 Found 106 hits with Last Name = 'li' and Initial = 'zm'
Found 106 hits with Last Name = 'li' and Initial = 'zm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50486243
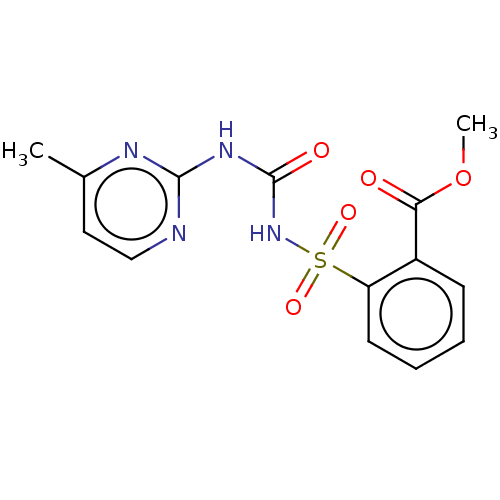
(MONOSULFURON ESTER)Show InChI InChI=1S/C14H14N4O5S/c1-9-7-8-15-13(16-9)17-14(20)18-24(21,22)11-6-4-3-5-10(11)12(19)23-2/h3-8H,1-2H3,(H2,15,16,17,18,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetohydroxyacid synthase colorimetric assay |
J Agric Food Chem 60: 8286-93 (2012)
Article DOI: 10.1021/jf302206x
BindingDB Entry DOI: 10.7270/Q2FX7DB2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Signal transducer and activator of transcription 3
(Homo sapiens (Human)) | BDBM50470518
(CHEMBL4294523)Show SMILES CNc1ccc(Br)cc(C(=O)\C=C\c2c(Cl)cccc2Cl)c1=O Show InChI InChI=1S/C17H12BrCl2NO2/c1-21-15-7-5-10(18)9-12(17(15)23)16(22)8-6-11-13(19)3-2-4-14(11)20/h2-9H,1H3,(H,21,23)/b8-6+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of Cy5-labeled double-strand DNA probe binding to STAT3 (unknown origin) expressed in H1299 cell lysates assessed as decrease in DNA bindi... |
Eur J Med Chem 157: 887-897 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.037
BindingDB Entry DOI: 10.7270/Q27M0BNN |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 3
(Homo sapiens (Human)) | BDBM50470513
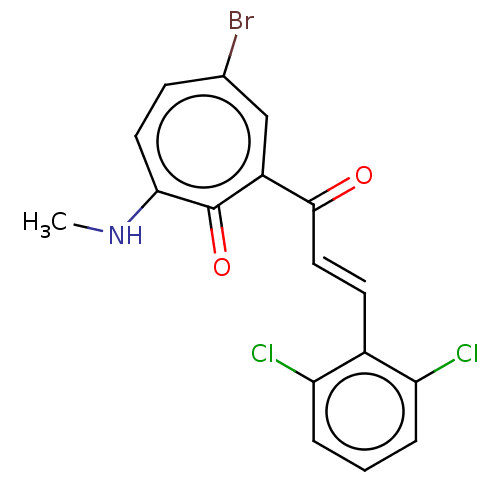
(CHEMBL4290712)Show InChI InChI=1S/C18H15Br2NO2/c1-2-21-16-9-8-14(20)11-15(18(16)23)17(22)10-5-12-3-6-13(19)7-4-12/h3-11H,2H2,1H3,(H,21,23)/b10-5+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of Cy5-labeled double-strand DNA probe binding to STAT3 (unknown origin) expressed in H1299 cell lysates assessed as decrease in DNA bindi... |
Eur J Med Chem 157: 887-897 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.037
BindingDB Entry DOI: 10.7270/Q27M0BNN |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 3
(Homo sapiens (Human)) | BDBM50470517

(CHEMBL4294114)Show InChI InChI=1S/C17H13BrClNO2/c1-20-15-7-6-12(18)10-14(17(15)22)16(21)8-5-11-3-2-4-13(19)9-11/h2-10H,1H3,(H,20,22)/b8-5+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of Cy5-labeled double-strand DNA probe binding to STAT3 (unknown origin) expressed in H1299 cell lysates assessed as decrease in DNA bindi... |
Eur J Med Chem 157: 887-897 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.037
BindingDB Entry DOI: 10.7270/Q27M0BNN |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 3
(Homo sapiens (Human)) | BDBM50470512
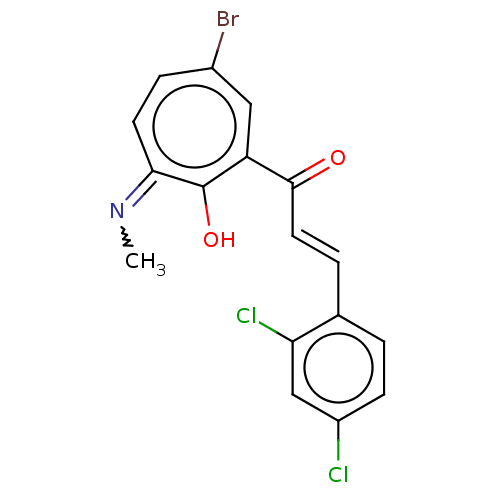
(CHEMBL1416382)Show SMILES CN=c1ccc(Br)cc(C(=O)\C=C\c2ccc(Cl)cc2Cl)c1O Show InChI InChI=1S/C17H12BrCl2NO2/c1-21-15-6-4-11(18)8-13(17(15)23)16(22)7-3-10-2-5-12(19)9-14(10)20/h2-9H,1H3,(H,21,23)/b7-3+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of Cy5-labeled double-strand DNA probe binding to STAT3 (unknown origin) expressed in H1299 cell lysates assessed as decrease in DNA bindi... |
Eur J Med Chem 157: 887-897 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.037
BindingDB Entry DOI: 10.7270/Q27M0BNN |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50486242

(CHEMBL2230130)Show InChI InChI=1S/C8H7N3S2/c1-2-4-7(5-3-1)12-13-8-9-6-10-11-8/h1-6H,(H,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetohydroxyacid synthase colorimetric assay |
J Agric Food Chem 60: 8286-93 (2012)
Article DOI: 10.1021/jf302206x
BindingDB Entry DOI: 10.7270/Q2FX7DB2 |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 3
(Homo sapiens (Human)) | BDBM50470515
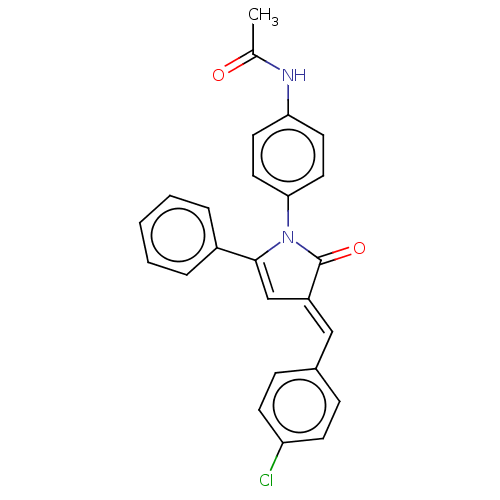
(CHEMBL4291509)Show SMILES CC(=O)Nc1ccc(cc1)N1C(=O)\C(=C\c2ccc(Cl)cc2)C=C1c1ccccc1 |c:24| Show InChI InChI=1S/C25H19ClN2O2/c1-17(29)27-22-11-13-23(14-12-22)28-24(19-5-3-2-4-6-19)16-20(25(28)30)15-18-7-9-21(26)10-8-18/h2-16H,1H3,(H,27,29)/b20-15+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of Cy5-labeled double-strand DNA probe binding to STAT3 (unknown origin) expressed in H1299 cell lysates assessed as decrease in DNA bindi... |
Eur J Med Chem 157: 887-897 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.037
BindingDB Entry DOI: 10.7270/Q27M0BNN |
More data for this
Ligand-Target Pair | |
Ketol-acid reductoisomerase (NADP(+))
(Escherichia coli) | BDBM82145
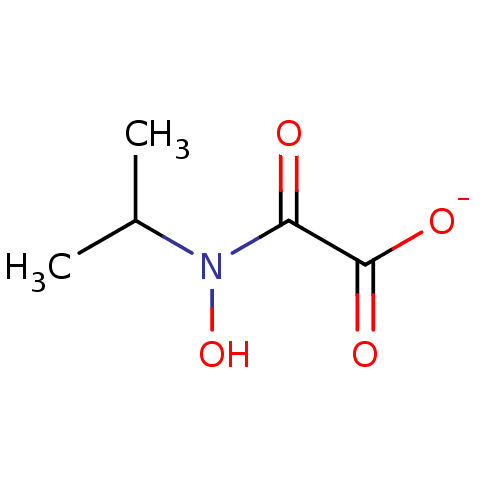
(N-hydroxy-N-isopropyloxamate, IpOHA)Show InChI InChI=1S/C5H9NO4/c1-3(2)6(10)4(7)5(8)9/h3,10H,1-2H3,(H,8,9)/p-1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2.75E+3 | -32.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 30 |
Nankai University
| Assay Description
Inhibition of E. coli KARI (ketol-acid reductoisomerase) is time dependent, and activity was measured by the decrease in A340 at 30 C in solutions co... |
Chem Biol Drug Des 75: 228-32 (2010)
Article DOI: 10.1111/j.1747-0285.2009.00924.x
BindingDB Entry DOI: 10.7270/Q2BV7F45 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50486241

