 Found 482 hits with Last Name = 'li' and Initial = 'zh'
Found 482 hits with Last Name = 'li' and Initial = 'zh' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Farnesyl diphosphate synthase
(Trypanosoma cruzi) | BDBM25298
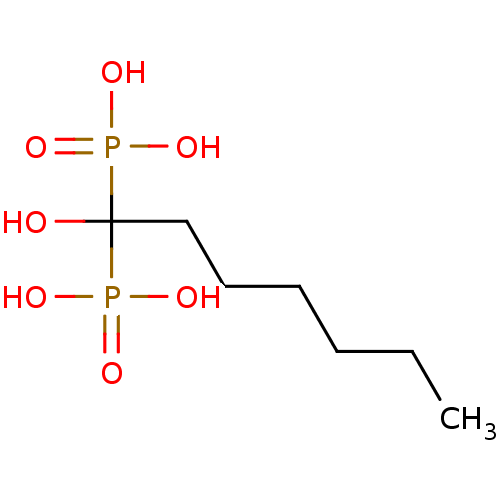
((1-hydroxy-1-phosphonoheptyl)phosphonic acid | CHE...)Show InChI InChI=1S/C7H18O7P2/c1-2-3-4-5-6-7(8,15(9,10)11)16(12,13)14/h8H,2-6H2,1H3,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Buenos Aires
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi FPPS |
Bioorg Med Chem 16: 3283-90 (2008)
Article DOI: 10.1016/j.bmc.2007.12.010
BindingDB Entry DOI: 10.7270/Q2JD4XPC |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM50288336
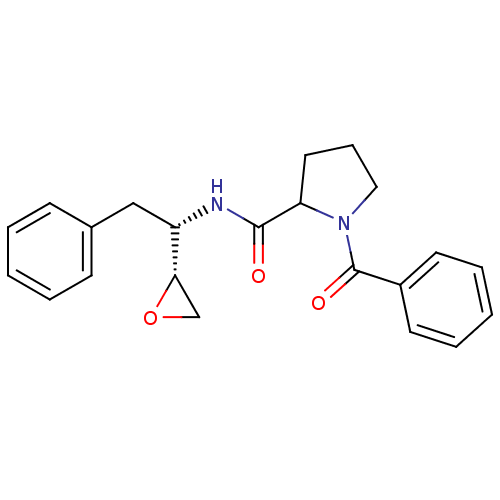
(1-Benzoyl-pyrrolidine-2-carboxylic acid ((S)-1-(R)...)Show SMILES O=C(N[C@@H](Cc1ccccc1)[C@@H]1CO1)C1CCCN1C(=O)c1ccccc1 Show InChI InChI=1S/C22H24N2O3/c25-21(23-18(20-15-27-20)14-16-8-3-1-4-9-16)19-12-7-13-24(19)22(26)17-10-5-2-6-11-17/h1-6,8-11,18-20H,7,12-15H2,(H,23,25)/t18-,19?,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 8.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against alpha-chymotrypsin |
Bioorg Med Chem Lett 6: 2837-2840 (1996)
Article DOI: 10.1016/S0960-894X(96)00536-7
BindingDB Entry DOI: 10.7270/Q2C53KTP |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50283299
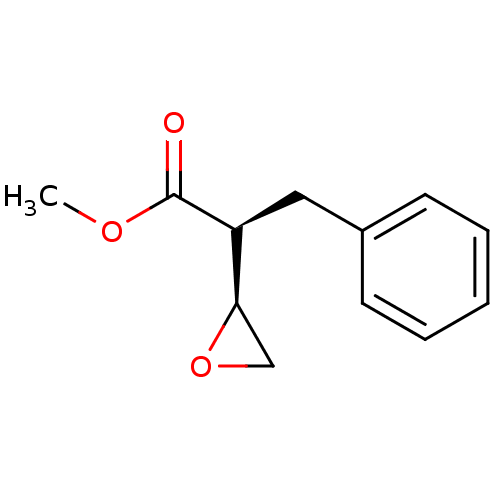
((S)-2-(S)-Oxiranyl-3-phenyl-propionic acid methyl ...)Show InChI InChI=1S/C12H14O3/c1-14-12(13)10(11-8-15-11)7-9-5-3-2-4-6-9/h2-6,10-11H,7-8H2,1H3/t10-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 9.95E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against alpha-chymotrypsin |
Bioorg Med Chem Lett 4: 2297-2302 (1994)
Article DOI: 10.1016/0960-894X(94)85028-3
BindingDB Entry DOI: 10.7270/Q21N8138 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50283299

((S)-2-(S)-Oxiranyl-3-phenyl-propionic acid methyl ...)Show InChI InChI=1S/C12H14O3/c1-14-12(13)10(11-8-15-11)7-9-5-3-2-4-6-9/h2-6,10-11H,7-8H2,1H3/t10-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 9.95E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against alpha-chymotrypsin |
Bioorg Med Chem Lett 4: 2297-2302 (1994)
Article DOI: 10.1016/0960-894X(94)85028-3
BindingDB Entry DOI: 10.7270/Q21N8138 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM82258

(CAS_114798-26-4 | Losartan | NSC_3961)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H23ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3,(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemical Engineering& the Environment
Curated by ChEMBL
| Assay Description
Inhibition of angiotensin AT1 receptor |
Bioorg Med Chem 20: 4208-16 (2012)
Article DOI: 10.1016/j.bmc.2012.05.056
BindingDB Entry DOI: 10.7270/Q2GT5P7J |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50043280

