 Found 437 hits with Last Name = 'long' and Initial = 'st'
Found 437 hits with Last Name = 'long' and Initial = 'st' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin K
(Homo sapiens (Human)) | BDBM19783
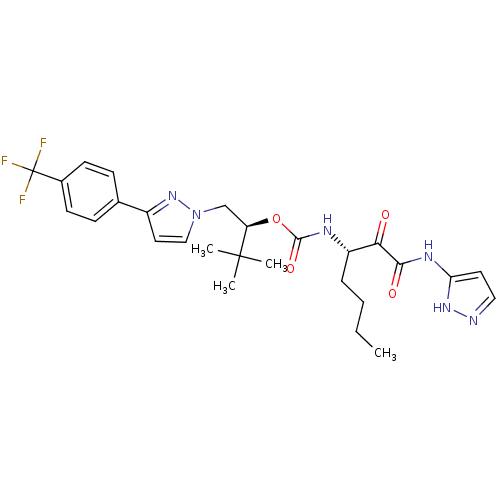
((2S)-3,3-dimethyl-1-{3-[4-(trifluoromethyl)phenyl]...)Show SMILES CCCC[C@H](NC(=O)O[C@H](Cn1ccc(n1)-c1ccc(cc1)C(F)(F)F)C(C)(C)C)C(=O)C(=O)Nc1ccn[nH]1 |r| Show InChI InChI=1S/C27H33F3N6O4/c1-5-6-7-20(23(37)24(38)33-22-12-14-31-34-22)32-25(39)40-21(26(2,3)4)16-36-15-13-19(35-36)17-8-10-18(11-9-17)27(28,29)30/h8-15,20-21H,5-7,16H2,1-4H3,(H,32,39)(H2,31,33,34,38)/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0720 | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut... |
Bioorg Med Chem Lett 17: 22-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.102
BindingDB Entry DOI: 10.7270/Q2NZ85XC |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50084655
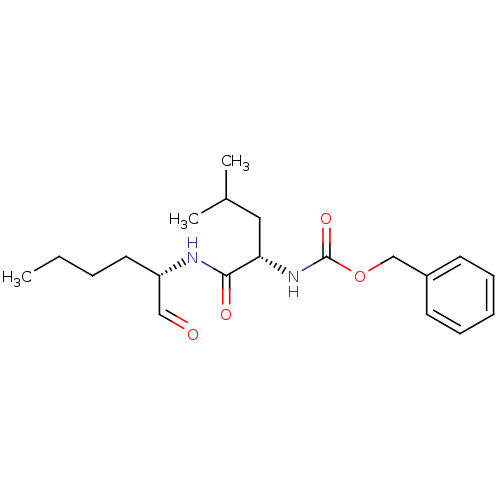
(CHEMBL92708 | Calpeptin | Z-Leu-Nle-CHO | [(S)-1-(...)Show SMILES CCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C=O |r| Show InChI InChI=1S/C20H30N2O4/c1-4-5-11-17(13-23)21-19(24)18(12-15(2)3)22-20(25)26-14-16-9-7-6-8-10-16/h6-10,13,15,17-18H,4-5,11-12,14H2,1-3H3,(H,21,24)(H,22,25)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against human cathepsin K |
Bioorg Med Chem Lett 14: 719-22 (2004)
BindingDB Entry DOI: 10.7270/Q2QV3KX1 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM19783

((2S)-3,3-dimethyl-1-{3-[4-(trifluoromethyl)phenyl]...)Show SMILES CCCC[C@H](NC(=O)O[C@H](Cn1ccc(n1)-c1ccc(cc1)C(F)(F)F)C(C)(C)C)C(=O)C(=O)Nc1ccn[nH]1 |r| Show InChI InChI=1S/C27H33F3N6O4/c1-5-6-7-20(23(37)24(38)33-22-12-14-31-34-22)32-25(39)40-21(26(2,3)4)16-36-15-13-19(35-36)17-8-10-18(11-9-17)27(28,29)30/h8-15,20-21H,5-7,16H2,1-4H3,(H,32,39)(H2,31,33,34,38)/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut... |
Bioorg Med Chem Lett 17: 22-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.102
BindingDB Entry DOI: 10.7270/Q2NZ85XC |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50138858
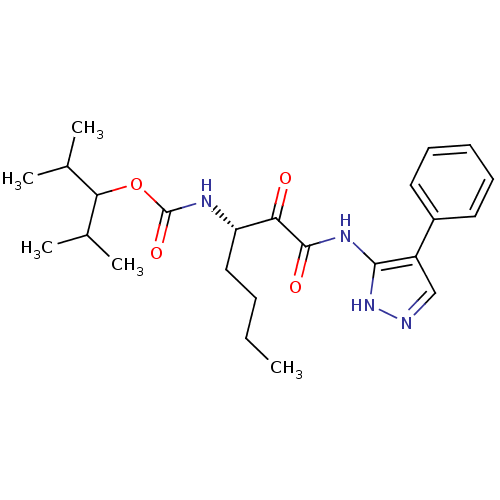
(CHEMBL154579 | [(S)-1-(4-Phenyl-1H-pyrazol-3-ylami...)Show SMILES CCCC[C@H](NC(=O)OC(C(C)C)C(C)C)C(=O)C(=O)Nc1[nH]ncc1-c1ccccc1 Show InChI InChI=1S/C24H34N4O4/c1-6-7-13-19(26-24(31)32-21(15(2)3)16(4)5)20(29)23(30)27-22-18(14-25-28-22)17-11-9-8-10-12-17/h8-12,14-16,19,21H,6-7,13H2,1-5H3,(H,26,31)(H2,25,27,28,30)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% of cysteine protease cathepsin K of human |
J Med Chem 47: 588-99 (2004)
Article DOI: 10.1021/jm030373l
BindingDB Entry DOI: 10.7270/Q29S1QF7 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50138853
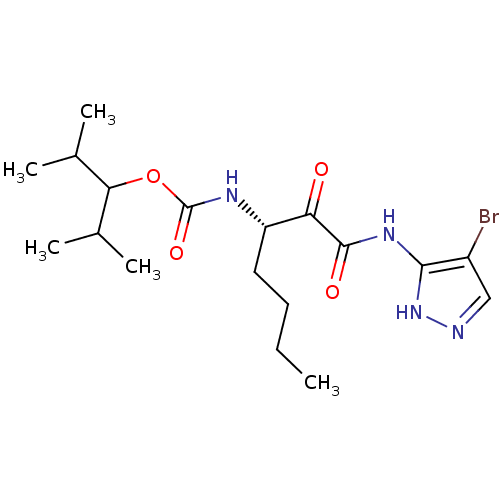
(CHEMBL157072 | [(S)-1-(4-Bromo-1H-pyrazol-3-ylamin...)Show SMILES CCCC[C@H](NC(=O)OC(C(C)C)C(C)C)C(=O)C(=O)Nc1[nH]ncc1Br Show InChI InChI=1S/C18H29BrN4O4/c1-6-7-8-13(21-18(26)27-15(10(2)3)11(4)5)14(24)17(25)22-16-12(19)9-20-23-16/h9-11,13,15H,6-8H2,1-5H3,(H,21,26)(H2,20,22,23,25)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% of cysteine protease cathepsin K of human |
J Med Chem 47: 588-99 (2004)
Article DOI: 10.1021/jm030373l
BindingDB Entry DOI: 10.7270/Q29S1QF7 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50138876
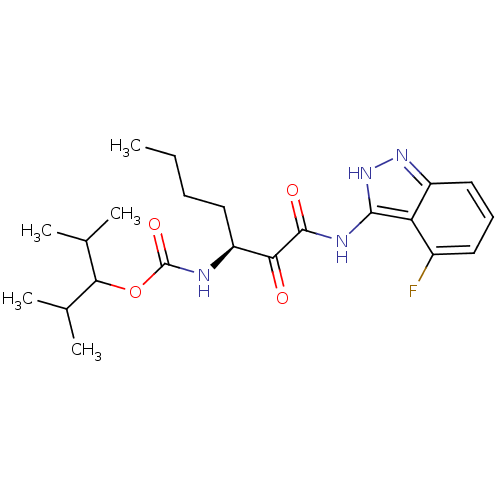
(CHEMBL345569 | [(S)-1-(4-Fluoro-1H-indazol-3-ylami...)Show SMILES CCCC[C@H](NC(=O)OC(C(C)C)C(C)C)C(=O)C(=O)Nc1[nH]nc2cccc(F)c12 Show InChI InChI=1S/C22H31FN4O4/c1-6-7-10-16(24-22(30)31-19(12(2)3)13(4)5)18(28)21(29)25-20-17-14(23)9-8-11-15(17)26-27-20/h8-9,11-13,16,19H,6-7,10H2,1-5H3,(H,24,30)(H2,25,26,27,29)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% of cysteine protease cathepsin K of human |
J Med Chem 47: 588-99 (2004)
Article DOI: 10.1021/jm030373l
BindingDB Entry DOI: 10.7270/Q29S1QF7 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50177501