(CHEBI:5869 | IMAZAQUIN)Show SMILES CC(C)C1(C)N=C(NC1=O)c1nc2ccccc2cc1C(O)=O |c:5| Show InChI InChI=1S/C17H17N3O3/c1-9(2)17(3)16(23)19-14(20-17)13-11(15(21)22)8-10-6-4-5-7-12(10)18-13/h4-9H,1-3H3,(H,21,22)(H,19,20,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetohydroxyacid synthase colorimetric assay |
J Agric Food Chem 60: 8286-93 (2012)
Article DOI: 10.1021/jf302206x
BindingDB Entry DOI: 10.7270/Q2FX7DB2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Signal transducer and activator of transcription 3
(Homo sapiens (Human)) | BDBM50470514
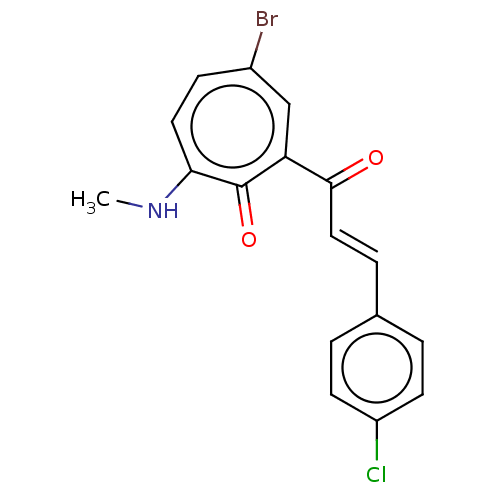
(CHEMBL4283640)Show InChI InChI=1S/C17H13BrClNO2/c1-20-15-8-5-12(18)10-14(17(15)22)16(21)9-4-11-2-6-13(19)7-3-11/h2-10H,1H3,(H,20,22)/b9-4+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of Cy5-labeled double-strand DNA probe binding to STAT3 (unknown origin) expressed in H1299 cell lysates assessed as decrease in DNA bindi... |
Eur J Med Chem 157: 887-897 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.037
BindingDB Entry DOI: 10.7270/Q27M0BNN |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50486245
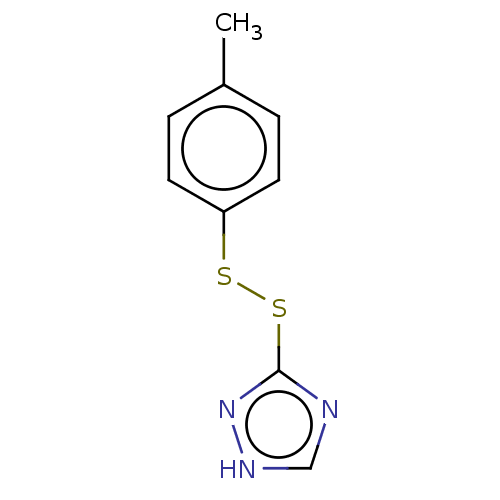
(CHEMBL2230131)Show InChI InChI=1S/C9H9N3S2/c1-7-2-4-8(5-3-7)13-14-9-10-6-11-12-9/h2-6H,1H3,(H,10,11,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 4.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetohydroxyacid synthase colorimetric assay |
J Agric Food Chem 60: 8286-93 (2012)
Article DOI: 10.1021/jf302206x
BindingDB Entry DOI: 10.7270/Q2FX7DB2 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50486244
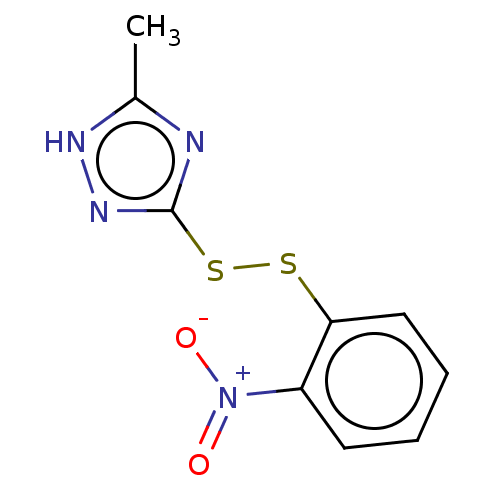
(CHEMBL2229985)Show InChI InChI=1S/C9H8N4O2S2/c1-6-10-9(12-11-6)17-16-8-5-3-2-4-7(8)13(14)15/h2-5H,1H3,(H,10,11,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 5.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of wild type Arabidopsis thaliana acetohydroxyacid synthase colorimetric assay |
J Agric Food Chem 60: 8286-93 (2012)
Article DOI: 10.1021/jf302206x
BindingDB Entry DOI: 10.7270/Q2FX7DB2 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50486243