(4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1H-benzimidaz...)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)-c1nc2ccccc2n1C Show InChI InChI=1S/C33H30N4O2/c1-4-9-30-35-31-21(2)18-24(32-34-27-12-7-8-13-28(27)36(32)3)19-29(31)37(30)20-22-14-16-23(17-15-22)25-10-5-6-11-26(25)33(38)39/h5-8,10-19H,4,9,20H2,1-3H3,(H,38,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT2 receptor after 180 mins by gamma counting |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Lymphokine-activated killer T-cell-originated protein kinase
(Homo sapiens (Human)) | BDBM50520955

(CHEMBL4445123)Show SMILES C[C@@H](CN)c1ccc(cc1)-c1c(O)cc(C)c2[nH]c(=O)c3ccccc3c12 |r| Show InChI InChI=1S/C23H22N2O2/c1-13-11-19(26)20(16-9-7-15(8-10-16)14(2)12-24)21-17-5-3-4-6-18(17)23(27)25-22(13)21/h3-11,14,26H,12,24H2,1-2H3,(H,25,27)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human TOPK using MBP as substrate after 2 hrs in presence of [gamma-33P]-ATP by filter-binding method |
Eur J Med Chem 162: 407-422 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.007
BindingDB Entry DOI: 10.7270/Q28K7DGV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50388737
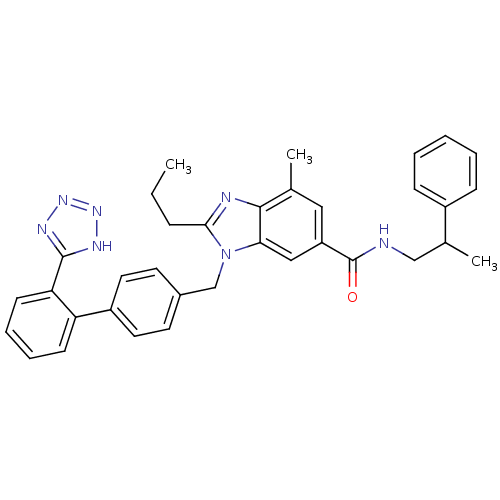
(CHEMBL2058860)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)C(=O)NCC(C)c1ccccc1 Show InChI InChI=1S/C35H35N7O/c1-4-10-32-37-33-23(2)19-28(35(43)36-21-24(3)26-11-6-5-7-12-26)20-31(33)42(32)22-25-15-17-27(18-16-25)29-13-8-9-14-30(29)34-38-40-41-39-34/h5-9,11-20,24H,4,10,21-22H2,1-3H3,(H,36,43)(H,38,39,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemical Engineering& the Environment
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1Ile8-Ang2 from angiotensin AT1 receptor after 180 mins by gamma counting |
Bioorg Med Chem 20: 4208-16 (2012)
Article DOI: 10.1016/j.bmc.2012.05.056
BindingDB Entry DOI: 10.7270/Q2GT5P7J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50043280

(4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1H-benzimidaz...)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)-c1nc2ccccc2n1C Show InChI InChI=1S/C33H30N4O2/c1-4-9-30-35-31-21(2)18-24(32-34-27-12-7-8-13-28(27)36(32)3)19-29(31)37(30)20-22-14-16-23(17-15-22)25-10-5-6-11-26(25)33(38)39/h5-8,10-19H,4,9,20H2,1-3H3,(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemical Engineering& the Environment
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1Ile8-Ang2 from angiotensin AT1 receptor after 180 mins by gamma counting |
Bioorg Med Chem 20: 4208-16 (2012)
Article DOI: 10.1016/j.bmc.2012.05.056
BindingDB Entry DOI: 10.7270/Q2GT5P7J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50043280

(4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1H-benzimidaz...)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)-c1nc2ccccc2n1C Show InChI InChI=1S/C33H30N4O2/c1-4-9-30-35-31-21(2)18-24(32-34-27-12-7-8-13-28(27)36(32)3)19-29(31)37(30)20-22-14-16-23(17-15-22)25-10-5-6-11-26(25)33(38)39/h5-8,10-19H,4,9,20H2,1-3H3,(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT1 receptor after 180 mins by gamma counting |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50560691

(CHEMBL4788952)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(CN3CCN(CC3=O)C(=O)C3CC3)cc2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127479
BindingDB Entry DOI: 10.7270/Q2MG7T7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50560700
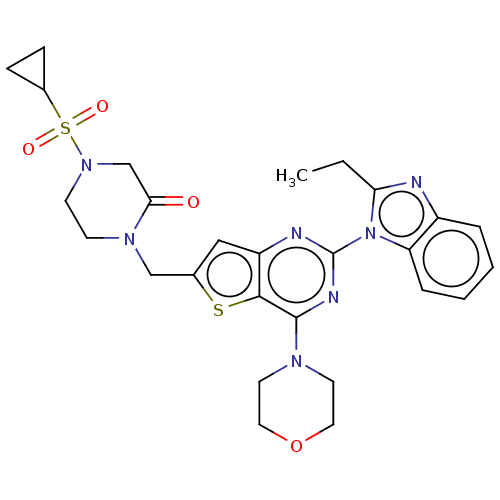
(CHEMBL4787716)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(CN3CCN(CC3=O)S(=O)(=O)C3CC3)cc2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127479
BindingDB Entry DOI: 10.7270/Q2MG7T7R |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM25121