(CHEMBL203663 | {(S)-1-[(morpholine-4-carbonyl)-hyd...)Show SMILES CCCC[C@H](NC(=O)O[C@H](Cc1ccccc1)C(C)C)C=NNC(=O)N1CCOCC1 |w:21.22| Show InChI InChI=1S/C23H36N4O4/c1-4-5-11-20(17-24-26-22(28)27-12-14-30-15-13-27)25-23(29)31-21(18(2)3)16-19-9-7-6-8-10-19/h6-10,17-18,20-21H,4-5,11-16H2,1-3H3,(H,25,29)(H,26,28)/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human cathepsin K in a fluorescence assay |
Bioorg Med Chem Lett 16: 978-83 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.108
BindingDB Entry DOI: 10.7270/Q2X929VS |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50148292

(((S)-1-Formyl-pentyl)-carbamic acid 1-benzyl-cyclo...)Show InChI InChI=1S/C19H27NO3/c1-2-3-11-17(15-21)20-18(22)23-19(12-7-8-13-19)14-16-9-5-4-6-10-16/h4-6,9-10,15,17H,2-3,7-8,11-14H2,1H3,(H,20,22)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human cathepsin K in a fluorescence assay |
Bioorg Med Chem Lett 16: 978-83 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.108
BindingDB Entry DOI: 10.7270/Q2X929VS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50138866
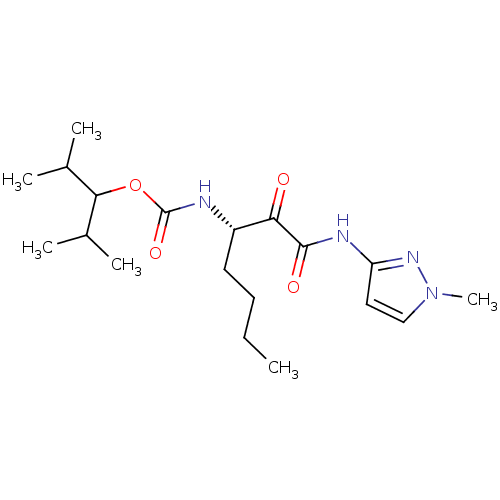
(CHEMBL156764 | [(S)-1-(1-Methyl-1H-pyrazol-3-ylami...)Show SMILES CCCC[C@H](NC(=O)OC(C(C)C)C(C)C)C(=O)C(=O)Nc1ccn(C)n1 Show InChI InChI=1S/C19H32N4O4/c1-7-8-9-14(20-19(26)27-17(12(2)3)13(4)5)16(24)18(25)21-15-10-11-23(6)22-15/h10-14,17H,7-9H2,1-6H3,(H,20,26)(H,21,22,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% of cysteine protease cathepsin K of human |
J Med Chem 47: 588-99 (2004)
Article DOI: 10.1021/jm030373l
BindingDB Entry DOI: 10.7270/Q29S1QF7 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50179446