(MONOSULFURON ESTER)Show InChI InChI=1S/C14H14N4O5S/c1-9-7-8-15-13(16-9)17-14(20)18-24(21,22)11-6-4-3-5-10(11)12(19)23-2/h3-8H,1-2H3,(H2,15,16,17,18,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 8.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana acetohydroxyacid synthase W574L mutant colorimetric assay |
J Agric Food Chem 60: 8286-93 (2012)
Article DOI: 10.1021/jf302206x
BindingDB Entry DOI: 10.7270/Q2FX7DB2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Signal transducer and activator of transcription 3
(Homo sapiens (Human)) | BDBM50470516
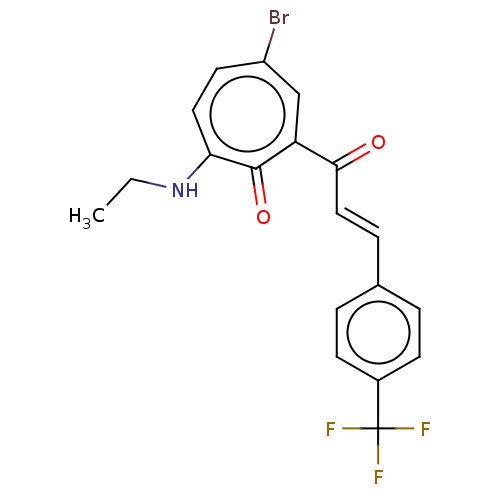
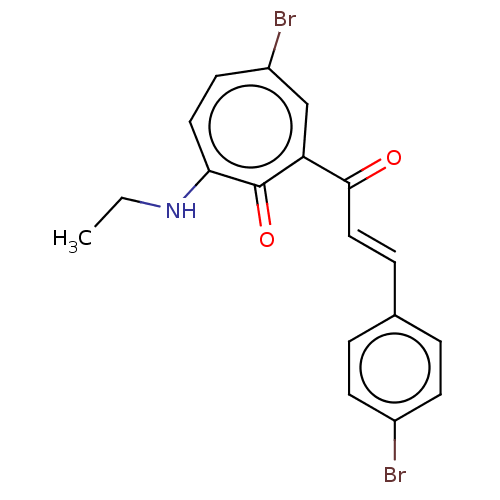
(CHEMBL4282824)Show SMILES CCNc1ccc(Br)cc(C(=O)\C=C\c2ccc(cc2)C(F)(F)F)c1=O Show InChI InChI=1S/C19H15BrF3NO2/c1-2-24-16-9-8-14(20)11-15(18(16)26)17(25)10-5-12-3-6-13(7-4-12)19(21,22)23/h3-11H,2H2,1H3,(H,24,26)/b10-5+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of Cy5-labeled double-strand DNA probe binding to STAT3 (unknown origin) expressed in H1299 cell lysates assessed as decrease in DNA bindi... |
Eur J Med Chem 157: 887-897 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.037
BindingDB Entry DOI: 10.7270/Q27M0BNN |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50486242

(CHEMBL2230130)Show InChI InChI=1S/C8H7N3S2/c1-2-4-7(5-3-1)12-13-8-9-6-10-11-8/h1-6H,(H,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 2.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana acetohydroxyacid synthase W574L mutant colorimetric assay |
J Agric Food Chem 60: 8286-93 (2012)
Article DOI: 10.1021/jf302206x
BindingDB Entry DOI: 10.7270/Q2FX7DB2 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50486245

(CHEMBL2230131)Show InChI InChI=1S/C9H9N3S2/c1-7-2-4-8(5-3-7)13-14-9-10-6-11-12-9/h2-6H,1H3,(H,10,11,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 2.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana acetohydroxyacid synthase W574L mutant colorimetric assay |
J Agric Food Chem 60: 8286-93 (2012)
Article DOI: 10.1021/jf302206x
BindingDB Entry DOI: 10.7270/Q2FX7DB2 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50486244

(CHEMBL2229985)Show InChI InChI=1S/C9H8N4O2S2/c1-6-10-9(12-11-6)17-16-8-5-3-2-4-7(8)13(14)15/h2-5H,1H3,(H,10,11,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 2.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana acetohydroxyacid synthase W574L mutant colorimetric assay |
J Agric Food Chem 60: 8286-93 (2012)
Article DOI: 10.1021/jf302206x
BindingDB Entry DOI: 10.7270/Q2FX7DB2 |
More data for this
Ligand-Target Pair | |
Ketol-acid reductoisomerase (NADP(+))
(Escherichia coli) | BDBM82144
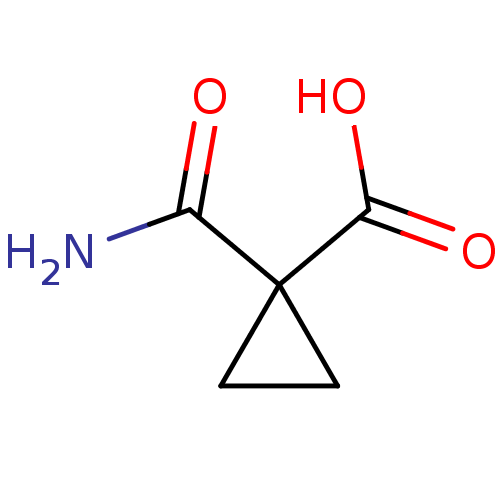
(Cyclopropane, 5)Show InChI InChI=1S/C5H7NO3/c6-3(7)5(1-2-5)4(8)9/h1-2H2,(H2,6,7)(H,8,9) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.12E+4 | -26.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 30 |
Nankai University
| Assay Description
Inhibition of E. coli KARI (ketol-acid reductoisomerase) is time dependent, and activity was measured by the decrease in A340 at 30 C in solutions co... |
Chem Biol Drug Des 75: 228-32 (2010)
Article DOI: 10.1111/j.1747-0285.2009.00924.x
BindingDB Entry DOI: 10.7270/Q2BV7F45 |
More data for this
Ligand-Target Pair | |
Ketol-acid reductoisomerase (NADP(+))
(Escherichia coli) | BDBM82142
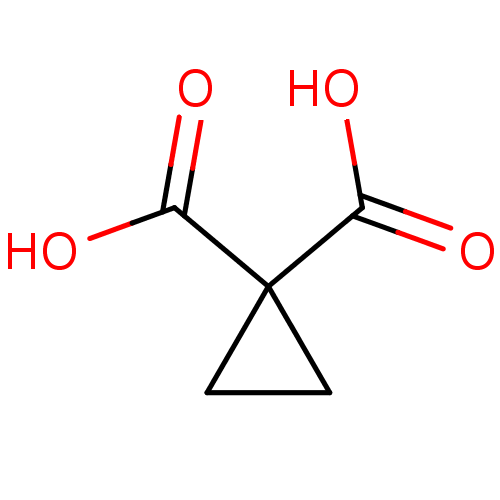
(Cyclopropane, 3)Show InChI InChI=1S/C5H6O4/c6-3(7)5(1-2-5)4(8)9/h1-2H2,(H,6,7)(H,8,9) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 7.66E+4 | -23.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 30 |
Nankai University
| Assay Description
Inhibition of E. coli KARI (ketol-acid reductoisomerase) is time dependent, and activity was measured by the decrease in A340 at 30 C in solutions co... |
Chem Biol Drug Des 75: 228-32 (2010)
Article DOI: 10.1111/j.1747-0285.2009.00924.x
BindingDB Entry DOI: 10.7270/Q2BV7F45 |
More data for this
Ligand-Target Pair | |
Ketol-acid reductoisomerase (NADP(+))
(Escherichia coli) | BDBM82143

(Cyclopropane, 4)Show InChI InChI=1S/C5H5NO2/c6-3-5(1-2-5)4(7)8/h1-2H2,(H,7,8) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 9.53E+4 | -23.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 30 |
Nankai University
| Assay Description
Inhibition of E. coli KARI (ketol-acid reductoisomerase) is time dependent, and activity was measured by the decrease in A340 at 30 C in solutions co... |
Chem Biol Drug Des 75: 228-32 (2010)
Article DOI: 10.1111/j.1747-0285.2009.00924.x
BindingDB Entry DOI: 10.7270/Q2BV7F45 |
More data for this
Ligand-Target Pair | |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50486241