(4-{[(7R)-8-cyclopentyl-7-ethyl-5-methyl-6-oxo-5,6,...)Show SMILES CC[C@H]1N(C2CCCC2)c2nc(Nc3ccc(cc3OC)C(=O)NC3CCN(C)CC3)ncc2N(C)C1=O |r| Show InChI InChI=1S/C28H39N7O3/c1-5-22-27(37)34(3)23-17-29-28(32-25(23)35(22)20-8-6-7-9-20)31-21-11-10-18(16-24(21)38-4)26(36)30-19-12-14-33(2)15-13-19/h10-11,16-17,19-20,22H,5-9,12-15H2,1-4H3,(H,30,36)(H,29,31,32)/t22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRD4 (unknown origin) by alpha-screen assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112152
BindingDB Entry DOI: 10.7270/Q20V8HF9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50363833

(CHEMBL1945010)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCCc1cc(OC)ccc1OC Show InChI InChI=1S/C36H37N3O5/c1-5-8-33-38-34-23(2)19-27(35(40)37-18-17-26-20-28(43-3)15-16-32(26)44-4)21-31(34)39(33)22-24-11-13-25(14-12-24)29-9-6-7-10-30(29)36(41)42/h6-7,9-16,19-21H,5,8,17-18,22H2,1-4H3,(H,37,40)(H,41,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT1 receptor after 180 mins by gamma counting |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50388736
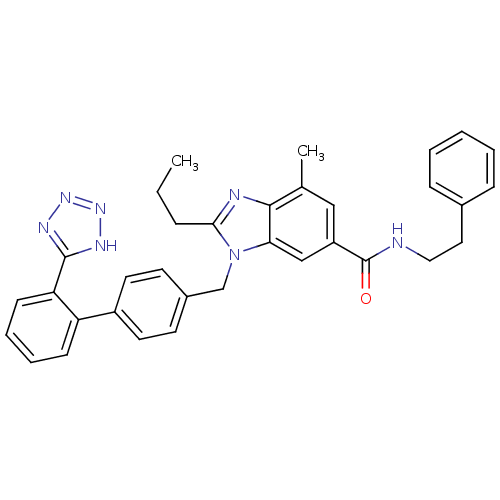
(CHEMBL2058859)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C34H33N7O/c1-3-9-31-36-32-23(2)20-27(34(42)35-19-18-24-10-5-4-6-11-24)21-30(32)41(31)22-25-14-16-26(17-15-25)28-12-7-8-13-29(28)33-37-39-40-38-33/h4-8,10-17,20-21H,3,9,18-19,22H2,1-2H3,(H,35,42)(H,37,38,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemical Engineering& the Environment
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1Ile8-Ang2 from angiotensin AT1 receptor after 180 mins by gamma counting |
Bioorg Med Chem 20: 4208-16 (2012)
Article DOI: 10.1016/j.bmc.2012.05.056
BindingDB Entry DOI: 10.7270/Q2GT5P7J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50560692

