

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Human rhinovirus B) | BDBM50065588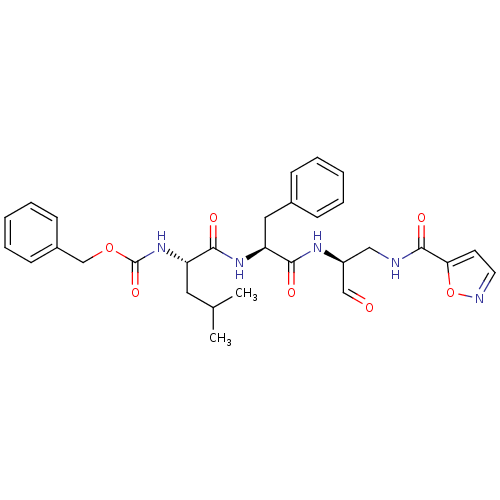 (CHEMBL96803 | [(S)-1-((S)-1-{(S)-1-Formyl-2-[(isox...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity against HRV-14 3C protease. | J Med Chem 41: 2786-805 (1998) Article DOI: 10.1021/jm980071x BindingDB Entry DOI: 10.7270/Q2F76BPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50065603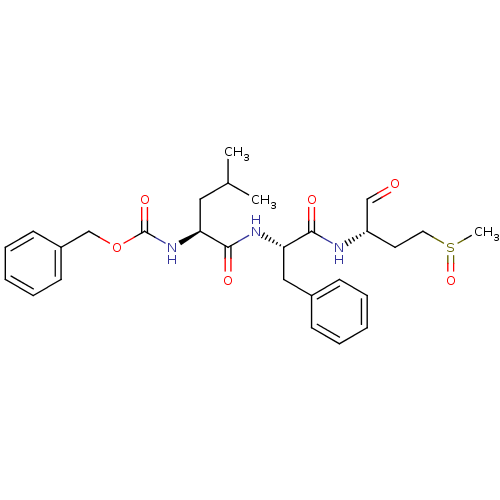 (CHEMBL96185 | {(S)-1-[(S)-1-((S)-1-Formyl-3-methan...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity against HRV-14 3C protease. | J Med Chem 41: 2786-805 (1998) Article DOI: 10.1021/jm980071x BindingDB Entry DOI: 10.7270/Q2F76BPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50065598 (CHEMBL419332 | {(S)-1-[(S)-1-((S)-3-Dimethylcarbam...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity against HRV-14 3C protease. | J Med Chem 41: 2786-805 (1998) Article DOI: 10.1021/jm980071x BindingDB Entry DOI: 10.7270/Q2F76BPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50065590 (((S)-1-{(S)-1-[(S)-1-(Acetylamino-methyl)-2-oxo-et...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity against HRV-14 3C protease. | J Med Chem 41: 2786-805 (1998) Article DOI: 10.1021/jm980071x BindingDB Entry DOI: 10.7270/Q2F76BPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50065586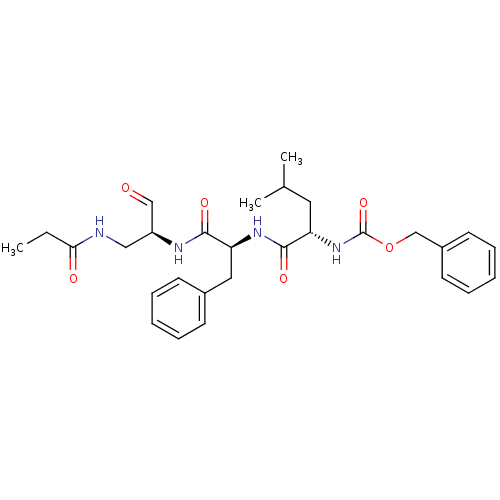 (((S)-3-Methyl-1-{(S)-1-[(S)-2-oxo-1-(propionylamin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity against HRV-14 3C protease. | J Med Chem 41: 2786-805 (1998) Article DOI: 10.1021/jm980071x BindingDB Entry DOI: 10.7270/Q2F76BPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50065595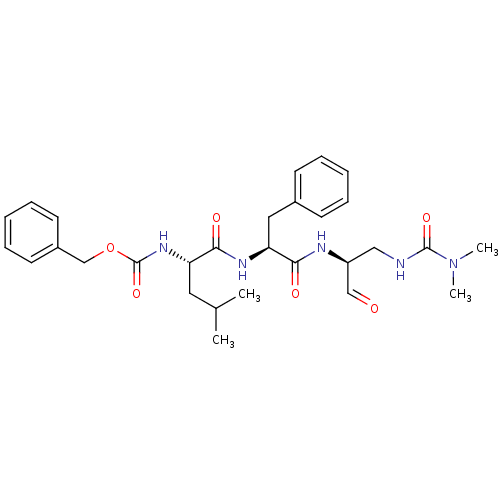 (((S)-1-{(S)-1-[(S)-2-(3,3-Dimethyl-ureido)-1-formy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity against HRV-14 3C protease. | J Med Chem 41: 2786-805 (1998) Article DOI: 10.1021/jm980071x BindingDB Entry DOI: 10.7270/Q2F76BPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50065599 (CHEMBL94652 | {(S)-1-[(S)-1-((S)-2-Benzoylamino-1-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity against HRV-14 3C protease. | J Med Chem 41: 2786-805 (1998) Article DOI: 10.1021/jm980071x BindingDB Entry DOI: 10.7270/Q2F76BPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50065601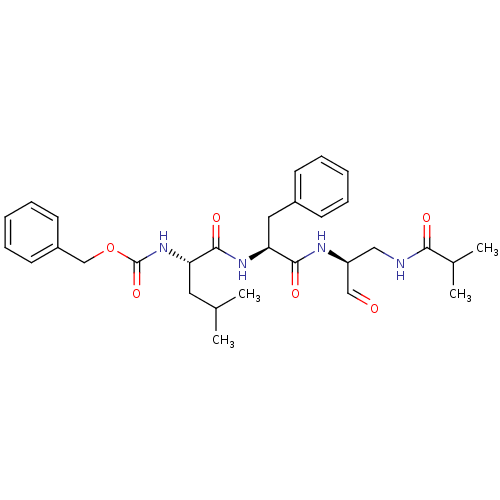 (CHEMBL95031 | {(S)-1-[(S)-1-((S)-1-Formyl-2-isobut...