(CHEBI:5869 | IMAZAQUIN)Show SMILES CC(C)C1(C)N=C(NC1=O)c1nc2ccccc2cc1C(O)=O |c:5| Show InChI InChI=1S/C17H17N3O3/c1-9(2)17(3)16(23)19-14(20-17)13-11(15(21)22)8-10-6-4-5-7-12(10)18-13/h4-9H,1-3H3,(H,21,22)(H,19,20,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 4.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nankai University
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana acetohydroxyacid synthase W574L mutant colorimetric assay |
J Agric Food Chem 60: 8286-93 (2012)
Article DOI: 10.1021/jf302206x
BindingDB Entry DOI: 10.7270/Q2FX7DB2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50259656
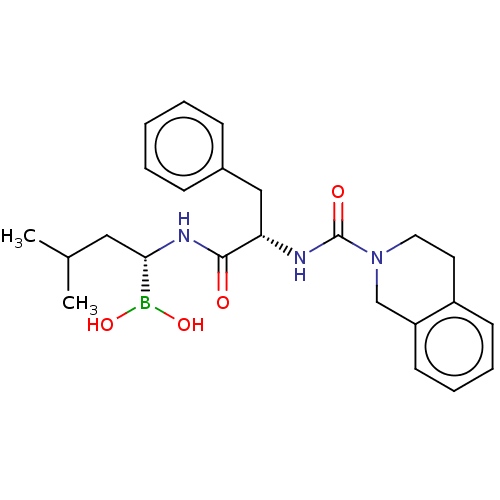
(CHEMBL4089402)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)N1CCc2ccccc2C1)B(O)O |r| Show InChI InChI=1S/C24H32BN3O4/c1-17(2)14-22(25(31)32)27-23(29)21(15-18-8-4-3-5-9-18)26-24(30)28-13-12-19-10-6-7-11-20(19)16-28/h3-11,17,21-22,31-32H,12-16H2,1-2H3,(H,26,30)(H,27,29)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.000200 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Dissociation constant against galectin-3 using competitive fluorescence polarization |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50259645
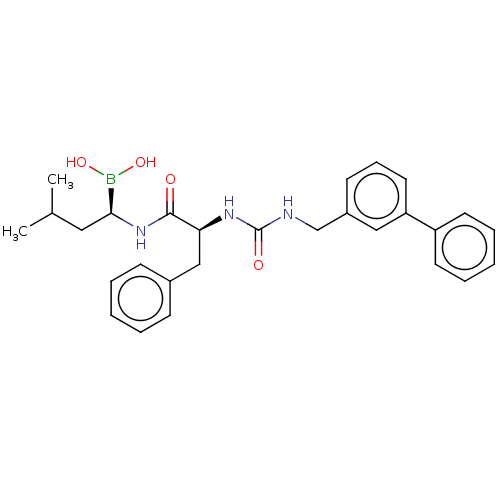
(CHEMBL4070336)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCc1cccc(c1)-c1ccccc1)B(O)O |r| Show InChI InChI=1S/C28H34BN3O4/c1-20(2)16-26(29(35)36)32-27(33)25(18-21-10-5-3-6-11-21)31-28(34)30-19-22-12-9-15-24(17-22)23-13-7-4-8-14-23/h3-15,17,20,25-26,35-36H,16,18-19H2,1-2H3,(H,32,33)(H2,30,31,34)/t25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of 20S proteasome in human HL60 cells using Suc-LLVYaminoluciferin as substrate after 2 hrs by fluorescence ... |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50259647
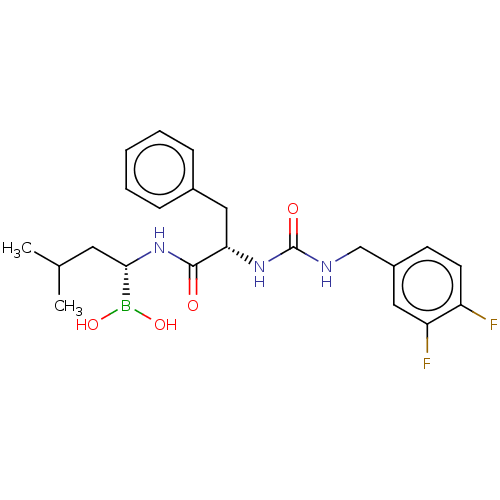
(CHEMBL4100727)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCc1ccc(F)c(F)c1)B(O)O |r| Show InChI InChI=1S/C22H28BF2N3O4/c1-14(2)10-20(23(31)32)28-21(29)19(12-15-6-4-3-5-7-15)27-22(30)26-13-16-8-9-17(24)18(25)11-16/h3-9,11,14,19-20,31-32H,10,12-13H2,1-2H3,(H,28,29)(H2,26,27,30)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of 20S proteasome in human HL60 cells using Suc-LLVYaminoluciferin as substrate after 2 hrs by fluorescence ... |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50259657

(CHEMBL4068221)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)N(C)Cc1ccccc1)B(O)O |r| Show InChI InChI=1S/C23H32BN3O4/c1-17(2)14-21(24(30)31)26-22(28)20(15-18-10-6-4-7-11-18)25-23(29)27(3)16-19-12-8-5-9-13-19/h4-13,17,20-21,30-31H,14-16H2,1-3H3,(H,25,29)(H,26,28)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Dissociation constant against galectin-3 using competitive fluorescence polarization |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50259659

(CHEMBL4076838)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCc1cccc(c1)[N+]([O-])=O)B(O)O |r| Show InChI InChI=1S/C22H29BN4O6/c1-15(2)11-20(23(30)31)26-21(28)19(13-16-7-4-3-5-8-16)25-22(29)24-14-17-9-6-10-18(12-17)27(32)33/h3-10,12,15,19-20,30-31H,11,13-14H2,1-2H3,(H,26,28)(H2,24,25,29)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Dissociation constant against galectin-3 using competitive fluorescence polarization |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50069989

((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O |r| Show InChI InChI=1S/C19H25BN4O4/c1-13(2)10-17(20(27)28)24-18(25)15(11-14-6-4-3-5-7-14)23-19(26)16-12-21-8-9-22-16/h3-9,12-13,15,17,27-28H,10-11H2,1-2H3,(H,23,26)(H,24,25)/t15-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of 20S proteasome in human HL60 cells using Suc-LLVYaminoluciferin as substrate after 2 hrs by fluorescence ... |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50259642