(CHEMBL4752490)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(CN3CCN(CC3=O)C(=O)C3CCC3)cc2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127479
BindingDB Entry DOI: 10.7270/Q2MG7T7R |
More data for this
Ligand-Target Pair | |
Lymphokine-activated killer T-cell-originated protein kinase
(Homo sapiens (Human)) | BDBM50520970
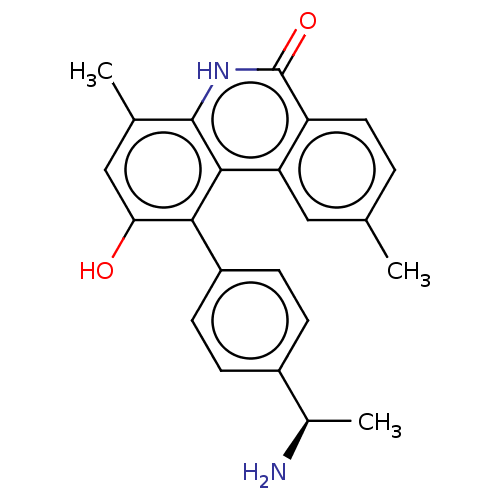
(CHEMBL4452726)Show SMILES C[C@@H](N)c1ccc(cc1)-c1c(O)cc(C)c2[nH]c(=O)c3ccc(C)cc3c12 |r| Show InChI InChI=1S/C23H22N2O2/c1-12-4-9-17-18(10-12)21-20(16-7-5-15(6-8-16)14(3)24)19(26)11-13(2)22(21)25-23(17)27/h4-11,14,26H,24H2,1-3H3,(H,25,27)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human TOPK using MBP as substrate after 2 hrs in presence of [gamma-33P]-ATP by filter-binding method |
Eur J Med Chem 162: 407-422 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.007
BindingDB Entry DOI: 10.7270/Q28K7DGV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50560684
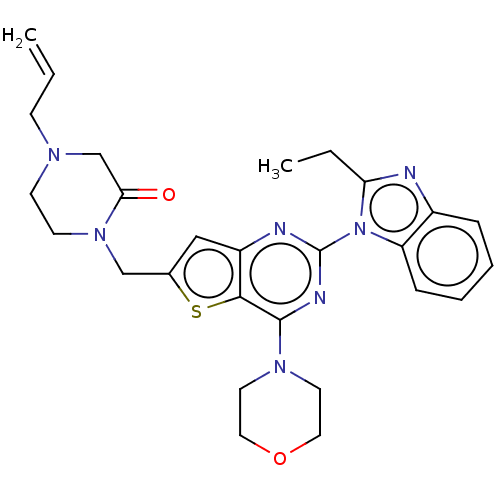
(CHEMBL4788725)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(CN3CCN(CC=C)CC3=O)cc2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127479
BindingDB Entry DOI: 10.7270/Q2MG7T7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50560685
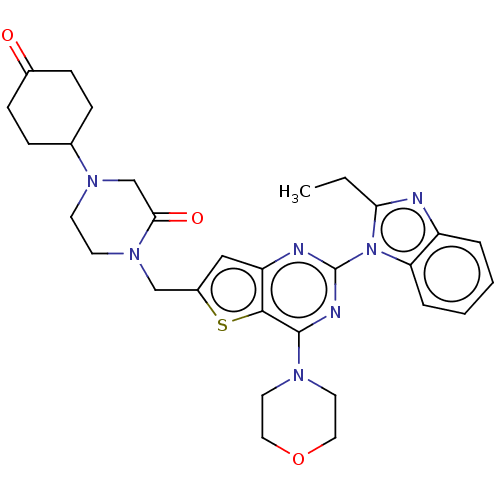
(CHEMBL4743492)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(CN3CCN(CC3=O)C3CCC(=O)CC3)cc2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127479
BindingDB Entry DOI: 10.7270/Q2MG7T7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50560693
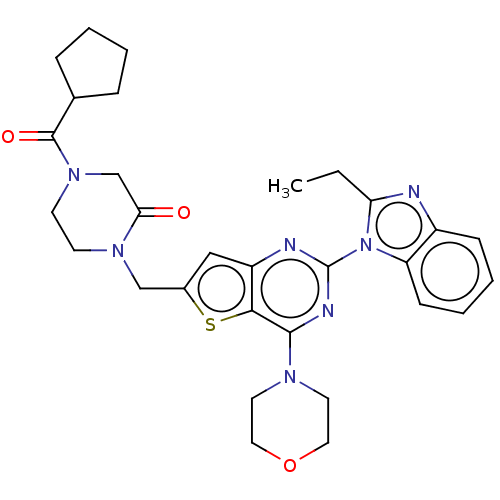
(CHEMBL4762689)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(CN3CCN(CC3=O)C(=O)C3CCCC3)cc2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127479
BindingDB Entry DOI: 10.7270/Q2MG7T7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50560671
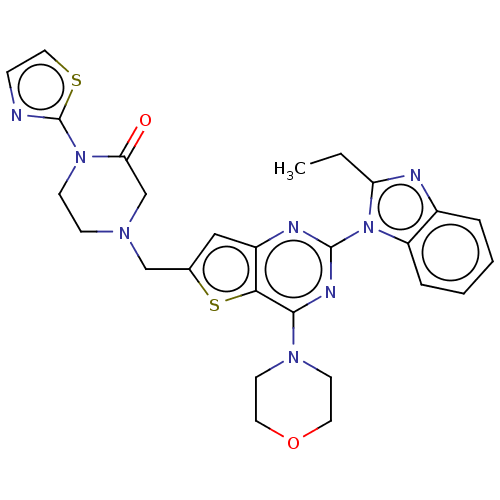
(CHEMBL4741654)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(CN3CCN(c4nccs4)C(=O)C3)cc2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127479
BindingDB Entry DOI: 10.7270/Q2MG7T7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50560666
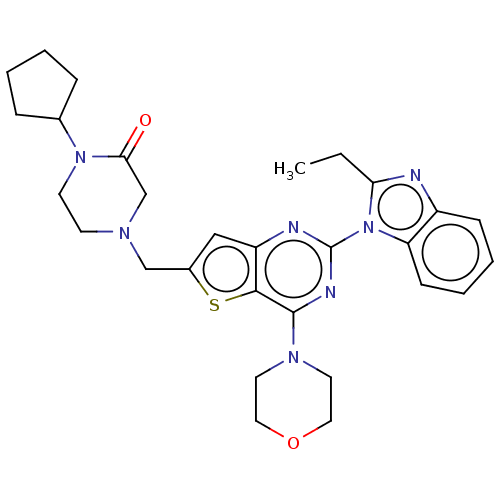
(CHEMBL4750636)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(CN3CCN(C4CCCC4)C(=O)C3)cc2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127479
BindingDB Entry DOI: 10.7270/Q2MG7T7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50560667

(CHEMBL4796048)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(CN3CCN([C@H]4CC[C@H](O)CC4)C(=O)C3)cc2n1 |r,wU:28.30,wD:31.34,(54.67,-34.34,;53.21,-34.82,;52.06,-33.78,;50.55,-34.1,;49.79,-32.77,;48.29,-32.45,;47.81,-31,;48.84,-29.85,;50.34,-30.17,;50.82,-31.63,;52.22,-32.25,;53.56,-31.48,;53.56,-29.94,;54.89,-29.17,;54.89,-27.63,;56.23,-26.86,;56.23,-25.33,;54.89,-24.55,;53.56,-25.33,;53.56,-26.87,;56.23,-29.93,;57.69,-29.44,;58.61,-30.69,;60.15,-30.68,;60.91,-29.34,;60.13,-28.02,;60.9,-26.68,;62.45,-26.68,;63.21,-25.35,;62.43,-24.02,;63.21,-22.68,;64.75,-22.68,;65.52,-21.35,;65.52,-24.02,;64.75,-25.35,;63.22,-28.01,;64.76,-28.01,;62.46,-29.34,;57.71,-31.94,;56.24,-31.47,;54.89,-32.25,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127479
BindingDB Entry DOI: 10.7270/Q2MG7T7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50560683

(CHEMBL4762501)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(CN3CCN(CC3=O)C3CCC4(CC3)OCCO4)cc2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127479
BindingDB Entry DOI: 10.7270/Q2MG7T7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50560672