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity against HRV-14 3C protease. | J Med Chem 41: 2786-805 (1998) Article DOI: 10.1021/jm980071x BindingDB Entry DOI: 10.7270/Q2F76BPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50065590 (((S)-1-{(S)-1-[(S)-1-(Acetylamino-methyl)-2-oxo-et...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound was measured against HRV-16 3C protease | J Med Chem 41: 2786-805 (1998) Article DOI: 10.1021/jm980071x BindingDB Entry DOI: 10.7270/Q2F76BPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50065591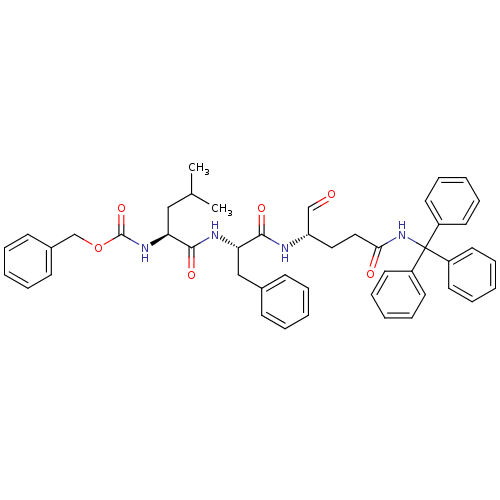 (((S)-1-{(S)-1-[(S)-1-Formyl-3-(trityl-carbamoyl)-p...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity against HRV-14 3C protease. | J Med Chem 41: 2786-805 (1998) Article DOI: 10.1021/jm980071x BindingDB Entry DOI: 10.7270/Q2F76BPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50065596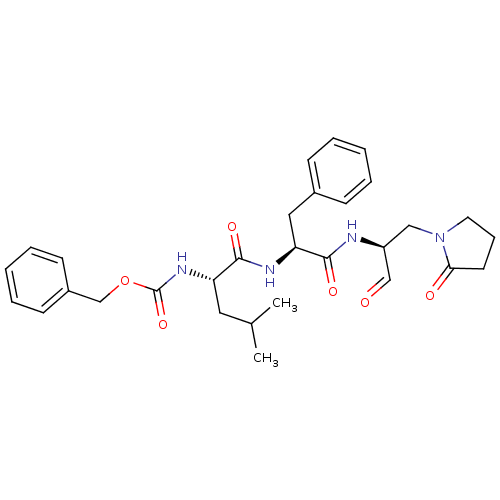 (((S)-1-{(S)-1-[(S)-1-Formyl-2-(2-oxo-pyrrolidin-1-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity against HRV-14 3C protease. | J Med Chem 41: 2786-805 (1998) Article DOI: 10.1021/jm980071x BindingDB Entry DOI: 10.7270/Q2F76BPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50065594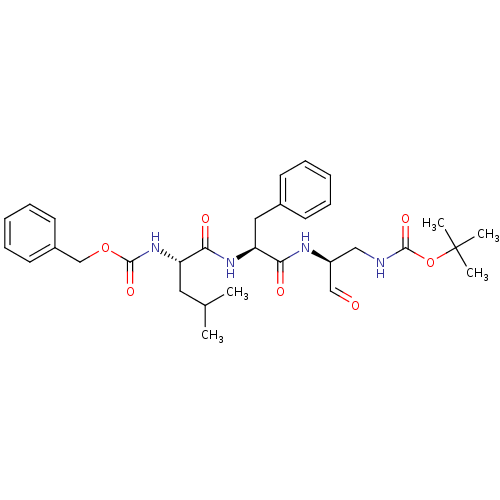 (((S)-1-{(S)-1-[(S)-1-(tert-Butoxycarbonylamino-met...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity against HRV-14 3C protease. | J Med Chem 41: 2786-805 (1998) Article DOI: 10.1021/jm980071x BindingDB Entry DOI: 10.7270/Q2F76BPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50065590 (((S)-1-{(S)-1-[(S)-1-(Acetylamino-methyl)-2-oxo-et...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound was measured against HRV-2 3C protease | J Med Chem 41: 2786-805 (1998) Article DOI: 10.1021/jm980071x BindingDB Entry DOI: 10.7270/Q2F76BPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50065589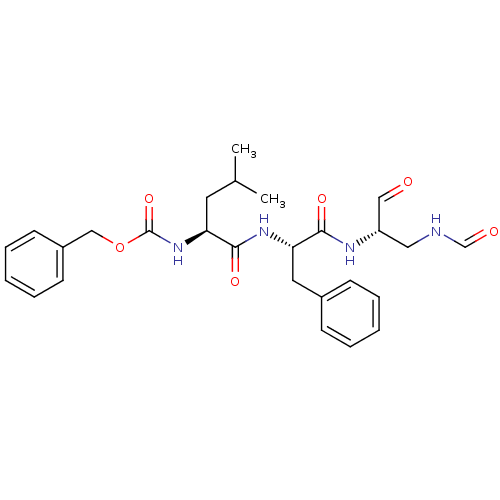 (CHEMBL95120 | {(S)-1-[(S)-1-((S)-1-Formylaminometh...