(CHEMBL4077037)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCc1cccc(C)c1C)B(O)O |r| Show InChI InChI=1S/C24H34BN3O4/c1-16(2)13-22(25(31)32)28-23(29)21(14-19-10-6-5-7-11-19)27-24(30)26-15-20-12-8-9-17(3)18(20)4/h5-12,16,21-22,31-32H,13-15H2,1-4H3,(H,28,29)(H2,26,27,30)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of 20S proteasome in human HL60 cells using Suc-LLVYaminoluciferin as substrate after 2 hrs by fluorescence ... |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50446191
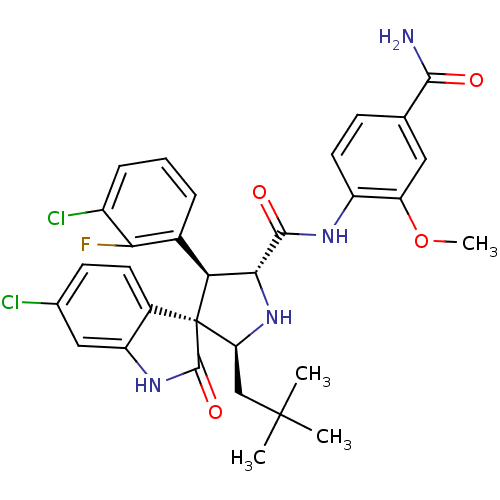
(CHEMBL3109036)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@]2([C@H]1c1cccc(Cl)c1F)C(=O)Nc1cc(Cl)ccc21)C(N)=O |r| Show InChI InChI=1S/C31H31Cl2FN4O4/c1-30(2,3)14-23-31(18-10-9-16(32)13-21(18)37-29(31)41)24(17-6-5-7-19(33)25(17)34)26(38-23)28(40)36-20-11-8-15(27(35)39)12-22(20)42-4/h5-13,23-24,26,38H,14H2,1-4H3,(H2,35,39)(H,36,40)(H,37,41)/t23-,24-,26+,31+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged MDM2 (unknown origin) assessed as inhibition of interaction with biotinylated p53 after 1 hr by HTRF assay |
ACS Med Chem Lett 5: 124-7 (2014)
Article DOI: 10.1021/ml400359z
BindingDB Entry DOI: 10.7270/Q20V8F7S |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50437206
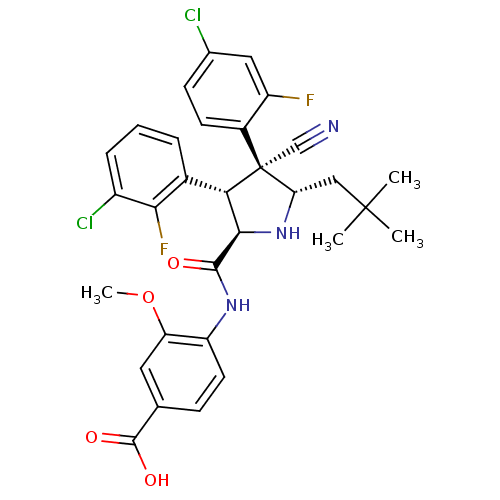
(CHEMBL2402737)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](C#N)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| Show InChI InChI=1S/C31H29Cl2F2N3O4/c1-30(2,3)14-24-31(15-36,19-10-9-17(32)13-21(19)34)25(18-6-5-7-20(33)26(18)35)27(38-24)28(39)37-22-11-8-16(29(40)41)12-23(22)42-4/h5-13,24-25,27,38H,14H2,1-4H3,(H,37,39)(H,40,41)/t24-,25-,27+,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged MDM2 (unknown origin) assessed as inhibition of interaction with p53 after 1 hr by HTRF assay |
J Med Chem 56: 5979-83 (2014)
Article DOI: 10.1021/jm400487c
BindingDB Entry DOI: 10.7270/Q2V40WMK |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50446197
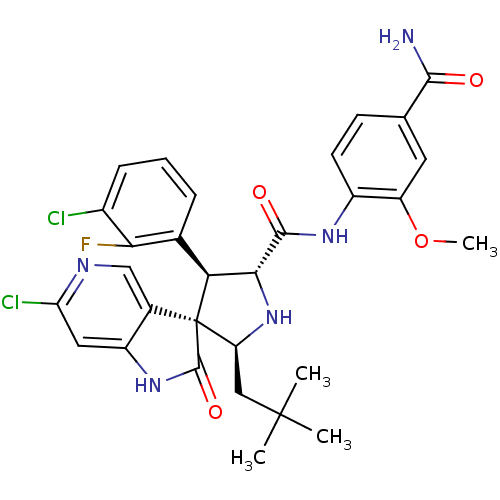
(CHEMBL3109042)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@]2([C@H]1c1cccc(Cl)c1F)C(=O)Nc1cc(Cl)ncc21)C(N)=O |r| Show InChI InChI=1S/C30H30Cl2FN5O4/c1-29(2,3)12-21-30(16-13-35-22(32)11-19(16)37-28(30)41)23(15-6-5-7-17(31)24(15)33)25(38-21)27(40)36-18-9-8-14(26(34)39)10-20(18)42-4/h5-11,13,21,23,25,38H,12H2,1-4H3,(H2,34,39)(H,36,40)(H,37,41)/t21-,23-,25+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged MDM2 (unknown origin) assessed as inhibition of interaction with biotinylated p53 after 1 hr by HTRF assay |
ACS Med Chem Lett 5: 124-7 (2014)
Article DOI: 10.1021/ml400359z
BindingDB Entry DOI: 10.7270/Q20V8F7S |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50446195

(CHEMBL3109040)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@]2([C@H]1c1cccc(Cl)c1F)C(=O)Nc1cc(Cl)sc21)C(N)=O |r| Show InChI InChI=1S/C29H29Cl2FN4O4S/c1-28(2,3)12-19-29(24-17(35-27(29)39)11-20(31)41-24)21(14-6-5-7-15(30)22(14)32)23(36-19)26(38)34-16-9-8-13(25(33)37)10-18(16)40-4/h5-11,19,21,23,36H,12H2,1-4H3,(H2,33,37)(H,34,38)(H,35,39)/t19-,21-,23+,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged MDM2 (unknown origin) assessed as inhibition of interaction with biotinylated p53 after 1 hr by HTRF assay |
ACS Med Chem Lett 5: 124-7 (2014)
Article DOI: 10.1021/ml400359z
BindingDB Entry DOI: 10.7270/Q20V8F7S |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50446196
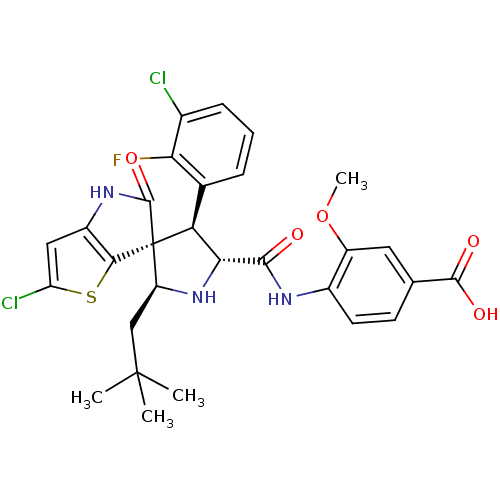
(CHEMBL3109041)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@]2([C@H]1c1cccc(Cl)c1F)C(=O)Nc1cc(Cl)sc21)C(O)=O |r| Show InChI InChI=1S/C29H28Cl2FN3O5S/c1-28(2,3)12-19-29(24-17(34-27(29)39)11-20(31)41-24)21(14-6-5-7-15(30)22(14)32)23(35-19)25(36)33-16-9-8-13(26(37)38)10-18(16)40-4/h5-11,19,21,23,35H,12H2,1-4H3,(H,33,36)(H,34,39)(H,37,38)/t19-,21-,23+,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged MDM2 (unknown origin) assessed as inhibition of interaction with biotinylated p53 after 1 hr by HTRF assay |
ACS Med Chem Lett 5: 124-7 (2014)
Article DOI: 10.1021/ml400359z
BindingDB Entry DOI: 10.7270/Q20V8F7S |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50259655
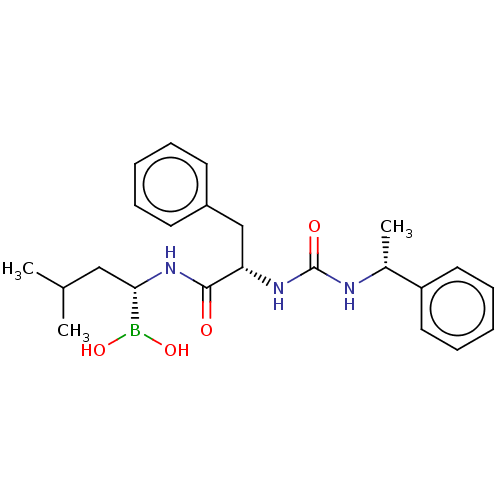
(CHEMBL4080306)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)N[C@H](C)c1ccccc1)B(O)O |r| Show InChI InChI=1S/C23H32BN3O4/c1-16(2)14-21(24(30)31)27-22(28)20(15-18-10-6-4-7-11-18)26-23(29)25-17(3)19-12-8-5-9-13-19/h4-13,16-17,20-21,30-31H,14-15H2,1-3H3,(H,27,28)(H2,25,26,29)/t17-,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Dissociation constant against galectin-3 using competitive fluorescence polarization |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50259641
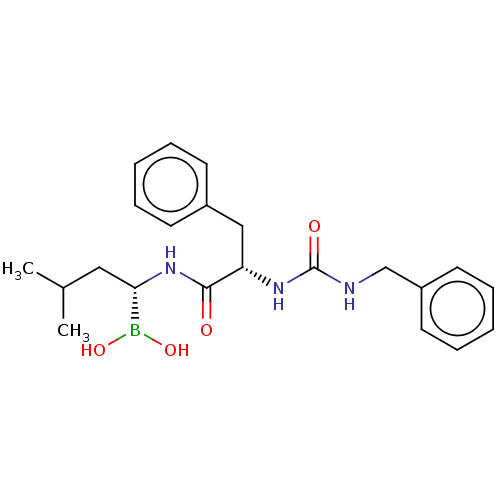
(CHEMBL484701)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCc1ccccc1)B(O)O |r| Show InChI InChI=1S/C22H30BN3O4/c1-16(2)13-20(23(29)30)26-21(27)19(14-17-9-5-3-6-10-17)25-22(28)24-15-18-11-7-4-8-12-18/h3-12,16,19-20,29-30H,13-15H2,1-2H3,(H,26,27)(H2,24,25,28)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of 20S proteasome in human HL60 cells using Suc-LLVYaminoluciferin as substrate after 2 hrs by fluorescence ... |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50446204