((S)-4,4-dimethyl-2-oxo-1-((4-(trifluoromethyl)phen...)Show SMILES CCCC[C@H](NC(=O)O[C@@H]1C(=O)N(CC1(C)C)C(=O)Nc1ccc(cc1)C(F)(F)F)C(=O)C(=O)Nc1ccn[nH]1 Show InChI InChI=1S/C25H29F3N6O6/c1-4-5-6-16(18(35)20(36)32-17-11-12-29-33-17)31-23(39)40-19-21(37)34(13-24(19,2)3)22(38)30-15-9-7-14(8-10-15)25(26,27)28/h7-12,16,19H,4-6,13H2,1-3H3,(H,30,38)(H,31,39)(H2,29,32,33,36)/t16-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 16: 1735-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.101
BindingDB Entry DOI: 10.7270/Q23J3CJX |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50138869
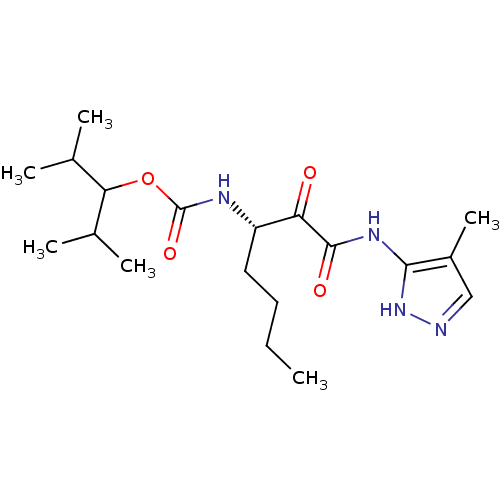
(CHEMBL154959 | [(S)-1-(4-Methyl-1H-pyrazol-3-ylami...)Show SMILES CCCC[C@H](NC(=O)OC(C(C)C)C(C)C)C(=O)C(=O)Nc1[nH]ncc1C Show InChI InChI=1S/C19H32N4O4/c1-7-8-9-14(21-19(26)27-16(11(2)3)12(4)5)15(24)18(25)22-17-13(6)10-20-23-17/h10-12,14,16H,7-9H2,1-6H3,(H,21,26)(H2,20,22,23,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% of cysteine protease cathepsin K of human |
J Med Chem 47: 588-99 (2004)
Article DOI: 10.1021/jm030373l
BindingDB Entry DOI: 10.7270/Q29S1QF7 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50148298
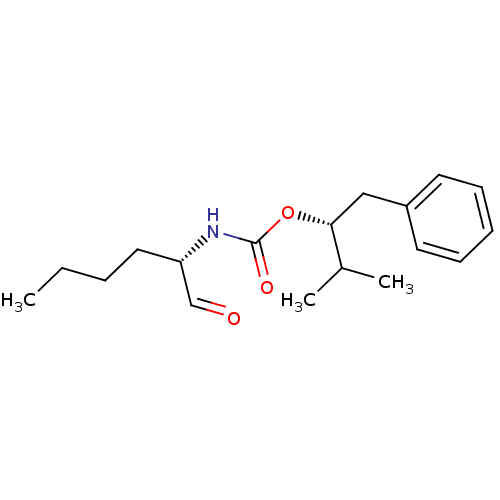
(((S)-1-Formyl-pentyl)-carbamic acid (R)-1-benzyl-2...)Show InChI InChI=1S/C18H27NO3/c1-4-5-11-16(13-20)19-18(21)22-17(14(2)3)12-15-9-7-6-8-10-15/h6-10,13-14,16-17H,4-5,11-12H2,1-3H3,(H,19,21)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human cathepsin K in a fluorescence assay |
Bioorg Med Chem Lett 16: 978-83 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.108
BindingDB Entry DOI: 10.7270/Q2X929VS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50138879
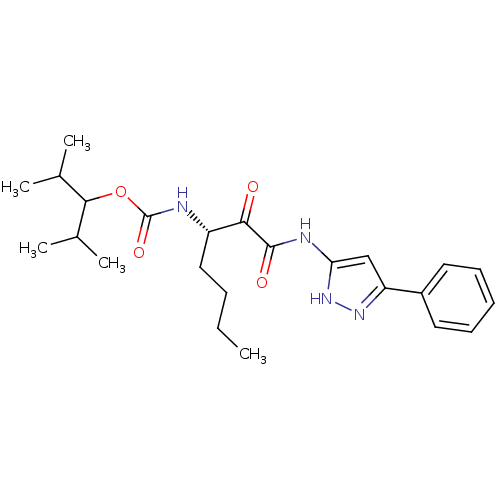
(CHEMBL345982 | [(S)-1-(5-Phenyl-1H-pyrazol-3-ylami...)Show SMILES CCCC[C@H](NC(=O)OC(C(C)C)C(C)C)C(=O)C(=O)Nc1cc(n[nH]1)-c1ccccc1 Show InChI InChI=1S/C24H34N4O4/c1-6-7-13-18(25-24(31)32-22(15(2)3)16(4)5)21(29)23(30)26-20-14-19(27-28-20)17-11-9-8-10-12-17/h8-12,14-16,18,22H,6-7,13H2,1-5H3,(H,25,31)(H2,26,27,28,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% of cysteine protease cathepsin K of human |
J Med Chem 47: 588-99 (2004)
Article DOI: 10.1021/jm030373l
BindingDB Entry DOI: 10.7270/Q29S1QF7 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50139490
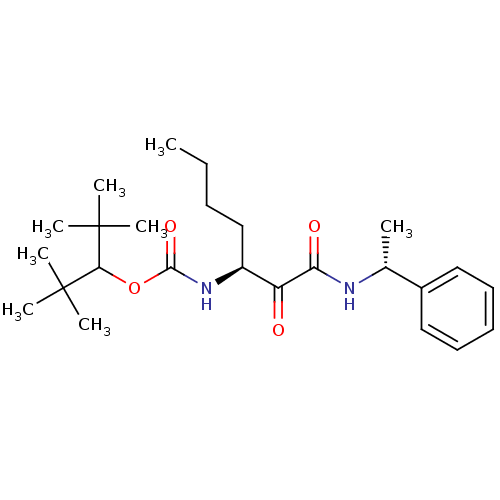
(2,2,4,4-tetramethylpentan-3-yl(S)-1,2-dioxo-1-((R)...)Show SMILES CCCC[C@H](NC(=O)OC(C(C)(C)C)C(C)(C)C)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C25H40N2O4/c1-9-10-16-19(27-23(30)31-22(24(3,4)5)25(6,7)8)20(28)21(29)26-17(2)18-14-12-11-13-15-18/h11-15,17,19,22H,9-10,16H2,1-8H3,(H,26,29)(H,27,30)/t17-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against human cathepsin K |
Bioorg Med Chem Lett 14: 719-22 (2004)
BindingDB Entry DOI: 10.7270/Q2QV3KX1 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50138871
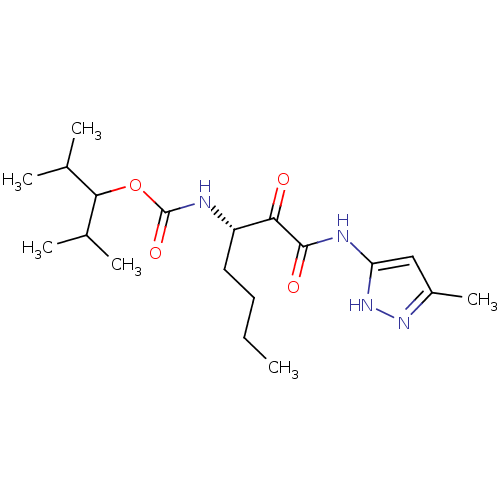
(CHEMBL154818 | [(S)-1-(5-Methyl-1H-pyrazol-3-ylami...)Show SMILES CCCC[C@H](NC(=O)OC(C(C)C)C(C)C)C(=O)C(=O)Nc1cc(C)n[nH]1 Show InChI InChI=1S/C19H32N4O4/c1-7-8-9-14(20-19(26)27-17(11(2)3)12(4)5)16(24)18(25)21-15-10-13(6)22-23-15/h10-12,14,17H,7-9H2,1-6H3,(H,20,26)(H2,21,22,23,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% of cysteine protease cathepsin K of human |
J Med Chem 47: 588-99 (2004)
Article DOI: 10.1021/jm030373l
BindingDB Entry DOI: 10.7270/Q29S1QF7 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50138868
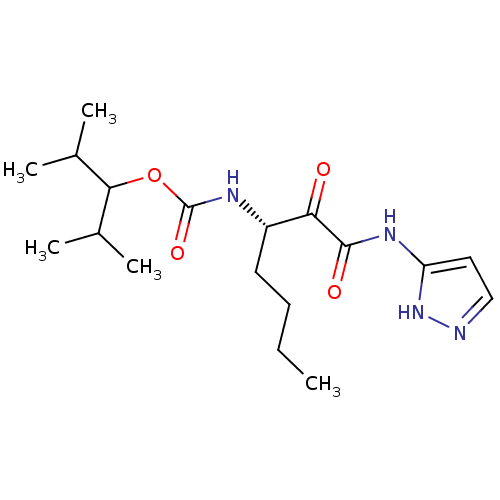
(CHEMBL154862 | [(S)-1-(1H-Pyrazol-3-ylaminooxalyl)...)Show SMILES CCCC[C@H](NC(=O)OC(C(C)C)C(C)C)C(=O)C(=O)Nc1ccn[nH]1 Show InChI InChI=1S/C18H30N4O4/c1-6-7-8-13(15(23)17(24)21-14-9-10-19-22-14)20-18(25)26-16(11(2)3)12(4)5/h9-13,16H,6-8H2,1-5H3,(H,20,25)(H2,19,21,22,24)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% of cysteine protease cathepsin K of human |
J Med Chem 47: 588-99 (2004)
Article DOI: 10.1021/jm030373l
BindingDB Entry DOI: 10.7270/Q29S1QF7 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50139481

