BindingDB Entry DOI: 10.7270/Q2NG4T34 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50261858

(CHEMBL4062258)Show SMILES Cn1cc(cn1)-c1c(Cl)c2c(Cl)cc(nc2n1C1CCCNC1)C(N)=O Show InChI InChI=1S/C17H18Cl2N6O/c1-24-8-9(6-22-24)15-14(19)13-11(18)5-12(16(20)26)23-17(13)25(15)10-3-2-4-21-7-10/h5-6,8,10,21H,2-4,7H2,1H3,(H2,20,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com.
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay |
Bioorg Med Chem Lett 27: 4735-4740 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.068
BindingDB Entry DOI: 10.7270/Q2NG4T34 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50377767

(CHEMBL429498)Show SMILES Clc1ccc(CC(=O)Nc2cccc(c2)-c2[nH]c(nc2-c2ccnc(NCCN3CCNC3=O)n2)C2CC2)cc1 Show InChI InChI=1S/C29H29ClN8O2/c30-21-8-4-18(5-9-21)16-24(39)34-22-3-1-2-20(17-22)25-26(37-27(36-25)19-6-7-19)23-10-11-31-28(35-23)32-12-14-38-15-13-33-29(38)40/h1-5,8-11,17,19H,6-7,12-16H2,(H,33,40)(H,34,39)(H,36,37)(H,31,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of C-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518520

(CHEMBL4593810)Show SMILES CC(O)C#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C17H18Cl2N4O2/c1-9(24)4-5-13-15(19)14-11(18)7-12(16(20)25)22-17(14)23(13)10-3-2-6-21-8-10/h7,9-10,21,24H,2-3,6,8H2,1H3,(H2,20,25)/t9?,10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50518511
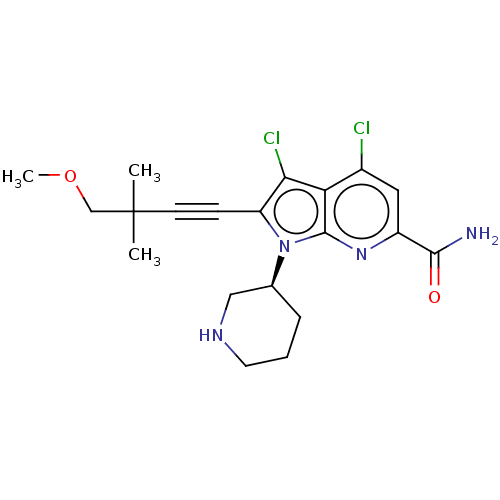
(CHEMBL4457529)Show SMILES COCC(C)(C)C#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C20H24Cl2N4O2/c1-20(2,11-28-3)7-6-15-17(22)16-13(21)9-14(18(23)27)25-19(16)26(15)12-5-4-8-24-10-12/h9,12,24H,4-5,8,10-11H2,1-3H3,(H2,23,27)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50518504
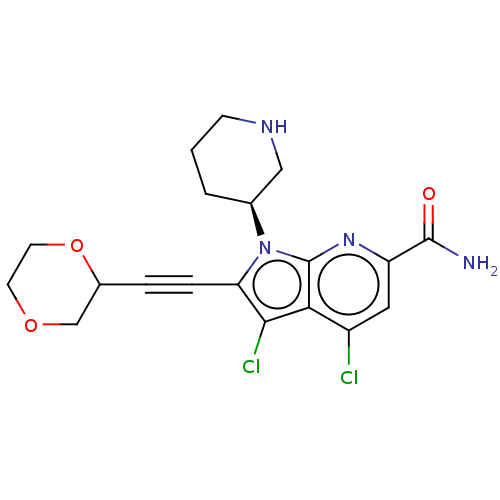
(CHEMBL4466764)Show SMILES NC(=O)c1cc(Cl)c2c(Cl)c(C#CC3COCCO3)n([C@H]3CCCNC3)c2n1 |r| Show InChI InChI=1S/C19H20Cl2N4O3/c20-13-8-14(18(22)26)24-19-16(13)17(21)15(4-3-12-10-27-6-7-28-12)25(19)11-2-1-5-23-9-11/h8,11-12,23H,1-2,5-7,9-10H2,(H2,22,26)/t11-,12?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518513

(CHEMBL4528544)Show SMILES NC(=O)c1cc(Cl)c2c(Cl)c(C#CC3CCOCC3)n([C@H]3CCCNC3)c2n1 |r| Show InChI InChI=1S/C20H22Cl2N4O2/c21-14-10-15(19(23)27)25-20-17(14)18(22)16(4-3-12-5-8-28-9-6-12)26(20)13-2-1-7-24-11-13/h10,12-13,24H,1-2,5-9,11H2,(H2,23,27)/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518511

(CHEMBL4457529)Show SMILES COCC(C)(C)C#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C20H24Cl2N4O2/c1-20(2,11-28-3)7-6-15-17(22)16-13(21)9-14(18(23)27)25-19(16)26(15)12-5-4-8-24-10-12/h9,12,24H,4-5,8,10-11H2,1-3H3,(H2,23,27)/t12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518518

(CHEMBL4470576)Show SMILES CC1(COC1)C#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C19H20Cl2N4O2/c1-19(9-27-10-19)5-4-14-16(21)15-12(20)7-13(17(22)26)24-18(15)25(14)11-3-2-6-23-8-11/h7,11,23H,2-3,6,8-10H2,1H3,(H2,22,26)/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data