(CHEMBL3109055)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@]2([C@H]1c1cccc(Cl)c1F)C(=O)Nc1nc(Cl)ccc21)C(N)=O |r| Show InChI InChI=1S/C30H30Cl2FN5O4/c1-29(2,3)13-20-30(16-9-11-21(32)37-26(16)38-28(30)41)22(15-6-5-7-17(31)23(15)33)24(36-20)27(40)35-18-10-8-14(25(34)39)12-19(18)42-4/h5-12,20,22,24,36H,13H2,1-4H3,(H2,34,39)(H,35,40)(H,37,38,41)/t20-,22-,24+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged MDM2 (unknown origin) assessed as inhibition of interaction with biotinylated p53 after 1 hr by HTRF assay |
ACS Med Chem Lett 5: 124-7 (2014)
Article DOI: 10.1021/ml400359z
BindingDB Entry DOI: 10.7270/Q20V8F7S |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50259646
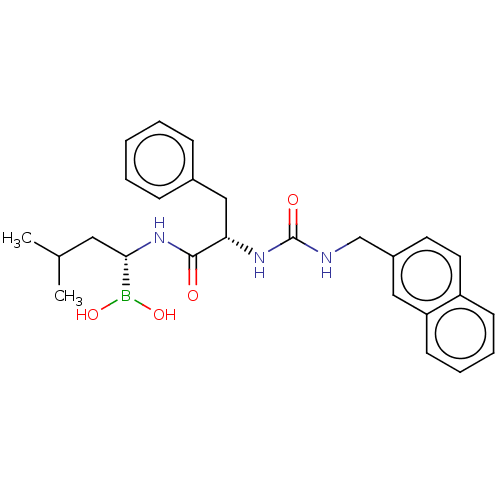
(CHEMBL4075225)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCc1ccc2ccccc2c1)B(O)O |r| Show InChI InChI=1S/C26H32BN3O4/c1-18(2)14-24(27(33)34)30-25(31)23(16-19-8-4-3-5-9-19)29-26(32)28-17-20-12-13-21-10-6-7-11-22(21)15-20/h3-13,15,18,23-24,33-34H,14,16-17H2,1-2H3,(H,30,31)(H2,28,29,32)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of 20S proteasome in human HL60 cells using Suc-LLVYaminoluciferin as substrate after 2 hrs by fluorescence ... |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50446207
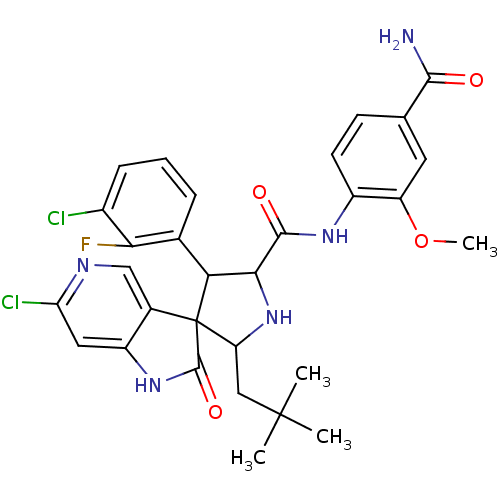
(CHEMBL3109051)Show SMILES COc1cc(ccc1NC(=O)C1NC(CC(C)(C)C)C2(C1c1cccc(Cl)c1F)C(=O)Nc1cc(Cl)ncc21)C(N)=O Show InChI InChI=1S/C30H30Cl2FN5O4/c1-29(2,3)12-21-30(16-13-35-22(32)11-19(16)37-28(30)41)23(15-6-5-7-17(31)24(15)33)25(38-21)27(40)36-18-9-8-14(26(34)39)10-20(18)42-4/h5-11,13,21,23,25,38H,12H2,1-4H3,(H2,34,39)(H,36,40)(H,37,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged MDM2 (unknown origin) assessed as inhibition of interaction with biotinylated p53 after 1 hr by HTRF assay |
ACS Med Chem Lett 5: 124-7 (2014)
Article DOI: 10.1021/ml400359z
BindingDB Entry DOI: 10.7270/Q20V8F7S |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50446205
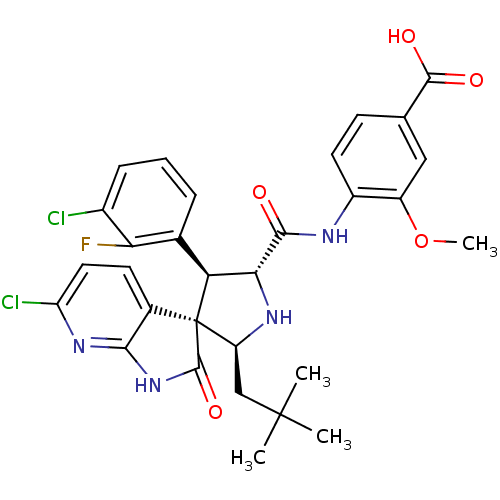
(CHEMBL3109049)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@]2([C@H]1c1cccc(Cl)c1F)C(=O)Nc1nc(Cl)ccc21)C(O)=O |r| Show InChI InChI=1S/C30H29Cl2FN4O5/c1-29(2,3)13-20-30(16-9-11-21(32)36-25(16)37-28(30)41)22(15-6-5-7-17(31)23(15)33)24(35-20)26(38)34-18-10-8-14(27(39)40)12-19(18)42-4/h5-12,20,22,24,35H,13H2,1-4H3,(H,34,38)(H,39,40)(H,36,37,41)/t20-,22-,24+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged MDM2 (unknown origin) assessed as inhibition of interaction with biotinylated p53 after 1 hr by HTRF assay |
ACS Med Chem Lett 5: 124-7 (2014)
Article DOI: 10.1021/ml400359z
BindingDB Entry DOI: 10.7270/Q20V8F7S |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50434287