(CHEMBL4799262)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(CN3CCN(C(=O)C3)c3cccnc3)cc2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127479
BindingDB Entry DOI: 10.7270/Q2MG7T7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50560697
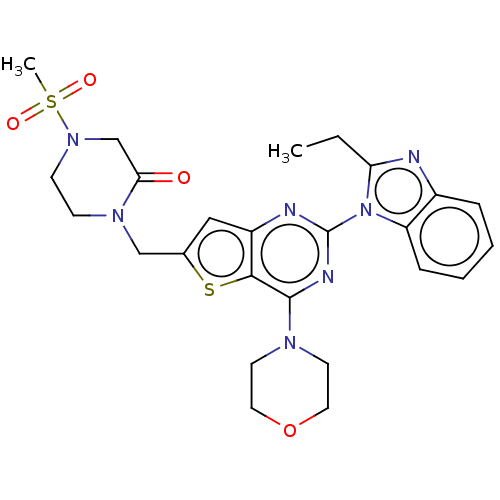
(CHEMBL4783917)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(CN3CCN(CC3=O)S(C)(=O)=O)cc2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127479
BindingDB Entry DOI: 10.7270/Q2MG7T7R |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50363826
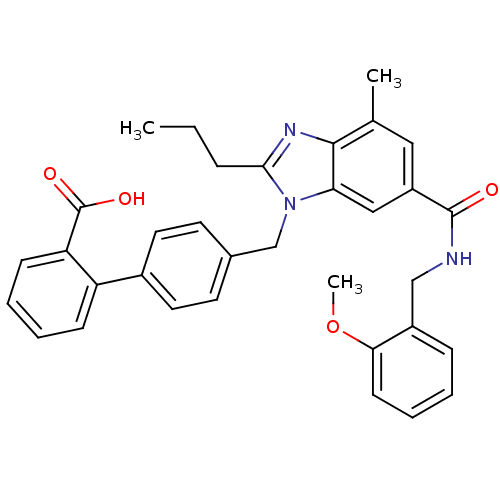
(CHEMBL1947129)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCc1ccccc1OC Show InChI InChI=1S/C34H33N3O4/c1-4-9-31-36-32-22(2)18-26(33(38)35-20-25-10-5-8-13-30(25)41-3)19-29(32)37(31)21-23-14-16-24(17-15-23)27-11-6-7-12-28(27)34(39)40/h5-8,10-19H,4,9,20-21H2,1-3H3,(H,35,38)(H,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT1 receptor after 180 mins by gamma counting |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50403068
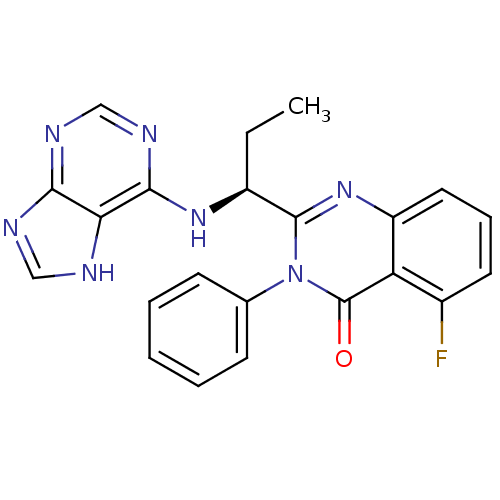
(CHEMBL2216870 | IDELALISIB | US9745321, CAL-101)Show SMILES CC[C@H](Nc1ncnc2nc[nH]c12)c1nc2cccc(F)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C22H18FN7O/c1-2-15(28-20-18-19(25-11-24-18)26-12-27-20)21-29-16-10-6-9-14(23)17(16)22(31)30(21)13-7-4-3-5-8-13/h3-12,15H,2H2,1H3,(H2,24,25,26,27,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human His-tagged PI3Kdelta expressed in baculovirus expression system using PIP2:PS as substrate incubated for 1 hr by invi... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127479
BindingDB Entry DOI: 10.7270/Q2MG7T7R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50560665

