 Found 317 hits with Last Name = 'lozama' and Initial = 'a'
Found 317 hits with Last Name = 'lozama' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50170672
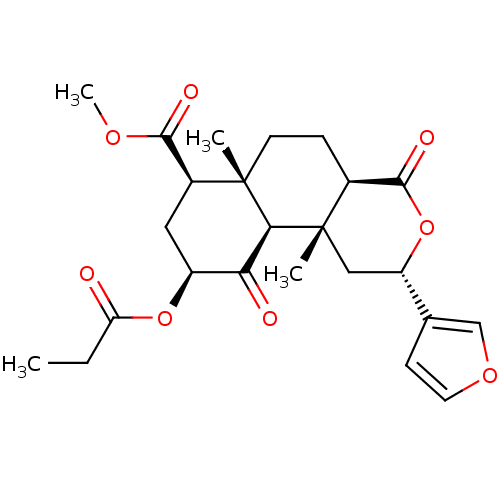
((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 2-(furan-3-yl)...)Show SMILES CCC(=O)O[C@H]1C[C@@H](C(=O)OC)[C@]2(C)CC[C@H]3C(=O)O[C@@H](C[C@]3(C)[C@H]2C1=O)c1ccoc1 |r| Show InChI InChI=1S/C24H30O8/c1-5-18(25)31-16-10-15(21(27)29-4)23(2)8-6-14-22(28)32-17(13-7-9-30-12-13)11-24(14,3)20(23)19(16)26/h7,9,12,14-17,20H,5-6,8,10-11H2,1-4H3/t14-,15-,16-,17-,20-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125]OXY from kappa opioid receptor |
J Nat Prod 69: 914-8 (2006)
Article DOI: 10.1021/np060094b
BindingDB Entry DOI: 10.7270/Q28W3D22 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50159165
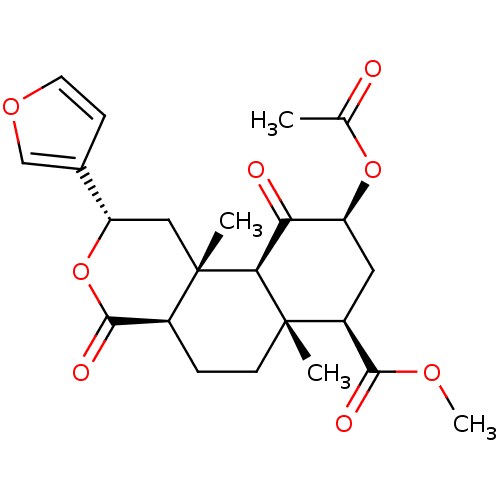
((2S,4aR,6aR,7R,9S,10aS,10bR)-9-(acetyloxy)-2-(fura...)Show SMILES COC(=O)[C@@H]1C[C@H](OC(C)=O)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 |r| Show InChI InChI=1S/C23H28O8/c1-12(24)30-16-9-15(20(26)28-4)22(2)7-5-14-21(27)31-17(13-6-8-29-11-13)10-23(14,3)19(22)18(16)25/h6,8,11,14-17,19H,5,7,9-10H2,1-4H3/t14-,15-,16-,17-,19-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant kappa opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay |
J Med Chem 51: 2421-31 (2008)
Article DOI: 10.1021/jm701162g
BindingDB Entry DOI: 10.7270/Q2DV1KR7 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50159165

((2S,4aR,6aR,7R,9S,10aS,10bR)-9-(acetyloxy)-2-(fura...)Show SMILES COC(=O)[C@@H]1C[C@H](OC(C)=O)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 |r| Show InChI InChI=1S/C23H28O8/c1-12(24)30-16-9-15(20(26)28-4)22(2)7-5-14-21(27)31-17(13-6-8-29-11-13)10-23(14,3)19(22)18(16)25/h6,8,11,14-17,19H,5,7,9-10H2,1-4H3/t14-,15-,16-,17-,19-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125]OXY from kappa opioid receptor |
J Nat Prod 69: 914-8 (2006)
Article DOI: 10.1021/np060094b
BindingDB Entry DOI: 10.7270/Q28W3D22 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50159165