(CHEMBL2386346)Show SMILES CCOc1cc(ccc1C1=N[C@@](C)(c2ccc(Cl)cc2)[C@](C)(N1C(=O)N1CCN(CCCS(C)(=O)=O)CC1)c1ccc(Cl)cc1)C(C)(C)C |r,t:10| Show InChI InChI=1S/C38H48Cl2N4O4S/c1-8-48-33-26-29(36(2,3)4)14-19-32(33)34-41-37(5,27-10-15-30(39)16-11-27)38(6,28-12-17-31(40)18-13-28)44(34)35(45)43-23-21-42(22-24-43)20-9-25-49(7,46)47/h10-19,26H,8-9,20-25H2,1-7H3/t37-,38+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged MDM2 (unknown origin) assessed as inhibition of interaction with p53 after 1 hr by HTRF assay |
J Med Chem 56: 5979-83 (2014)
Article DOI: 10.1021/jm400487c
BindingDB Entry DOI: 10.7270/Q2V40WMK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50259649
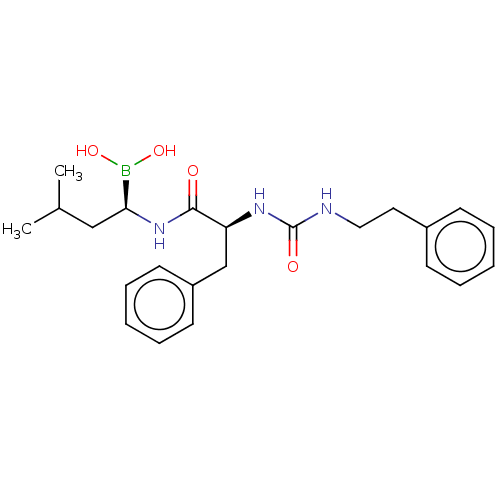
(CHEMBL4077282)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCCc1ccccc1)B(O)O |r| Show InChI InChI=1S/C23H32BN3O4/c1-17(2)15-21(24(30)31)27-22(28)20(16-19-11-7-4-8-12-19)26-23(29)25-14-13-18-9-5-3-6-10-18/h3-12,17,20-21,30-31H,13-16H2,1-2H3,(H,27,28)(H2,25,26,29)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Dissociation constant against galectin-3 using competitive fluorescence polarization |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50437209
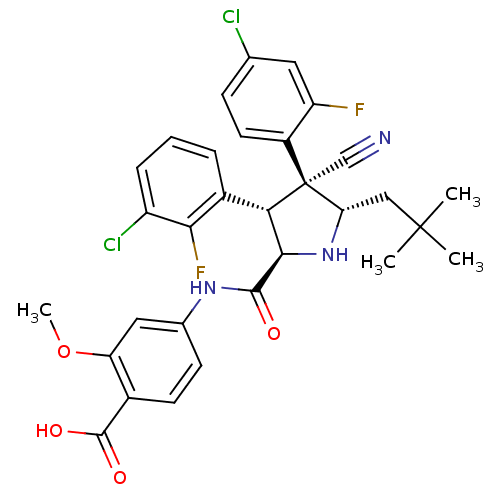
(CHEMBL2402734)Show SMILES COc1cc(NC(=O)[C@@H]2N[C@@H](CC(C)(C)C)[C@@](C#N)([C@H]2c2cccc(Cl)c2F)c2ccc(Cl)cc2F)ccc1C(O)=O |r| Show InChI InChI=1S/C31H29Cl2F2N3O4/c1-30(2,3)14-24-31(15-36,20-11-8-16(32)12-22(20)34)25(19-6-5-7-21(33)26(19)35)27(38-24)28(39)37-17-9-10-18(29(40)41)23(13-17)42-4/h5-13,24-25,27,38H,14H2,1-4H3,(H,37,39)(H,40,41)/t24-,25-,27+,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged MDM2 (unknown origin) assessed as inhibition of interaction with p53 after 1 hr by HTRF assay |
J Med Chem 56: 5979-83 (2014)
Article DOI: 10.1021/jm400487c
BindingDB Entry DOI: 10.7270/Q2V40WMK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-1
(Homo sapiens (Human)) | BDBM50259656

(CHEMBL4089402)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)N1CCc2ccccc2C1)B(O)O |r| Show InChI InChI=1S/C24H32BN3O4/c1-17(2)14-22(25(31)32)27-23(29)21(15-18-8-4-3-5-9-18)26-24(30)28-13-12-19-10-6-7-11-20(19)16-28/h3-11,17,21-22,31-32H,12-16H2,1-2H3,(H,26,30)(H,27,29)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of caspase-like activity of 20S proteasome in human HL60 cells using Z-nLPnLDaminoluciferin as substrate after 2 hrs by fluorescence analy... |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50259658