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity against HRV-14 3C protease. | J Med Chem 41: 2786-805 (1998) Article DOI: 10.1021/jm980071x BindingDB Entry DOI: 10.7270/Q2F76BPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50065597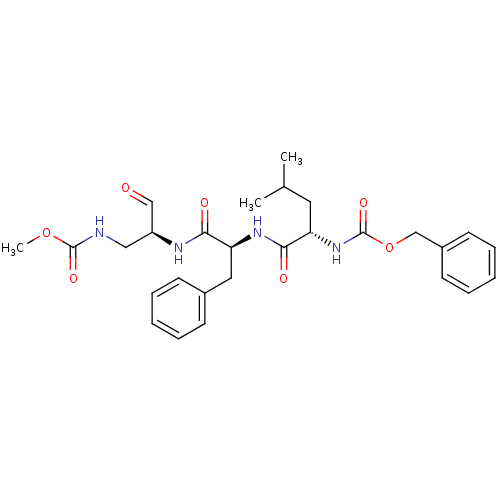 (((S)-1-{(S)-1-[(S)-1-(Methoxycarbonylamino-methyl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity against HRV-14 3C protease. | J Med Chem 41: 2786-805 (1998) Article DOI: 10.1021/jm980071x BindingDB Entry DOI: 10.7270/Q2F76BPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50065593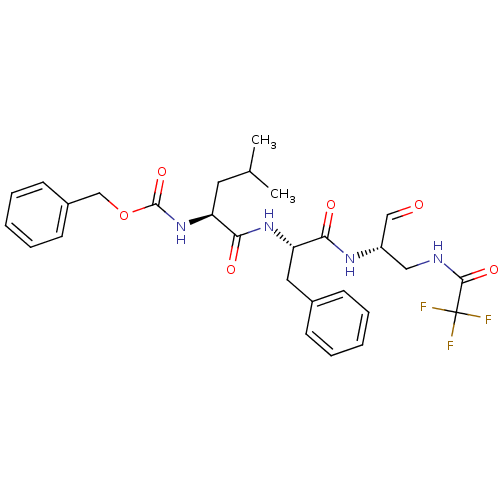 (((S)-1-{(S)-1-[(S)-1-Formyl-2-(2,2,2-trifluoro-ace...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity against HRV-14 3C protease. | J Med Chem 41: 2786-805 (1998) Article DOI: 10.1021/jm980071x BindingDB Entry DOI: 10.7270/Q2F76BPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50065590 (((S)-1-{(S)-1-[(S)-1-(Acetylamino-methyl)-2-oxo-et...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound was measured against HRV-89 3C protease | J Med Chem 41: 2786-805 (1998) Article DOI: 10.1021/jm980071x BindingDB Entry DOI: 10.7270/Q2F76BPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50065587 (CHEMBL97090 | {(S)-1-[(S)-1-((S)-3-Cyano-1-formyl-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity against HRV-14 3C protease. | J Med Chem 41: 2786-805 (1998) Article DOI: 10.1021/jm980071x BindingDB Entry DOI: 10.7270/Q2F76BPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50065600 (((S)-1-{(S)-1-[(S)-2-(Acetyl-methyl-amino)-1-formy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 469 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity against HRV-14 3C protease. | J Med Chem 41: 2786-805 (1998) Article DOI: 10.1021/jm980071x BindingDB Entry DOI: 10.7270/Q2F76BPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 2 (Homo sapiens) | BDBM604241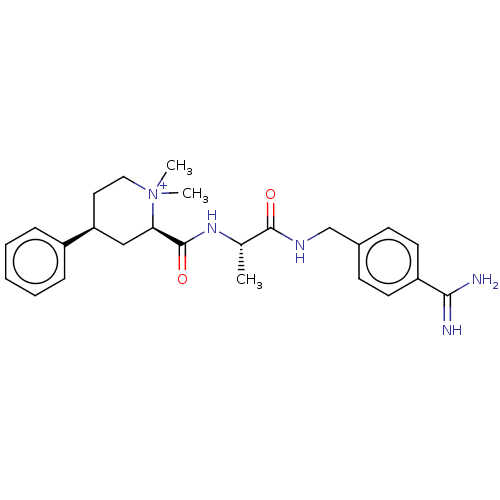 (Preparation of (2R,4S)-2-(((S)-1-((4-carbamimidoyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | <500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2P2733R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 2 (Homo sapiens) | BDBM604242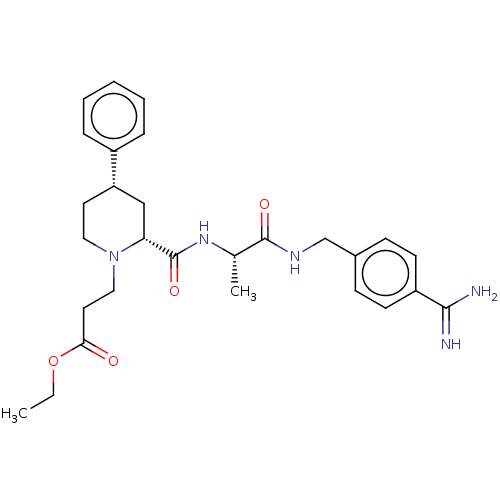 (Preparation of ethyl 3-((2R,4S)-2-(((S)-1-((4-carb...