((R)-2,2-dimethyl-6-phenylhexan-3-yl(S)-1,2-dioxo-1...)Show SMILES CCCC[C@H](NC(=O)O[C@H](CCCc1ccccc1)C(C)(C)C)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C30H42N2O4/c1-6-7-20-25(27(33)28(34)31-22(2)24-18-12-9-13-19-24)32-29(35)36-26(30(3,4)5)21-14-17-23-15-10-8-11-16-23/h8-13,15-16,18-19,22,25-26H,6-7,14,17,20-21H2,1-5H3,(H,31,34)(H,32,35)/t22-,25+,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against human cathepsin K |
Bioorg Med Chem Lett 14: 719-22 (2004)
BindingDB Entry DOI: 10.7270/Q2QV3KX1 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50179439
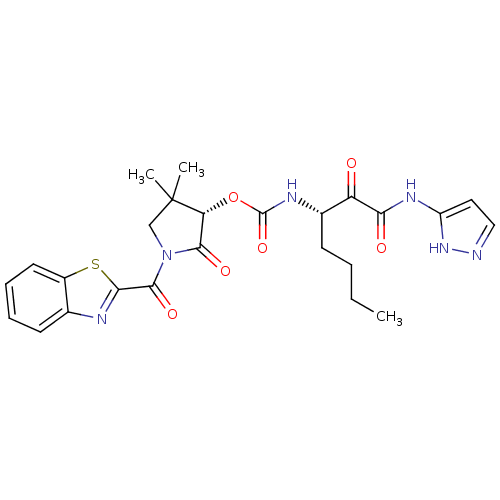
((S)-1-(benzo[d]thiazole-2-carbonyl)-4,4-dimethyl-2...)Show SMILES CCCC[C@H](NC(=O)O[C@@H]1C(=O)N(CC1(C)C)C(=O)c1nc2ccccc2s1)C(=O)C(=O)Nc1ccn[nH]1 Show InChI InChI=1S/C25H28N6O6S/c1-4-5-8-15(18(32)20(33)29-17-11-12-26-30-17)28-24(36)37-19-22(34)31(13-25(19,2)3)23(35)21-27-14-9-6-7-10-16(14)38-21/h6-7,9-12,15,19H,4-5,8,13H2,1-3H3,(H,28,36)(H2,26,29,30,33)/t15-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 16: 1735-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.101
BindingDB Entry DOI: 10.7270/Q23J3CJX |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50152524
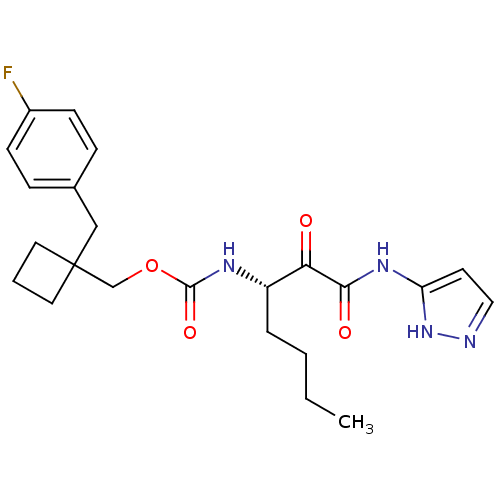
((S)-(1-(4-fluorobenzyl)cyclobutyl)methyl 1-(1H-pyr...)Show SMILES CCCC[C@H](NC(=O)OCC1(Cc2ccc(F)cc2)CCC1)C(=O)C(=O)Nc1ccn[nH]1 Show InChI InChI=1S/C23H29FN4O4/c1-2-3-5-18(20(29)21(30)27-19-10-13-25-28-19)26-22(31)32-15-23(11-4-12-23)14-16-6-8-17(24)9-7-16/h6-10,13,18H,2-5,11-12,14-15H2,1H3,(H,26,31)(H2,25,27,28,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Bioorg Med Chem Lett 14: 4897-902 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.031
BindingDB Entry DOI: 10.7270/Q26W99JR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50138870
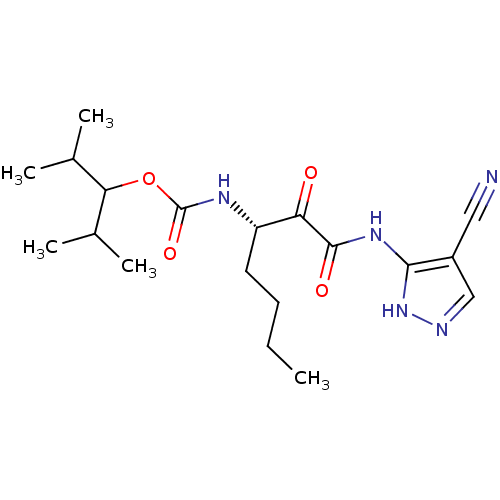
(CHEMBL347004 | [(S)-1-(4-Cyano-1H-pyrazol-3-ylamin...)Show SMILES CCCC[C@H](NC(=O)OC(C(C)C)C(C)C)C(=O)C(=O)Nc1[nH]ncc1C#N Show InChI InChI=1S/C19H29N5O4/c1-6-7-8-14(22-19(27)28-16(11(2)3)12(4)5)15(25)18(26)23-17-13(9-20)10-21-24-17/h10-12,14,16H,6-8H2,1-5H3,(H,22,27)(H2,21,23,24,26)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% of cysteine protease cathepsin K of human |
J Med Chem 47: 588-99 (2004)
Article DOI: 10.1021/jm030373l
BindingDB Entry DOI: 10.7270/Q29S1QF7 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50177494
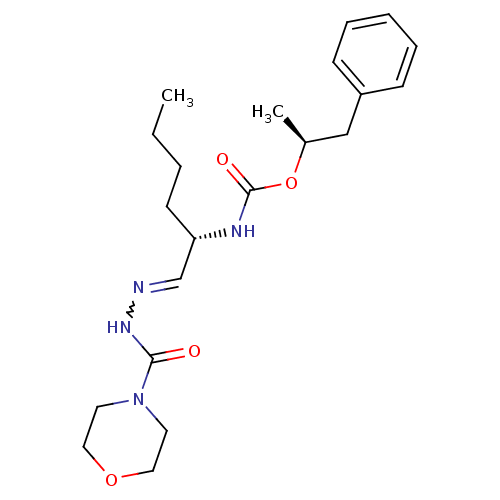
(CHEMBL204605 | {(S)-1-[(morpholine-4-carbonyl)-hyd...)Show SMILES CCCC[C@H](NC(=O)O[C@@H](C)Cc1ccccc1)C=NNC(=O)N1CCOCC1 |w:19.20| Show InChI InChI=1S/C21H32N4O4/c1-3-4-10-19(16-22-24-20(26)25-11-13-28-14-12-25)23-21(27)29-17(2)15-18-8-6-5-7-9-18/h5-9,16-17,19H,3-4,10-15H2,1-2H3,(H,23,27)(H,24,26)/t17-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human cathepsin K in a fluorescence assay |
Bioorg Med Chem Lett 16: 978-83 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.108
BindingDB Entry DOI: 10.7270/Q2X929VS |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50179454
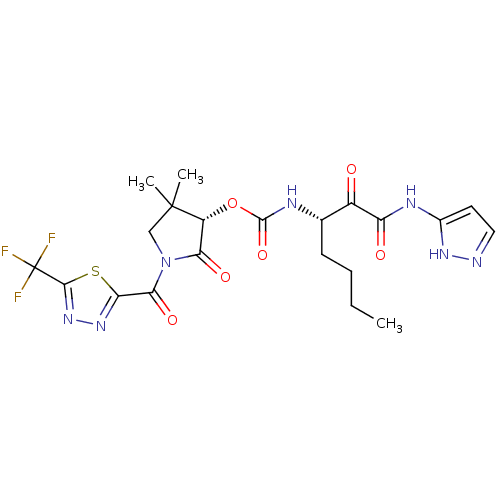
((S)-4,4-dimethyl-2-oxo-1-(2-(trifluoromethyl)-1,3,...)Show SMILES CCCC[C@H](NC(=O)O[C@@H]1C(=O)N(CC1(C)C)C(=O)c1nnc(s1)C(F)(F)F)C(=O)C(=O)Nc1ccn[nH]1 Show InChI InChI=1S/C21H24F3N7O6S/c1-4-5-6-10(12(32)14(33)27-11-7-8-25-28-11)26-19(36)37-13-16(34)31(9-20(13,2)3)17(35)15-29-30-18(38-15)21(22,23)24/h7-8,10,13H,4-6,9H2,1-3H3,(H,26,36)(H2,25,27,28,33)/t10-,13+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 16: 1735-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.101
BindingDB Entry DOI: 10.7270/Q23J3CJX |
More data for this
Ligand-Target Pair | |
Cathepsin K
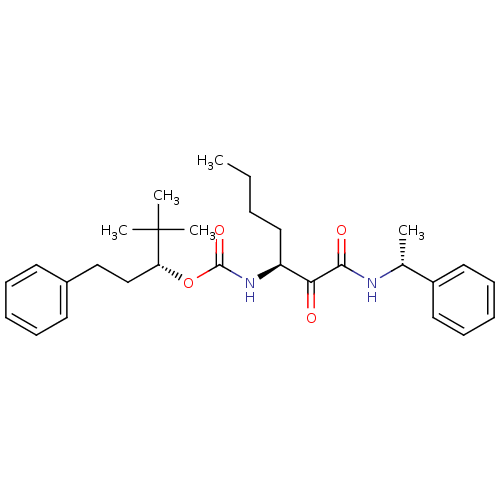
(Homo sapiens (Human)) | BDBM50139496
((R)-4,4-dimethyl-1-phenylpentan-3-yl(S)-1,2-dioxo-...)Show SMILES CCCC[C@H](NC(=O)O[C@H](CCc1ccccc1)C(C)(C)C)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C29H40N2O4/c1-6-7-18-24(26(32)27(33)30-21(2)23-16-12-9-13-17-23)31-28(34)35-25(29(3,4)5)20-19-22-14-10-8-11-15-22/h8-17,21,24-25H,6-7,18-20H2,1-5H3,(H,30,33)(H,31,34)/t21-,24+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against human cathepsin K |
Bioorg Med Chem Lett 14: 719-22 (2004)
BindingDB Entry DOI: 10.7270/Q2QV3KX1 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50139496