(CHEMBL4786126)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(CN3CCN(CC4CC4)C(=O)C3)cc2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127479
BindingDB Entry DOI: 10.7270/Q2MG7T7R |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50388738
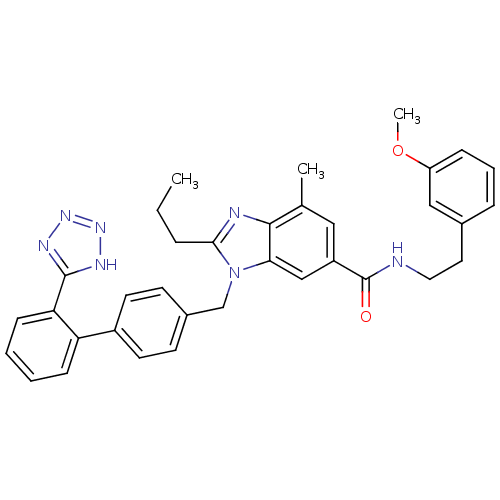
(CHEMBL2058861)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)C(=O)NCCc1cccc(OC)c1 Show InChI InChI=1S/C35H35N7O2/c1-4-8-32-37-33-23(2)19-27(35(43)36-18-17-24-9-7-10-28(20-24)44-3)21-31(33)42(32)22-25-13-15-26(16-14-25)29-11-5-6-12-30(29)34-38-40-41-39-34/h5-7,9-16,19-21H,4,8,17-18,22H2,1-3H3,(H,36,43)(H,38,39,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemical Engineering& the Environment
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1Ile8-Ang2 from angiotensin AT1 receptor after 180 mins by gamma counting |
Bioorg Med Chem 20: 4208-16 (2012)
Article DOI: 10.1016/j.bmc.2012.05.056
BindingDB Entry DOI: 10.7270/Q2GT5P7J |
More data for this
Ligand-Target Pair | |
Lymphokine-activated killer T-cell-originated protein kinase
(Homo sapiens (Human)) | BDBM50520960
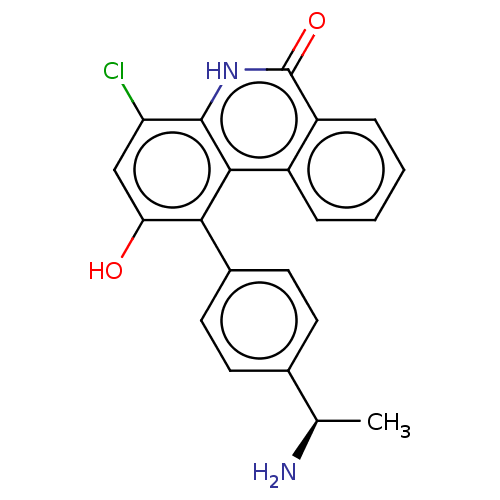
(CHEMBL4440222)Show SMILES C[C@@H](N)c1ccc(cc1)-c1c(O)cc(Cl)c2[nH]c(=O)c3ccccc3c12 |r| Show InChI InChI=1S/C21H17ClN2O2/c1-11(23)12-6-8-13(9-7-12)18-17(25)10-16(22)20-19(18)14-4-2-3-5-15(14)21(26)24-20/h2-11,25H,23H2,1H3,(H,24,26)/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human TOPK using MBP as substrate after 2 hrs in presence of [gamma-33P]-ATP by filter-binding method |
Eur J Med Chem 162: 407-422 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.007
BindingDB Entry DOI: 10.7270/Q28K7DGV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50560677
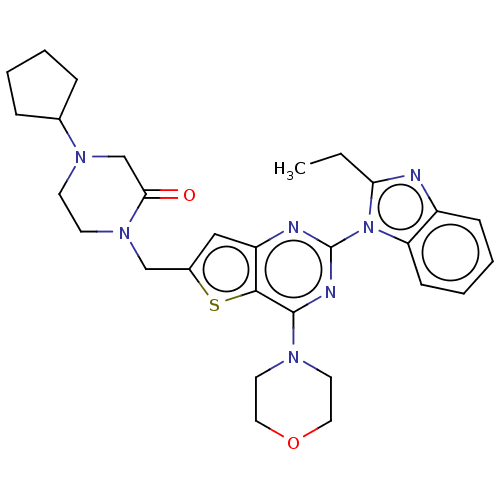
(CHEMBL4753342)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(CN3CCN(CC3=O)C3CCCC3)cc2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127479
BindingDB Entry DOI: 10.7270/Q2MG7T7R |
More data for this
Ligand-Target Pair | |
Lymphokine-activated killer T-cell-originated protein kinase
(Homo sapiens (Human)) | BDBM50520972
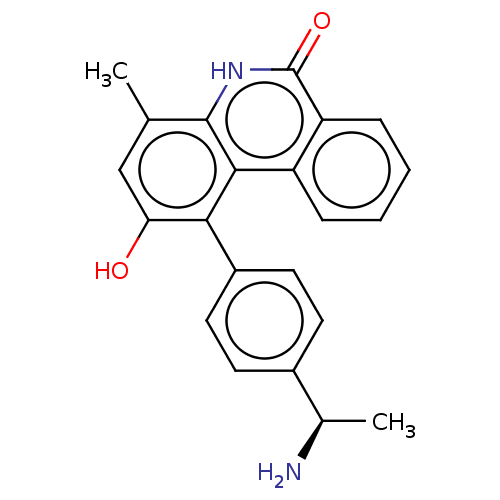
(CHEMBL4527920)Show SMILES C[C@@H](N)c1ccc(cc1)-c1c(O)cc(C)c2[nH]c(=O)c3ccccc3c12 |r| Show InChI InChI=1S/C22H20N2O2/c1-12-11-18(25)19(15-9-7-14(8-10-15)13(2)23)20-16-5-3-4-6-17(16)22(26)24-21(12)20/h3-11,13,25H,23H2,1-2H3,(H,24,26)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human TOPK using MBP as substrate after 2 hrs in presence of [gamma-33P]-ATP by filter-binding method |
Eur J Med Chem 162: 407-422 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.007
BindingDB Entry DOI: 10.7270/Q28K7DGV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50560694
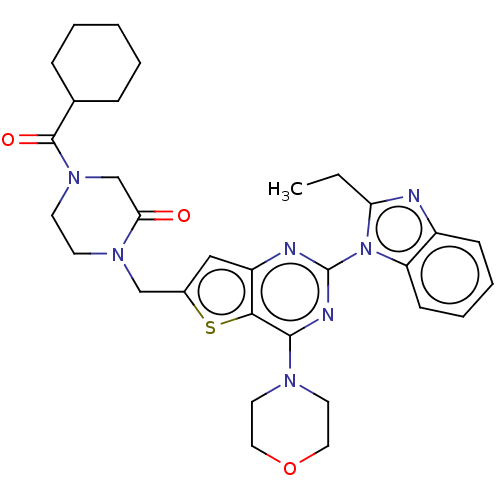
(CHEMBL4797595)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(CN3CCN(CC3=O)C(=O)C3CCCCC3)cc2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127479
BindingDB Entry DOI: 10.7270/Q2MG7T7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50560690
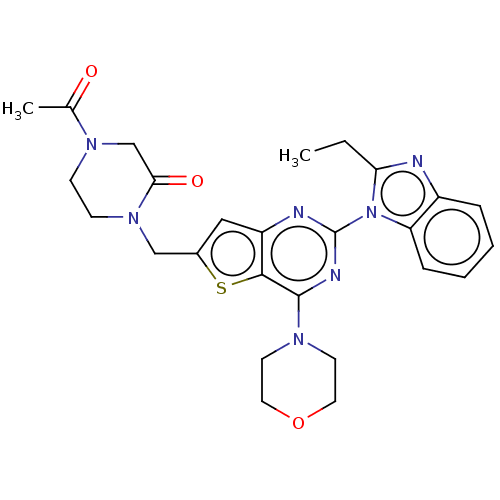
(CHEMBL4783142)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(CN3CCN(CC3=O)C(C)=O)cc2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127479
BindingDB Entry DOI: 10.7270/Q2MG7T7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50560670