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | <500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2P2733R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 2 (Homo sapiens) | BDBM604243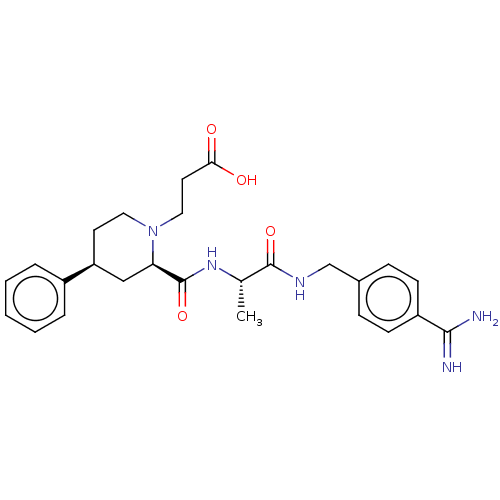 (Preparation of 3-((2R,4S)-2-(((S)-1-((4-carbamimid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | <500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2P2733R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 2 (Homo sapiens) | BDBM604244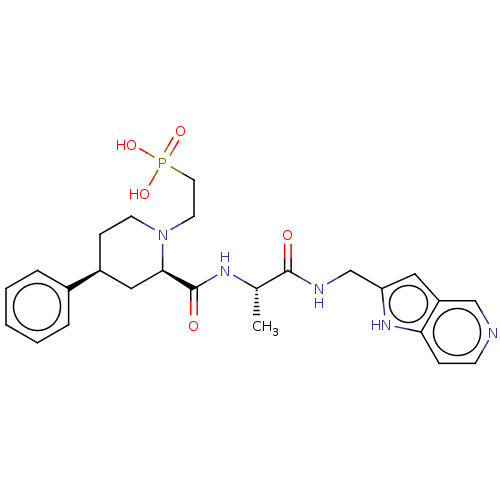 (US11661418, Compound I-15) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | <500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2P2733R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 2 (Homo sapiens) | BDBM604245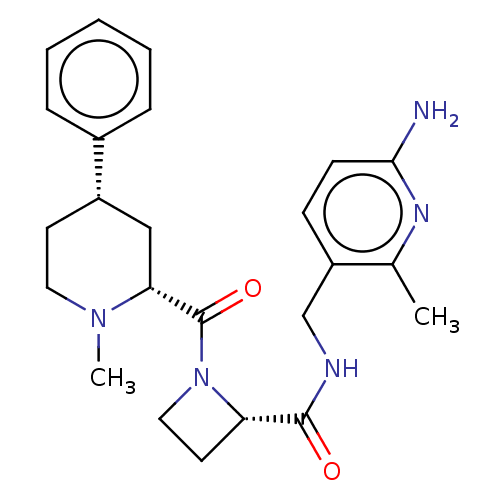 (US11661418, Compound I-16) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | <500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2P2733R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 2 (Homo sapiens) | BDBM604246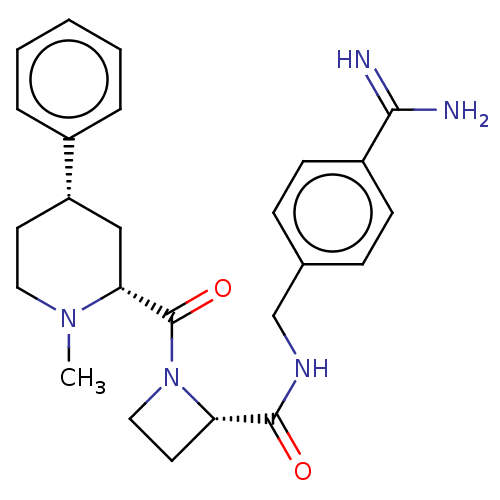 (Preparation of (S)—N-(4-carbamimidoylbenzyl)-1-((2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | <500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2P2733R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 2 (Homo sapiens) | BDBM604249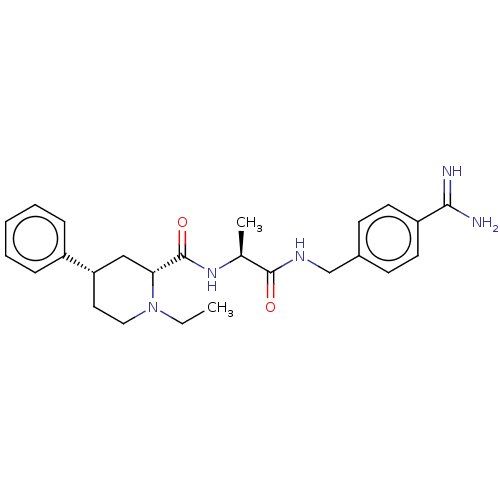 (Preparation of (2R,4S)—N—((S)-1-((4-carbamimidoylb...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | <500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2P2733R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 2 (Homo sapiens) | BDBM604250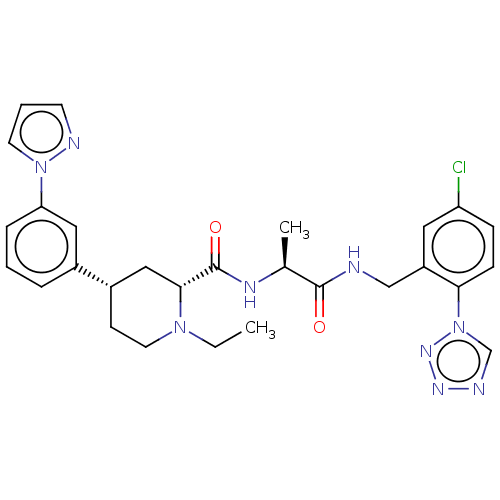 (Preparation of (2R,4S)-4-(3-(1H-pyrazol-1-yl)pheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | <500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2P2733R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 2 (Homo sapiens) | BDBM604251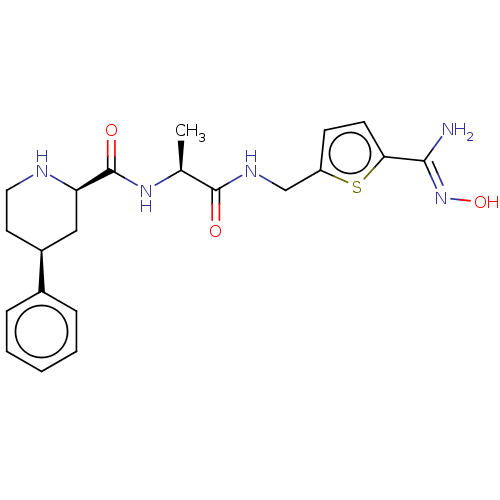 (Preparation of (2R,4S)—N—((S)-1-(((5-((Z)—N-hydrox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | <500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2P2733R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 2 (Homo sapiens) | BDBM595060 (US11584714, Compound 1088) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay was run at room temperature in an assay buffer containing 20 mM Hepes, pH 7.4, 140 mM NaCl and 0.1% Tween 20. Assay parameters were adjuste... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VH5SRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 2 (Mouse) | BDBM595060 (US11584714, Compound 1088) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay was run at room temperature in an assay buffer containing 20 mM Hepes, pH 7.4, 140 mM NaCl and 0.1% Tween 20. Assay parameters were adjuste... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VH5SRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 2 (Homo sapiens) | BDBM595062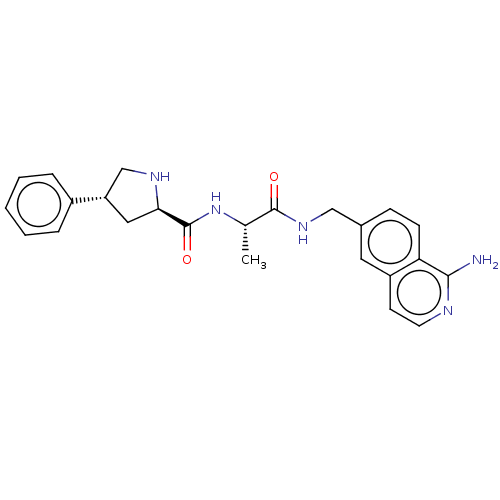 (US11584714, Compound 1090) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay was run at room temperature in an assay buffer containing 20 mM Hepes, pH 7.4, 140 mM NaCl and 0.1% Tween 20. Assay parameters were adjuste... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VH5SRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 2 (Mouse) | BDBM595062 (US11584714, Compound 1090) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay was run at room temperature in an assay buffer containing 20 mM Hepes, pH 7.4, 140 mM NaCl and 0.1% Tween 20. Assay parameters were adjuste... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VH5SRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 2 (Mouse) | BDBM595063 (US11584714, Compound 1091) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay was run at room temperature in an assay buffer containing 20 mM Hepes, pH 7.4, 140 mM NaCl and 0.1% Tween 20. Assay parameters were adjuste... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VH5SRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 2 (Homo sapiens) | BDBM595064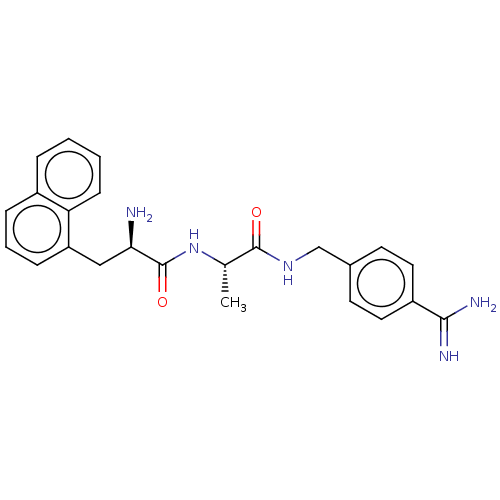 (US11584714, Compound 1092) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay was run at room temperature in an assay buffer containing 20 mM Hepes, pH 7.4, 140 mM NaCl and 0.1% Tween 20. Assay parameters were adjuste... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VH5SRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 2 (Mouse) | BDBM595064 (US11584714, Compound 1092) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay was run at room temperature in an assay buffer containing 20 mM Hepes, pH 7.4, 140 mM NaCl and 0.1% Tween 20. Assay parameters were adjuste... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VH5SRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| thrombin (Homo sapiens (Human)) | BDBM595064 (US11584714, Compound 1092) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The thrombin assay utilizes a fluorogenic peptide substrate (Boc-VPR-AMC (R&D Systems) and was run at room temperature in an assay buffer containing ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VH5SRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| thrombin (Homo sapiens (Human)) | BDBM595401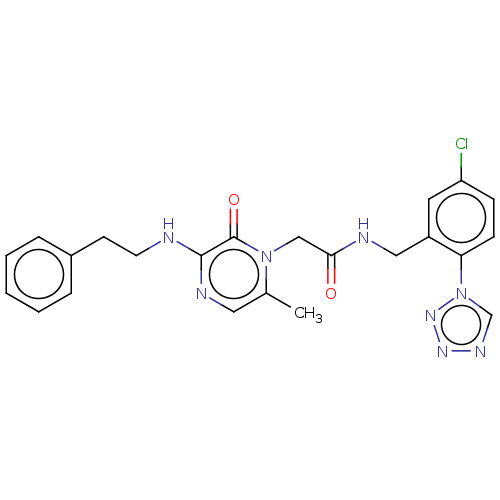 (US11584714, Compound 1489) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The thrombin assay utilizes a fluorogenic peptide substrate (Boc-VPR-AMC (R&D Systems) and was run at room temperature in an assay buffer containing ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VH5SRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 2 (Homo sapiens) | BDBM595402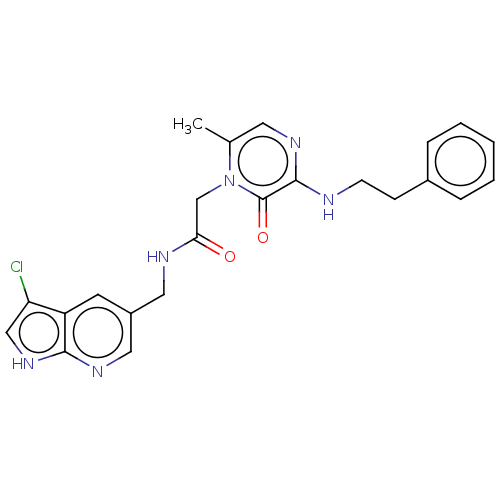 (US11584714, Compound 1490) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay was run at room temperature in an assay buffer containing 20 mM Hepes, pH 7.4, 140 mM NaCl and 0.1% Tween 20. Assay parameters were adjuste... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VH5SRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| thrombin (Homo sapiens (Human)) | BDBM595402 (US11584714, Compound 1490) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The thrombin assay utilizes a fluorogenic peptide substrate (Boc-VPR-AMC (R&D Systems) and was run at room temperature in an assay buffer containing ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VH5SRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 2 (Mouse) | BDBM595403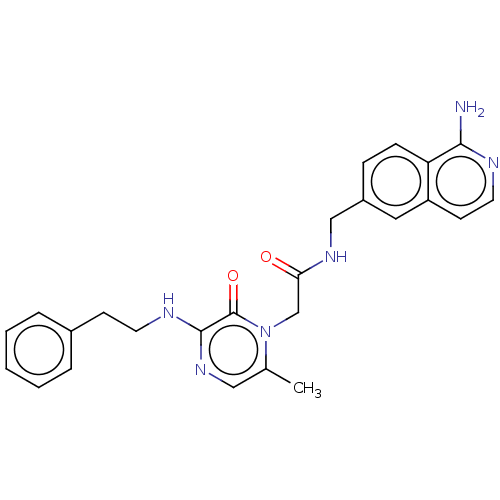 (US11584714, Compound 1491) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay was run at room temperature in an assay buffer containing 20 mM Hepes, pH 7.4, 140 mM NaCl and 0.1% Tween 20. Assay parameters were adjuste... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VH5SRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| thrombin (Homo sapiens (Human)) | BDBM595403 (US11584714, Compound 1491) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The thrombin assay utilizes a fluorogenic peptide substrate (Boc-VPR-AMC (R&D Systems) and was run at room temperature in an assay buffer containing ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VH5SRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 2 (Homo sapiens) | BDBM595404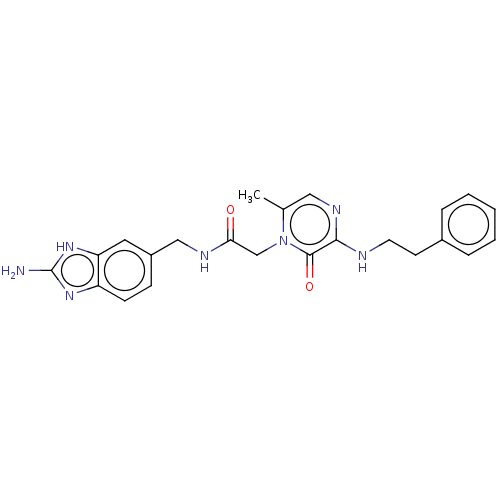 (US11584714, Compound 1492) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay was run at room temperature in an assay buffer containing 20 mM Hepes, pH 7.4, 140 mM NaCl and 0.1% Tween 20. Assay parameters were adjuste... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VH5SRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 2 (Mouse) | BDBM595404 (US11584714, Compound 1492) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay was run at room temperature in an assay buffer containing 20 mM Hepes, pH 7.4, 140 mM NaCl and 0.1% Tween 20. Assay parameters were adjuste... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VH5SRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| thrombin (Homo sapiens (Human)) | BDBM595404 (US11584714, Compound 1492) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The thrombin assay utilizes a fluorogenic peptide substrate (Boc-VPR-AMC (R&D Systems) and was run at room temperature in an assay buffer containing ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VH5SRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 2 (Homo sapiens) | BDBM595406 (US11584714, Compound 1494) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay was run at room temperature in an assay buffer containing 20 mM Hepes, pH 7.4, 140 mM NaCl and 0.1% Tween 20. Assay parameters were adjuste... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VH5SRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 2 (Mouse) | BDBM595406 (US11584714, Compound 1494) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay was run at room temperature in an assay buffer containing 20 mM Hepes, pH 7.4, 140 mM NaCl and 0.1% Tween 20. Assay parameters were adjuste... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VH5SRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 2 (Homo sapiens) | BDBM595408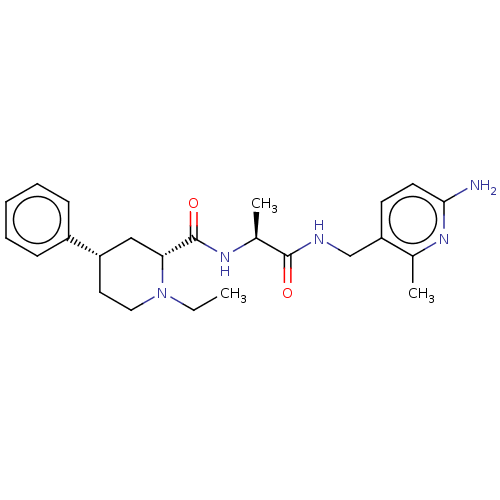 (US11584714, Compound 1496) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay was run at room temperature in an assay buffer containing 20 mM Hepes, pH 7.4, 140 mM NaCl and 0.1% Tween 20. Assay parameters were adjuste... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VH5SRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 2 (Homo sapiens) | BDBM595409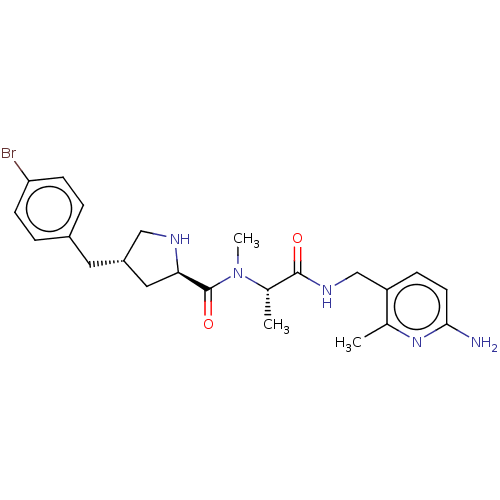 (US11584714, Compound 1497) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay was run at room temperature in an assay buffer containing 20 mM Hepes, pH 7.4, 140 mM NaCl and 0.1% Tween 20. Assay parameters were adjuste... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VH5SRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 2 (Homo sapiens) | BDBM604240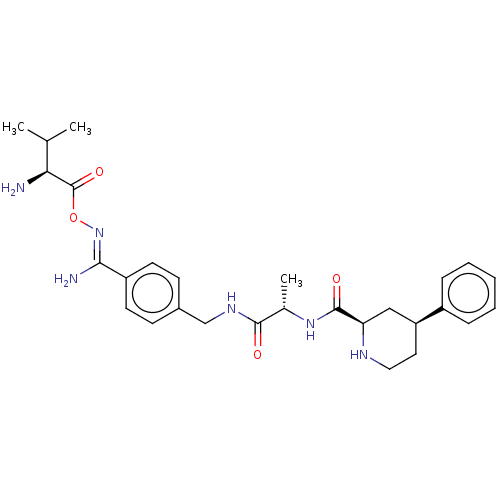 (Preparation of (2R,4S)—N—((S)-1-((4-((Z)—N′-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | <500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2P2733R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mannan-binding lectin serine protease 2 (Homo sapiens) | BDBM595385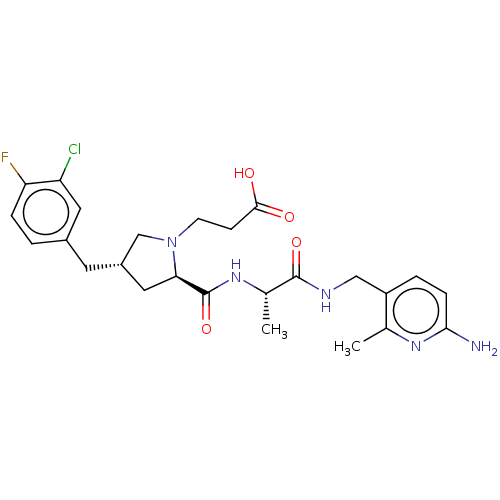 (US11584714, Compound 1473) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay was run at room temperature in an assay buffer containing 20 mM Hepes, pH 7.4, 140 mM NaCl and 0.1% Tween 20. Assay parameters were adjuste... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VH5SRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1768 total ) | Next | Last >> |