(CHEMBL4096371)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCc1ccco1)B(O)O |r| Show InChI InChI=1S/C20H28BN3O5/c1-14(2)11-18(21(27)28)24-19(25)17(12-15-7-4-3-5-8-15)23-20(26)22-13-16-9-6-10-29-16/h3-10,14,17-18,27-28H,11-13H2,1-2H3,(H,24,25)(H2,22,23,26)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Dissociation constant against galectin-3 using competitive fluorescence polarization |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50437208
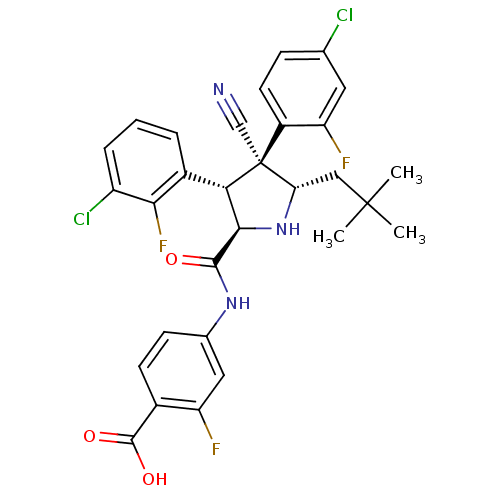
(CHEMBL2402735)Show SMILES CC(C)(C)C[C@@H]1N[C@H]([C@H](c2cccc(Cl)c2F)[C@@]1(C#N)c1ccc(Cl)cc1F)C(=O)Nc1ccc(C(O)=O)c(F)c1 |r| Show InChI InChI=1S/C30H26Cl2F3N3O3/c1-29(2,3)13-23-30(14-36,19-10-7-15(31)11-22(19)34)24(18-5-4-6-20(32)25(18)35)26(38-23)27(39)37-16-8-9-17(28(40)41)21(33)12-16/h4-12,23-24,26,38H,13H2,1-3H3,(H,37,39)(H,40,41)/t23-,24-,26+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged MDM2 (unknown origin) assessed as inhibition of interaction with p53 after 1 hr by HTRF assay |
J Med Chem 56: 5979-83 (2014)
Article DOI: 10.1021/jm400487c
BindingDB Entry DOI: 10.7270/Q2V40WMK |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50437210
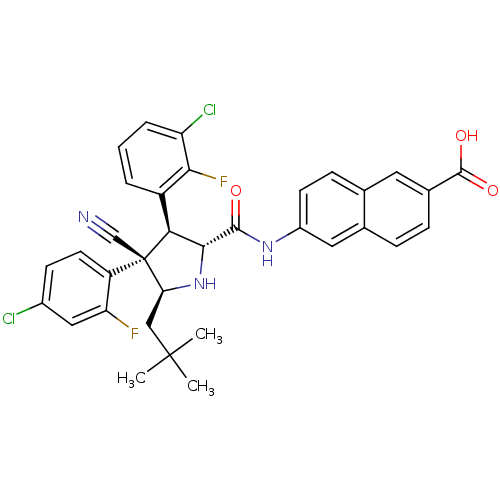
(CHEMBL2402733)Show SMILES CC(C)(C)C[C@@H]1N[C@H]([C@H](c2cccc(Cl)c2F)[C@@]1(C#N)c1ccc(Cl)cc1F)C(=O)Nc1ccc2cc(ccc2c1)C(O)=O |r| Show InChI InChI=1S/C34H29Cl2F2N3O3/c1-33(2,3)16-27-34(17-39,24-12-10-21(35)15-26(24)37)28(23-5-4-6-25(36)29(23)38)30(41-27)31(42)40-22-11-9-18-13-20(32(43)44)8-7-19(18)14-22/h4-15,27-28,30,41H,16H2,1-3H3,(H,40,42)(H,43,44)/t27-,28-,30+,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged MDM2 (unknown origin) assessed as inhibition of interaction with p53 after 1 hr by HTRF assay |
J Med Chem 56: 5979-83 (2014)
Article DOI: 10.1021/jm400487c
BindingDB Entry DOI: 10.7270/Q2V40WMK |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50437212
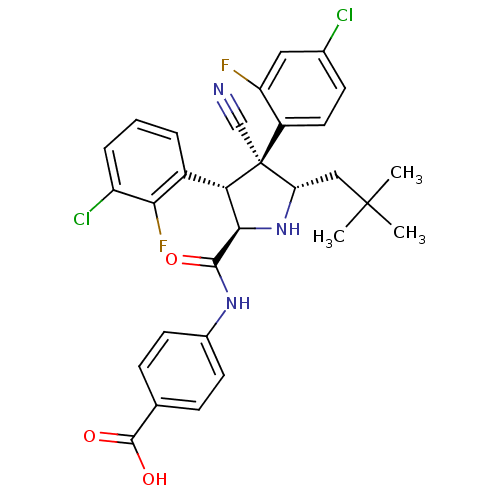
(CHEMBL2402731)Show SMILES CC(C)(C)C[C@@H]1N[C@H]([C@H](c2cccc(Cl)c2F)[C@@]1(C#N)c1ccc(Cl)cc1F)C(=O)Nc1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C30H27Cl2F2N3O3/c1-29(2,3)14-23-30(15-35,20-12-9-17(31)13-22(20)33)24(19-5-4-6-21(32)25(19)34)26(37-23)27(38)36-18-10-7-16(8-11-18)28(39)40/h4-13,23-24,26,37H,14H2,1-3H3,(H,36,38)(H,39,40)/t23-,24-,26+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged MDM2 (unknown origin) assessed as inhibition of interaction with p53 after 1 hr by HTRF assay |
J Med Chem 56: 5979-83 (2014)
Article DOI: 10.1021/jm400487c
BindingDB Entry DOI: 10.7270/Q2V40WMK |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50437211
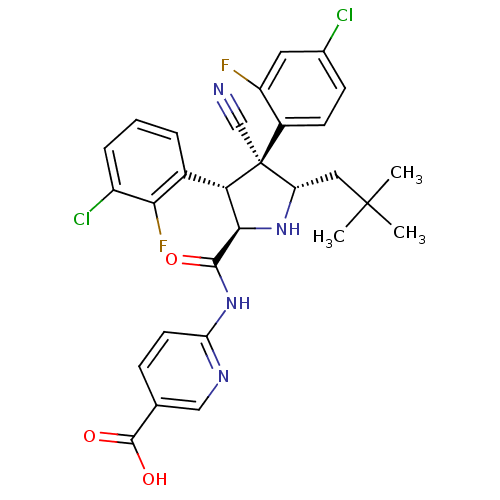
(CHEMBL2402732)Show SMILES CC(C)(C)C[C@@H]1N[C@H]([C@H](c2cccc(Cl)c2F)[C@@]1(C#N)c1ccc(Cl)cc1F)C(=O)Nc1ccc(cn1)C(O)=O |r| Show InChI InChI=1S/C29H26Cl2F2N4O3/c1-28(2,3)12-21-29(14-34,18-9-8-16(30)11-20(18)32)23(17-5-4-6-19(31)24(17)33)25(36-21)26(38)37-22-10-7-15(13-35-22)27(39)40/h4-11,13,21,23,25,36H,12H2,1-3H3,(H,39,40)(H,35,37,38)/t21-,23-,25+,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged MDM2 (unknown origin) assessed as inhibition of interaction with p53 after 1 hr by HTRF assay |
J Med Chem 56: 5979-83 (2014)
Article DOI: 10.1021/jm400487c
BindingDB Entry DOI: 10.7270/Q2V40WMK |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50446201
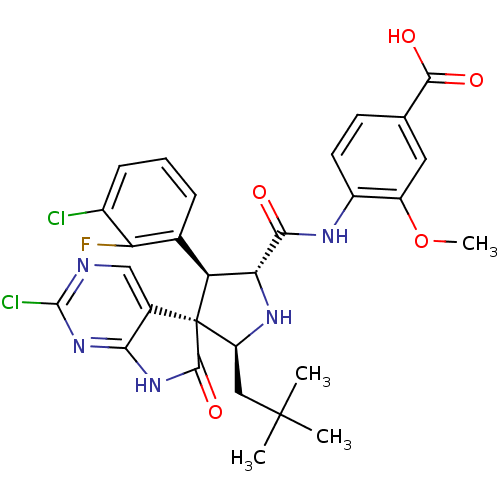
(CHEMBL3109046)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@]2([C@H]1c1cccc(Cl)c1F)C(=O)Nc1nc(Cl)ncc21)C(O)=O |r| Show InChI InChI=1S/C29H28Cl2FN5O5/c1-28(2,3)11-19-29(15-12-33-27(31)37-23(15)36-26(29)41)20(14-6-5-7-16(30)21(14)32)22(35-19)24(38)34-17-9-8-13(25(39)40)10-18(17)42-4/h5-10,12,19-20,22,35H,11H2,1-4H3,(H,34,38)(H,39,40)(H,33,36,37,41)/t19-,20-,22+,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged MDM2 (unknown origin) assessed as inhibition of interaction with biotinylated p53 after 1 hr by HTRF assay |
ACS Med Chem Lett 5: 124-7 (2014)
Article DOI: 10.1021/ml400359z
BindingDB Entry DOI: 10.7270/Q20V8F7S |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50437207
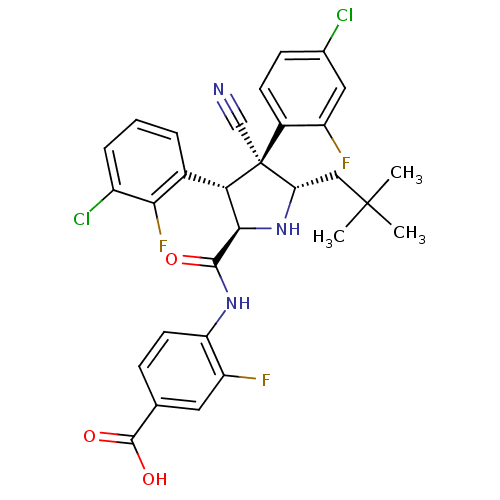
(CHEMBL2402736)Show SMILES CC(C)(C)C[C@@H]1N[C@H]([C@H](c2cccc(Cl)c2F)[C@@]1(C#N)c1ccc(Cl)cc1F)C(=O)Nc1ccc(cc1F)C(O)=O |r| Show InChI InChI=1S/C30H26Cl2F3N3O3/c1-29(2,3)13-23-30(14-36,18-9-8-16(31)12-20(18)33)24(17-5-4-6-19(32)25(17)35)26(38-23)27(39)37-22-10-7-15(28(40)41)11-21(22)34/h4-12,23-24,26,38H,13H2,1-3H3,(H,37,39)(H,40,41)/t23-,24-,26+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged MDM2 (unknown origin) assessed as inhibition of interaction with p53 after 1 hr by HTRF assay |
J Med Chem 56: 5979-83 (2014)
Article DOI: 10.1021/jm400487c
BindingDB Entry DOI: 10.7270/Q2V40WMK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data