(CHEMBL4761003)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(CN3CCN(c4cnn(C)c4)C(=O)C3)cc2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127479
BindingDB Entry DOI: 10.7270/Q2MG7T7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50560699

(CHEMBL4764973)Show SMILES CCCS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-n2c(CC)nc3ccccc23)C(=O)C1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127479
BindingDB Entry DOI: 10.7270/Q2MG7T7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50560689
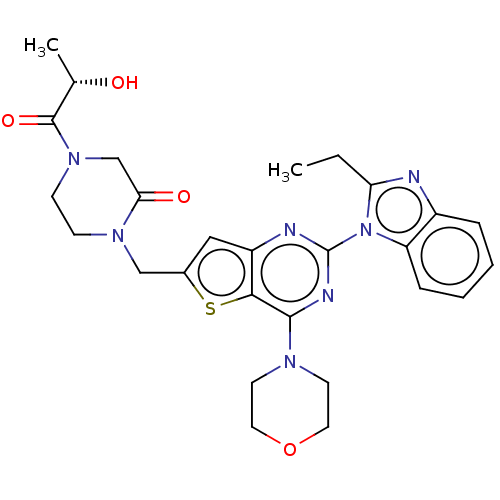
(CHEMBL4791430)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(CN3CCN(CC3=O)C(=O)[C@H](C)O)cc2n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127479
BindingDB Entry DOI: 10.7270/Q2MG7T7R |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50388741

(CHEMBL2058864)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)C(=O)NCCc1ccc(OC)c(OC)c1 Show InChI InChI=1S/C36H37N7O3/c1-5-8-33-38-34-23(2)19-27(36(44)37-18-17-24-13-16-31(45-3)32(20-24)46-4)21-30(34)43(33)22-25-11-14-26(15-12-25)28-9-6-7-10-29(28)35-39-41-42-40-35/h6-7,9-16,19-21H,5,8,17-18,22H2,1-4H3,(H,37,44)(H,39,40,41,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemical Engineering& the Environment
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1Ile8-Ang2 from angiotensin AT1 receptor after 180 mins by gamma counting |
Bioorg Med Chem 20: 4208-16 (2012)
Article DOI: 10.1016/j.bmc.2012.05.056
BindingDB Entry DOI: 10.7270/Q2GT5P7J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50560682

(CHEMBL4764632)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(CN3CCN(CC3=O)C3CCC(F)(F)CC3)cc2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127479
BindingDB Entry DOI: 10.7270/Q2MG7T7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394895

(CHEMBL2165500)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(CN3CCN(CC3)S(C)(=O)=O)cc2n1 Show InChI InChI=1S/C25H31N7O3S2/c1-3-22-26-19-6-4-5-7-21(19)32(22)25-27-20-16-18(17-29-8-10-31(11-9-29)37(2,33)34)36-23(20)24(28-25)30-12-14-35-15-13-30/h4-7,16H,3,8-15,17H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127479
BindingDB Entry DOI: 10.7270/Q2MG7T7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50560663