((R)-4,4-dimethyl-1-phenylpentan-3-yl(S)-1,2-dioxo-...)Show SMILES CCCC[C@H](NC(=O)O[C@H](CCc1ccccc1)C(C)(C)C)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C29H40N2O4/c1-6-7-18-24(26(32)27(33)30-21(2)23-16-12-9-13-17-23)31-28(34)35-25(29(3,4)5)20-19-22-14-10-8-11-15-22/h8-17,21,24-25H,6-7,18-20H2,1-5H3,(H,30,33)(H,31,34)/t21-,24+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Bioorg Med Chem Lett 14: 4897-902 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.031
BindingDB Entry DOI: 10.7270/Q26W99JR |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50177503
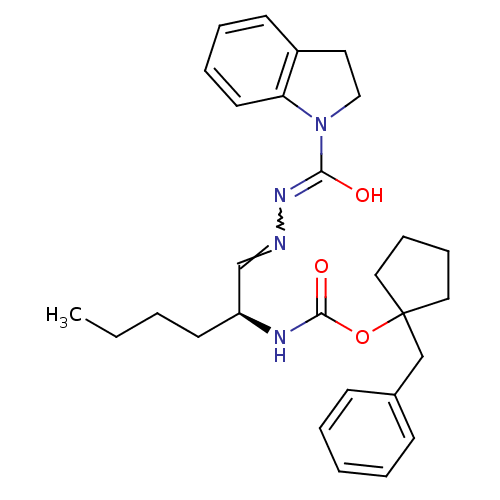
(CHEMBL426308 | {(S)-1-[(2,3-dihydro-indole-1-carbo...)Show SMILES CCCC[C@H](NC(=O)OC1(Cc2ccccc2)CCCC1)C=NN=C(O)N1CCc2ccccc12 |w:23.24| Show InChI InChI=1S/C28H36N4O3/c1-2-3-14-24(21-29-31-26(33)32-19-16-23-13-7-8-15-25(23)32)30-27(34)35-28(17-9-10-18-28)20-22-11-5-4-6-12-22/h4-8,11-13,15,21,24H,2-3,9-10,14,16-20H2,1H3,(H,30,34)(H,31,33)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human cathepsin K in a fluorescence assay |
Bioorg Med Chem Lett 16: 978-83 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.108
BindingDB Entry DOI: 10.7270/Q2X929VS |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19793

((2S)-1-(5,6-dichloro-1H-1,3-benzodiazol-1-yl)-3,3-...)Show SMILES C[C@H](NC(=O)O[C@H](Cn1cnc2cc(Cl)c(Cl)cc12)C(C)(C)C)C(=O)CNS(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C23H27Cl2N5O5S/c1-14(19(31)11-28-36(33,34)21-7-5-6-8-26-21)29-22(32)35-20(23(2,3)4)12-30-13-27-17-9-15(24)16(25)10-18(17)30/h5-10,13-14,20,28H,11-12H2,1-4H3,(H,29,32)/t14-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut... |
Bioorg Med Chem Lett 17: 22-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.102
BindingDB Entry DOI: 10.7270/Q2NZ85XC |
More data for this
Ligand-Target Pair | |
Cathepsin K
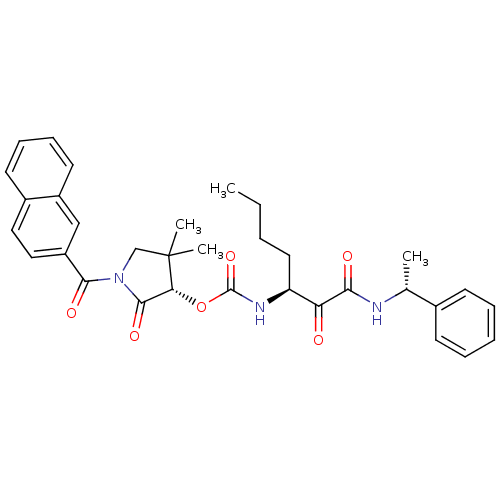
(Homo sapiens (Human)) | BDBM50179458
((S)-1-(2-naphthoyl)-4,4-dimethyl-2-oxopyrrolidin-3...)Show SMILES CCCC[C@H](NC(=O)O[C@@H]1C(=O)N(CC1(C)C)C(=O)c1ccc2ccccc2c1)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C33H37N3O6/c1-5-6-16-26(27(37)29(38)34-21(2)22-12-8-7-9-13-22)35-32(41)42-28-31(40)36(20-33(28,3)4)30(39)25-18-17-23-14-10-11-15-24(23)19-25/h7-15,17-19,21,26,28H,5-6,16,20H2,1-4H3,(H,34,38)(H,35,41)/t21-,26+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 16: 1735-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.101
BindingDB Entry DOI: 10.7270/Q23J3CJX |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50138846

(3-[(S)-3-(1-Isopropyl-2-methyl-propoxycarbonylamin...)Show SMILES CCCC[C@H](NC(=O)OC(C(C)C)C(C)C)C(=O)C(=O)Nc1[nH]ncc1C(=O)OCC Show InChI InChI=1S/C21H34N4O6/c1-7-9-10-15(23-21(29)31-17(12(3)4)13(5)6)16(26)19(27)24-18-14(11-22-25-18)20(28)30-8-2/h11-13,15,17H,7-10H2,1-6H3,(H,23,29)(H2,22,24,25,27)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% of cysteine protease cathepsin K of human |
J Med Chem 47: 588-99 (2004)
Article DOI: 10.1021/jm030373l
BindingDB Entry DOI: 10.7270/Q29S1QF7 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50179438
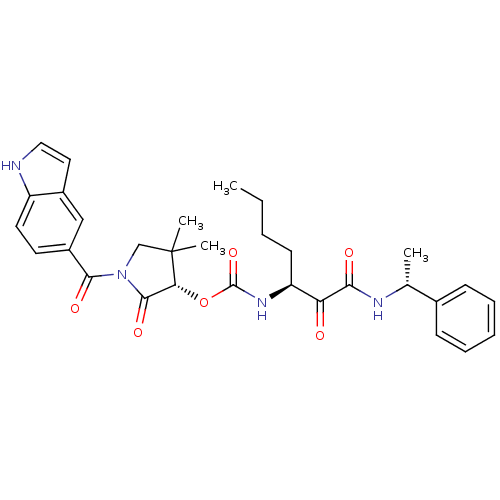
((S)-1-(1H-indole-5-carbonyl)-4,4-dimethyl-2-oxopyr...)Show SMILES CCCC[C@H](NC(=O)O[C@@H]1C(=O)N(CC1(C)C)C(=O)c1ccc2[nH]ccc2c1)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C31H36N4O6/c1-5-6-12-24(25(36)27(37)33-19(2)20-10-8-7-9-11-20)34-30(40)41-26-29(39)35(18-31(26,3)4)28(38)22-13-14-23-21(17-22)15-16-32-23/h7-11,13-17,19,24,26,32H,5-6,12,18H2,1-4H3,(H,33,37)(H,34,40)/t19-,24+,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 16: 1735-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.101
BindingDB Entry DOI: 10.7270/Q23J3CJX |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Rattus norvegicus) | BDBM50138858

(CHEMBL154579 | [(S)-1-(4-Phenyl-1H-pyrazol-3-ylami...)Show SMILES CCCC[C@H](NC(=O)OC(C(C)C)C(C)C)C(=O)C(=O)Nc1[nH]ncc1-c1ccccc1 Show InChI InChI=1S/C24H34N4O4/c1-6-7-13-19(26-24(31)32-21(15(2)3)16(4)5)20(29)23(30)27-22-18(14-25-28-22)17-11-9-8-10-12-17/h8-12,14-16,19,21H,6-7,13H2,1-5H3,(H,26,31)(H2,25,27,28,30)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of cystiene protease cathepsin K of rat |
J Med Chem 47: 588-99 (2004)
Article DOI: 10.1021/jm030373l
BindingDB Entry DOI: 10.7270/Q29S1QF7 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50148296

(((S)-1-Formyl-pentyl)-carbamic acid (S)-1-methyl-2...)Show InChI InChI=1S/C16H23NO3/c1-3-4-10-15(12-18)17-16(19)20-13(2)11-14-8-6-5-7-9-14/h5-9,12-13,15H,3-4,10-11H2,1-2H3,(H,17,19)/t13-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human cathepsin K in a fluorescence assay |
Bioorg Med Chem Lett 16: 978-83 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.108
BindingDB Entry DOI: 10.7270/Q2X929VS |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM19796
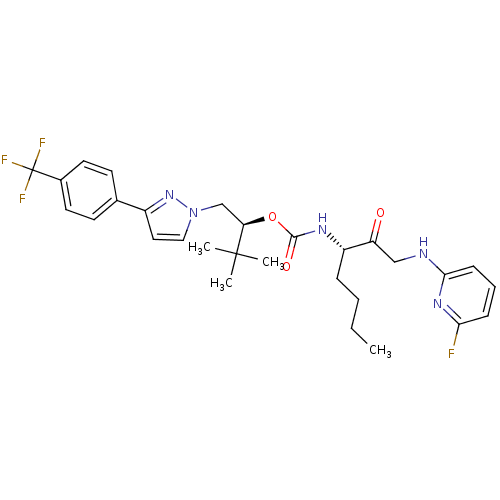
((2S)-3,3-dimethyl-1-{3-[4-(trifluoromethyl)phenyl]...)Show SMILES CCCC[C@H](NC(=O)O[C@H](Cn1ccc(n1)-c1ccc(cc1)C(F)(F)F)C(C)(C)C)C(=O)CNc1cccc(F)n1 |r| Show InChI InChI=1S/C29H35F4N5O3/c1-5-6-8-22(23(39)17-34-26-10-7-9-25(30)36-26)35-27(40)41-24(28(2,3)4)18-38-16-15-21(37-38)19-11-13-20(14-12-19)29(31,32)33/h7,9-16,22,24H,5-6,8,17-18H2,1-4H3,(H,34,36)(H,35,40)/t22-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut... |
Bioorg Med Chem Lett 17: 22-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.102
BindingDB Entry DOI: 10.7270/Q2NZ85XC |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50179445
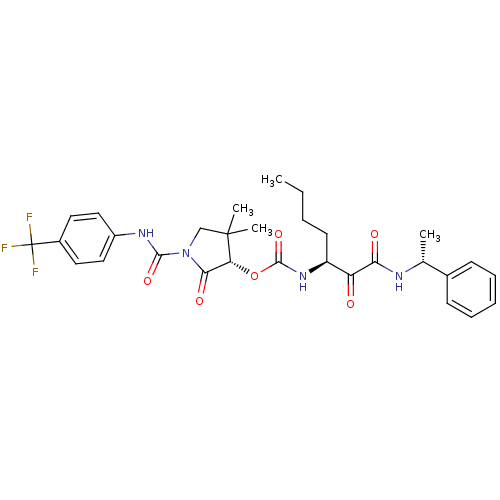
((S)-4,4-dimethyl-2-oxo-1-((4-(trifluoromethyl)phen...)Show SMILES CCCC[C@H](NC(=O)O[C@@H]1C(=O)N(CC1(C)C)C(=O)Nc1ccc(cc1)C(F)(F)F)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C30H35F3N4O6/c1-5-6-12-22(23(38)25(39)34-18(2)19-10-8-7-9-11-19)36-28(42)43-24-26(40)37(17-29(24,3)4)27(41)35-21-15-13-20(14-16-21)30(31,32)33/h7-11,13-16,18,22,24H,5-6,12,17H2,1-4H3,(H,34,39)(H,35,41)(H,36,42)/t18-,22+,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 16: 1735-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.101
BindingDB Entry DOI: 10.7270/Q23J3CJX |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50179437

((S)-4,4-dimethyl-2-oxo-3-[(S)-1-((R)-1-phenyl-ethy...)Show SMILES CCCC[C@H](NC(=O)O[C@@H]1C(=O)N(CC1(C)C)C(=O)OCc1ccc(cc1)-c1ccccc1)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C36H41N3O7/c1-5-6-17-29(30(40)32(41)37-24(2)26-13-9-7-10-14-26)38-34(43)46-31-33(42)39(23-36(31,3)4)35(44)45-22-25-18-20-28(21-19-25)27-15-11-8-12-16-27/h7-16,18-21,24,29,31H,5-6,17,22-23H2,1-4H3,(H,37,41)(H,38,43)/t24-,29+,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 16: 1735-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.101
BindingDB Entry DOI: 10.7270/Q23J3CJX |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50165427
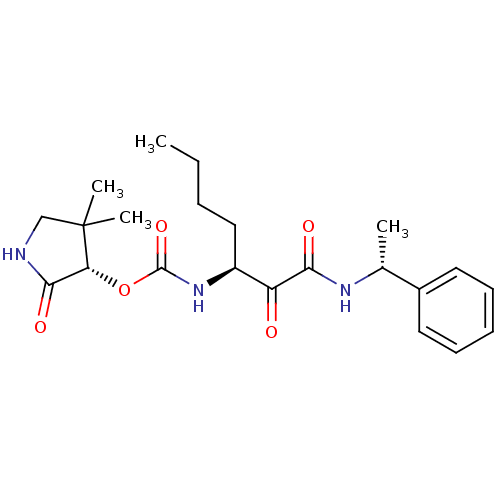
(CHEMBL194068 | [(S)-1-((R)-1-Phenyl-ethylaminooxal...)Show SMILES CCCC[C@H](NC(=O)O[C@@H]1C(=O)NCC1(C)C)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C22H31N3O5/c1-5-6-12-16(25-21(29)30-18-20(28)23-13-22(18,3)4)17(26)19(27)24-14(2)15-10-8-7-9-11-15/h7-11,14,16,18H,5-6,12-13H2,1-4H3,(H,23,28)(H,24,27)(H,25,29)/t14-,16+,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin K |
Bioorg Med Chem Lett 15: 2209-13 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.023
BindingDB Entry DOI: 10.7270/Q20C4V8V |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50138842

(2,4-dimethylpentan-3-yl(S)-1,2-dioxo-1-((R)-1-phen...)Show SMILES CCCC[C@H](NC(=O)OC(C(C)C)C(C)C)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C23H36N2O4/c1-7-8-14-19(25-23(28)29-21(15(2)3)16(4)5)20(26)22(27)24-17(6)18-12-10-9-11-13-18/h9-13,15-17,19,21H,7-8,14H2,1-6H3,(H,24,27)(H,25,28)/t17-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against human cathepsin K |
Bioorg Med Chem Lett 14: 719-22 (2004)
BindingDB Entry DOI: 10.7270/Q2QV3KX1 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50138842