(CHEMBL4747763)Show SMILES CCN1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-n2c(CC)nc3ccccc23)CC1=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127479
BindingDB Entry DOI: 10.7270/Q2MG7T7R |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50363828
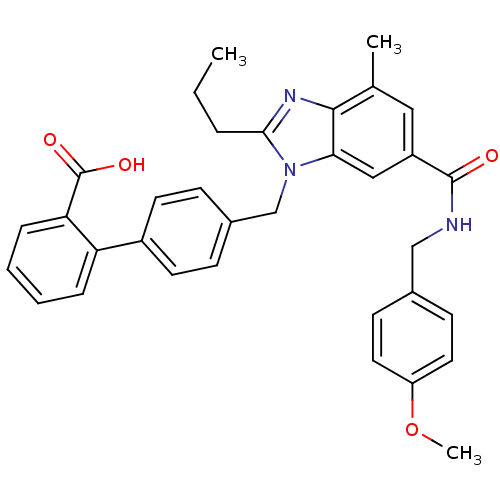
(CHEMBL1947131)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCc1ccc(OC)cc1 Show InChI InChI=1S/C34H33N3O4/c1-4-7-31-36-32-22(2)18-26(33(38)35-20-23-12-16-27(41-3)17-13-23)19-30(32)37(31)21-24-10-14-25(15-11-24)28-8-5-6-9-29(28)34(39)40/h5-6,8-19H,4,7,20-21H2,1-3H3,(H,35,38)(H,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT1 receptor after 180 mins by gamma counting |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50560698
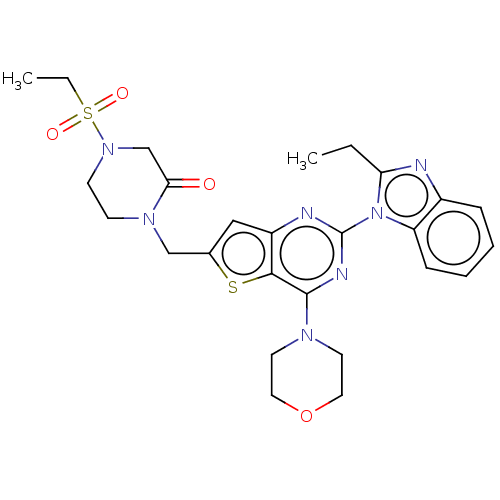
(CHEMBL4800610)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(CN3CCN(CC3=O)S(=O)(=O)CC)cc2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127479
BindingDB Entry DOI: 10.7270/Q2MG7T7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50560675
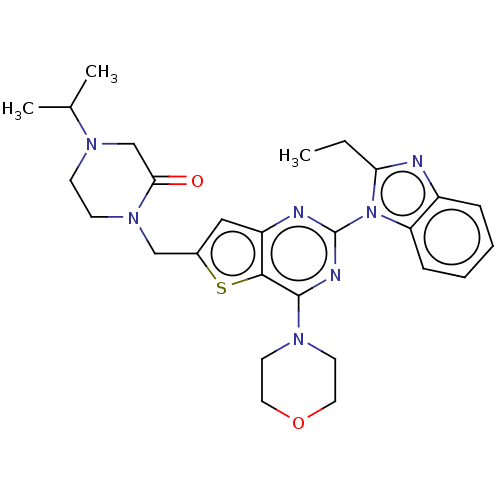
(CHEMBL4759104)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(CN3CCN(CC3=O)C(C)C)cc2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127479
BindingDB Entry DOI: 10.7270/Q2MG7T7R |
More data for this
Ligand-Target Pair | |
Lymphokine-activated killer T-cell-originated protein kinase
(Homo sapiens (Human)) | BDBM50520949

(CHEMBL4564624)Show SMILES C[C@@H](CN)c1ccc(cc1)-c1c(O)cc(Cl)c2[nH]c(=O)c3ccccc3c12 |r| Show InChI InChI=1S/C22H19ClN2O2/c1-12(11-24)13-6-8-14(9-7-13)19-18(26)10-17(23)21-20(19)15-4-2-3-5-16(15)22(27)25-21/h2-10,12,26H,11,24H2,1H3,(H,25,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human TOPK using MBP as substrate after 2 hrs in presence of [gamma-33P]-ATP by filter-binding method |
Eur J Med Chem 162: 407-422 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.007
BindingDB Entry DOI: 10.7270/Q28K7DGV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50403068

(CHEMBL2216870 | IDELALISIB | US9745321, CAL-101)Show SMILES CC[C@H](Nc1ncnc2nc[nH]c12)c1nc2cccc(F)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C22H18FN7O/c1-2-15(28-20-18-19(25-11-24-18)26-12-27-20)21-29-16-10-6-9-14(23)17(16)22(31)30(21)13-7-4-3-5-8-13/h3-12,15H,2H2,1H3,(H2,24,25,26,27,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127479
BindingDB Entry DOI: 10.7270/Q2MG7T7R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50560681

(CHEMBL4798399)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(CN3CCN(CC3=O)C3CCSCC3)cc2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127479
BindingDB Entry DOI: 10.7270/Q2MG7T7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50560701
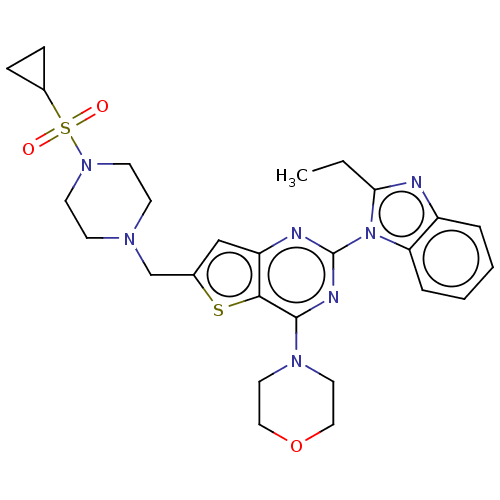
(CHEMBL4784160)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(CN3CCN(CC3)S(=O)(=O)C3CC3)cc2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127479
BindingDB Entry DOI: 10.7270/Q2MG7T7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50560662
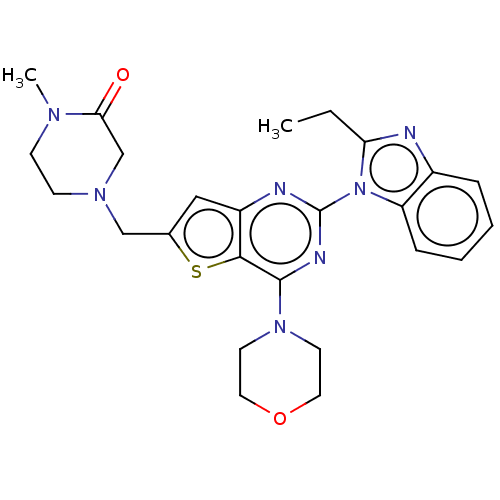
(CHEMBL4792029)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(CN3CCN(C)C(=O)C3)cc2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127479
BindingDB Entry DOI: 10.7270/Q2MG7T7R |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data