(2,4-dimethylpentan-3-yl(S)-1,2-dioxo-1-((R)-1-phen...)Show SMILES CCCC[C@H](NC(=O)OC(C(C)C)C(C)C)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C23H36N2O4/c1-7-8-14-19(25-23(28)29-21(15(2)3)16(4)5)20(26)22(27)24-17(6)18-12-10-9-11-13-18/h9-13,15-17,19,21H,7-8,14H2,1-6H3,(H,24,27)(H,25,28)/t17-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.54 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% of cysteine protease cathepsin K of human |
J Med Chem 47: 588-99 (2004)
Article DOI: 10.1021/jm030373l
BindingDB Entry DOI: 10.7270/Q29S1QF7 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50179440
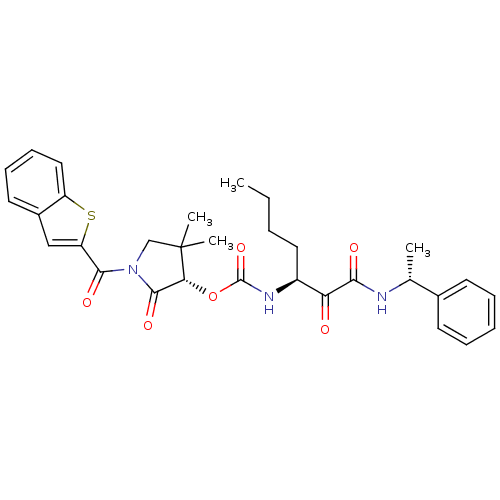
((S)-1-(benzo[b]thiophene-2-carbonyl)-4,4-dimethyl-...)Show SMILES CCCC[C@H](NC(=O)O[C@@H]1C(=O)N(CC1(C)C)C(=O)c1cc2ccccc2s1)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C31H35N3O6S/c1-5-6-15-22(25(35)27(36)32-19(2)20-12-8-7-9-13-20)33-30(39)40-26-29(38)34(18-31(26,3)4)28(37)24-17-21-14-10-11-16-23(21)41-24/h7-14,16-17,19,22,26H,5-6,15,18H2,1-4H3,(H,32,36)(H,33,39)/t19-,22+,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 16: 1735-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.101
BindingDB Entry DOI: 10.7270/Q23J3CJX |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50165424
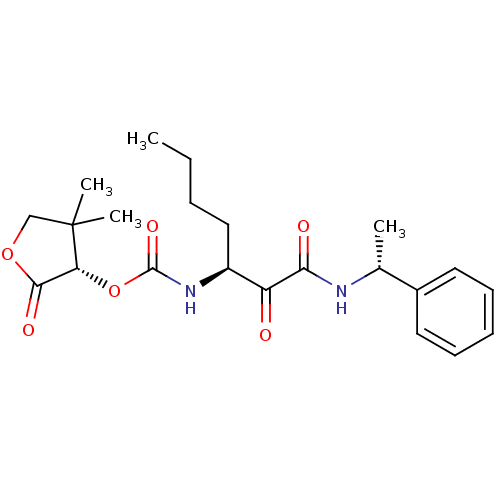
((S)-4,4-dimethyl-2-oxo-tetrahydrofuran-3-yl (S)-1,...)Show SMILES CCCC[C@H](NC(=O)O[C@@H]1C(=O)OCC1(C)C)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C22H30N2O6/c1-5-6-12-16(24-21(28)30-18-20(27)29-13-22(18,3)4)17(25)19(26)23-14(2)15-10-8-7-9-11-15/h7-11,14,16,18H,5-6,12-13H2,1-4H3,(H,23,26)(H,24,28)/t14-,16+,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin K |
Bioorg Med Chem Lett 15: 2209-13 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.023
BindingDB Entry DOI: 10.7270/Q20C4V8V |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50165424

((S)-4,4-dimethyl-2-oxo-tetrahydrofuran-3-yl (S)-1,...)Show SMILES CCCC[C@H](NC(=O)O[C@@H]1C(=O)OCC1(C)C)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C22H30N2O6/c1-5-6-12-16(24-21(28)30-18-20(27)29-13-22(18,3)4)17(25)19(26)23-14(2)15-10-8-7-9-11-15/h7-11,14,16,18H,5-6,12-13H2,1-4H3,(H,23,26)(H,24,28)/t14-,16+,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 16: 1735-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.101
BindingDB Entry DOI: 10.7270/Q23J3CJX |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50179442

((S)-4,4-dimethyl-2-oxo-1-(2-phenylacetyl)pyrrolidi...)Show SMILES CCCC[C@H](NC(=O)O[C@@H]1C(=O)N(CC1(C)C)C(=O)Cc1ccccc1)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C30H37N3O6/c1-5-6-17-23(25(35)27(36)31-20(2)22-15-11-8-12-16-22)32-29(38)39-26-28(37)33(19-30(26,3)4)24(34)18-21-13-9-7-10-14-21/h7-16,20,23,26H,5-6,17-19H2,1-4H3,(H,31,36)(H,32,38)/t20-,23+,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 16: 1735-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.101
BindingDB Entry DOI: 10.7270/Q23J3CJX |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19794
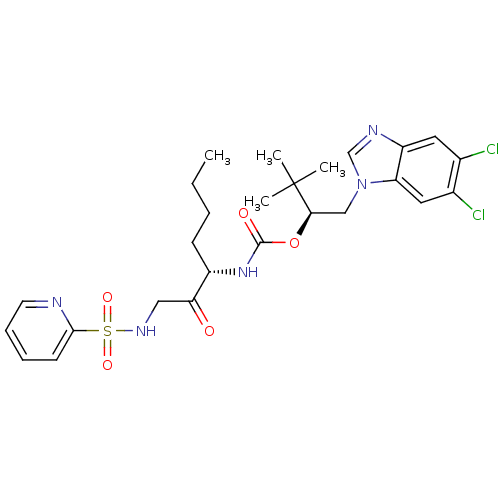
((2S)-1-(5,6-dichloro-1H-1,3-benzodiazol-1-yl)-3,3-...)Show SMILES CCCC[C@H](NC(=O)O[C@H](Cn1cnc2cc(Cl)c(Cl)cc12)C(C)(C)C)C(=O)CNS(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H33Cl2N5O5S/c1-5-6-9-19(22(34)14-31-39(36,37)24-10-7-8-11-29-24)32-25(35)38-23(26(2,3)4)15-33-16-30-20-12-17(27)18(28)13-21(20)33/h7-8,10-13,16,19,23,31H,5-6,9,14-15H2,1-4H3,(H,32,35)/t19-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut... |
Bioorg Med Chem Lett 17: 22-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.102
BindingDB Entry DOI: 10.7270/Q2NZ85XC |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19791
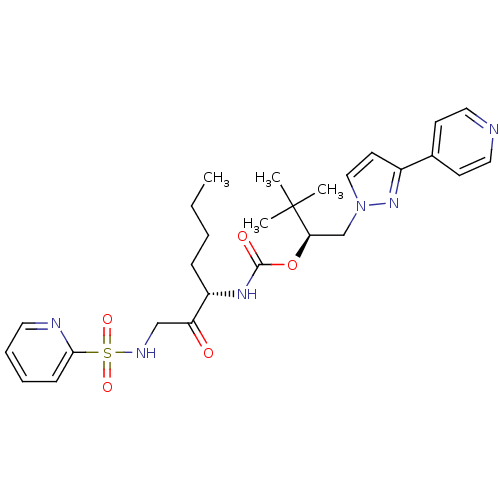
((2S)-3,3-dimethyl-1-[3-(pyridin-4-yl)-1H-pyrazol-1...)Show SMILES CCCC[C@H](NC(=O)O[C@H](Cn1ccc(n1)-c1ccncc1)C(C)(C)C)C(=O)CNS(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H36N6O5S/c1-5-6-9-22(23(34)18-30-39(36,37)25-10-7-8-14-29-25)31-26(35)38-24(27(2,3)4)19-33-17-13-21(32-33)20-11-15-28-16-12-20/h7-8,10-17,22,24,30H,5-6,9,18-19H2,1-4H3,(H,31,35)/t22-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut... |
Bioorg Med Chem Lett 17: 22-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.102
BindingDB Entry DOI: 10.7270/Q2NZ85XC |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50179453

((S)-1-(benzylcarbamoyl)-4,4-dimethyl-2-oxopyrrolid...)Show SMILES CCCC[C@H](NC(=O)O[C@@H]1C(=O)N(CC1(C)C)C(=O)NCc1ccccc1)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C30H38N4O6/c1-5-6-17-23(24(35)26(36)32-20(2)22-15-11-8-12-16-22)33-29(39)40-25-27(37)34(19-30(25,3)4)28(38)31-18-21-13-9-7-10-14-21/h7-16,20,23,25H,5-6,17-19H2,1-4H3,(H,31,38)(H,32,36)(H,33,39)/t20-,23+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 16: 1735-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.101
BindingDB Entry DOI: 10.7270/Q23J3CJX |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19786

((2S)-3,3-dimethyl-1-{3-[4-(trifluoromethyl)phenyl]...)Show SMILES CCCC[C@H](NC(=O)O[C@H](Cn1ccc(n1)-c1ccc(cc1)C(F)(F)F)C(C)(C)C)C(=O)CNS(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C29H36F3N5O5S/c1-5-6-9-23(24(38)18-34-43(40,41)26-10-7-8-16-33-26)35-27(39)42-25(28(2,3)4)19-37-17-15-22(36-37)20-11-13-21(14-12-20)29(30,31)32/h7-8,10-17,23,25,34H,5-6,9,18-19H2,1-4H3,(H,35,39)/t23-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut... |
Bioorg Med Chem Lett 17: 22-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.102
BindingDB Entry DOI: 10.7270/Q2NZ85XC |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50179441

((S)-4,4-dimethyl-2-oxo-1-(phenylcarbamoyl)pyrrolid...)Show SMILES CCCC[C@H](NC(=O)O[C@@H]1C(=O)N(CC1(C)C)C(=O)Nc1ccccc1)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C29H36N4O6/c1-5-6-17-22(23(34)25(35)30-19(2)20-13-9-7-10-14-20)32-28(38)39-24-26(36)33(18-29(24,3)4)27(37)31-21-15-11-8-12-16-21/h7-16,19,22,24H,5-6,17-18H2,1-4H3,(H,30,35)(H,31,37)(H,32,38)/t19-,22+,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 16: 1735-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.101
BindingDB Entry DOI: 10.7270/Q23J3CJX |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50138864
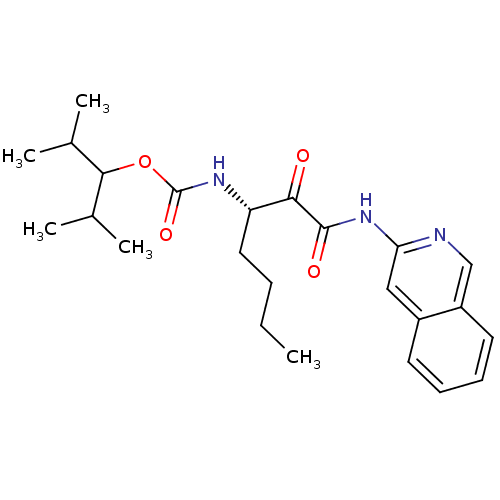
(CHEMBL351111 | [(S)-1-(Isoquinolin-3-ylaminooxalyl...)Show SMILES CCCC[C@H](NC(=O)OC(C(C)C)C(C)C)C(=O)C(=O)Nc1cc2ccccc2cn1 Show InChI InChI=1S/C24H33N3O4/c1-6-7-12-19(26-24(30)31-22(15(2)3)16(4)5)21(28)23(29)27-20-13-17-10-8-9-11-18(17)14-25-20/h8-11,13-16,19,22H,6-7,12H2,1-5H3,(H,26,30)(H,25,27,29)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% of cysteine protease cathepsin K of human |
J Med Chem 47: 588-99 (2004)
Article DOI: 10.1021/jm030373l
BindingDB Entry DOI: 10.7270/Q29S1QF7 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50179435
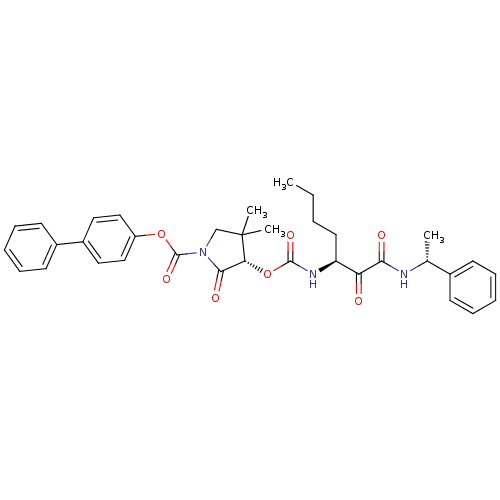
((S)-4,4-dimethyl-2-oxo-3-[(S)-1-((R)-1-phenyl-ethy...)Show SMILES CCCC[C@H](NC(=O)O[C@@H]1C(=O)N(CC1(C)C)C(=O)Oc1ccc(cc1)-c1ccccc1)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C35H39N3O7/c1-5-6-17-28(29(39)31(40)36-23(2)24-13-9-7-10-14-24)37-33(42)45-30-32(41)38(22-35(30,3)4)34(43)44-27-20-18-26(19-21-27)25-15-11-8-12-16-25/h7-16,18-21,23,28,30H,5-6,17,22H2,1-4H3,(H,36,40)(H,37,42)/t23-,28+,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 16: 1735-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.101
BindingDB Entry DOI: 10.7270/Q23J3CJX |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50138852
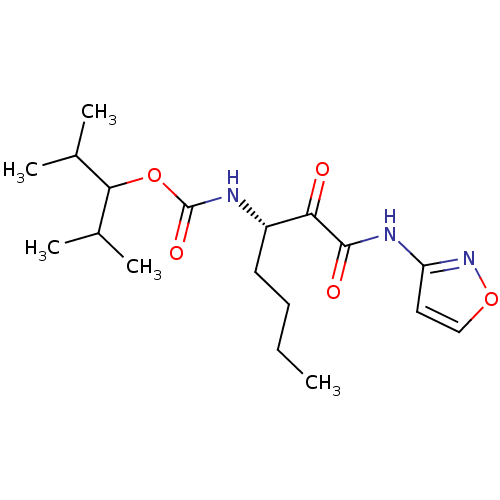
(CHEMBL356378 | [(S)-1-(Isoxazol-3-ylaminooxalyl)-p...)Show SMILES CCCC[C@H](NC(=O)OC(C(C)C)C(C)C)C(=O)C(=O)Nc1ccon1 Show InChI InChI=1S/C18H29N3O5/c1-6-7-8-13(15(22)17(23)20-14-9-10-25-21-14)19-18(24)26-16(11(2)3)12(4)5/h9-13,16H,6-8H2,1-5H3,(H,19,24)(H,20,21,23)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% of cysteine protease cathepsin K of human |
J Med Chem 47: 588-99 (2004)
Article DOI: 10.1021/jm030373l
BindingDB Entry DOI: 10.7270/Q29S1QF7 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50152524

((S)-(1-(4-fluorobenzyl)cyclobutyl)methyl 1-(1H-pyr...)Show SMILES CCCC[C@H](NC(=O)OCC1(Cc2ccc(F)cc2)CCC1)C(=O)C(=O)Nc1ccn[nH]1 Show InChI InChI=1S/C23H29FN4O4/c1-2-3-5-18(20(29)21(30)27-19-10-13-25-28-19)26-22(31)32-15-23(11-4-12-23)14-16-6-8-17(24)9-7-16/h6-10,13,18H,2-5,11-12,14-15H2,1H3,(H,26,31)(H2,25,27,28,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of 10 uM Cbz-Val-Val-Arg-AMC binding to human cathepsin S in fluorescence assay |
Bioorg Med Chem Lett 14: 4897-902 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.031
BindingDB Entry DOI: 10.7270/Q26W99JR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data