((2S,4aR,6aR,7R,9S,10aS,10bR)-9-(acetyloxy)-2-(fura...)Show SMILES COC(=O)[C@@H]1C[C@H](OC(C)=O)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 |r| Show InChI InChI=1S/C23H28O8/c1-12(24)30-16-9-15(20(26)28-4)22(2)7-5-14-21(27)31-17(13-6-8-29-11-13)10-23(14,3)19(22)18(16)25/h6,8,11,14-17,19H,5,7,9-10H2,1-4H3/t14-,15-,16-,17-,19-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125I]IOXY from human recombinant kappa opioid receptor expressed in CHO cells |
J Med Chem 51: 2421-31 (2008)
Article DOI: 10.1021/jm701162g
BindingDB Entry DOI: 10.7270/Q2DV1KR7 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50170676
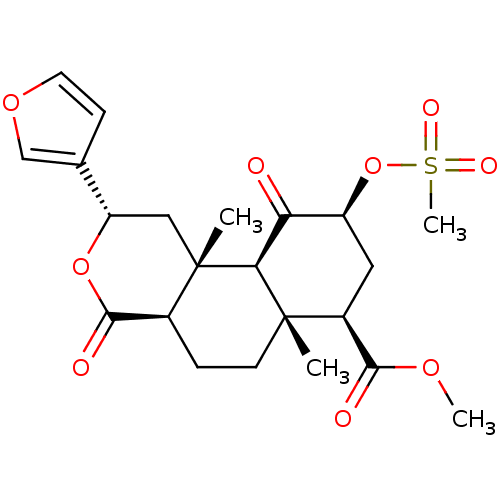
((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 2-(furan-3-yl)...)Show SMILES COC(=O)[C@@H]1C[C@H](OS(C)(=O)=O)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 |r| Show InChI InChI=1S/C22H28O9S/c1-21-7-5-13-20(25)30-16(12-6-8-29-11-12)10-22(13,2)18(21)17(23)15(31-32(4,26)27)9-14(21)19(24)28-3/h6,8,11,13-16,18H,5,7,9-10H2,1-4H3/t13-,14-,15-,16-,18-,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125]OXY from kappa opioid receptor |
J Nat Prod 69: 914-8 (2006)
Article DOI: 10.1021/np060094b
BindingDB Entry DOI: 10.7270/Q28W3D22 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50216132
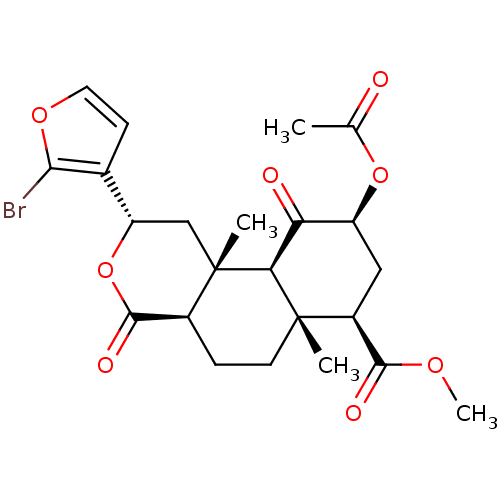
((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 9-acetoxy-2-(2...)Show SMILES COC(=O)[C@@H]1C[C@H](OC(C)=O)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1Br |r| Show InChI InChI=1S/C23H27BrO8/c1-11(25)31-15-9-14(20(27)29-4)22(2)7-5-13-21(28)32-16(12-6-8-30-19(12)24)10-23(13,3)18(22)17(15)26/h6,8,13-16,18H,5,7,9-10H2,1-4H3/t13-,14-,15-,16-,18-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125I]IOXY from human kappa opioid receptor expressed in CHO cells |
J Med Chem 50: 3596-603 (2007)
Article DOI: 10.1021/jm070393d
BindingDB Entry DOI: 10.7270/Q2ZK5HH0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50376845
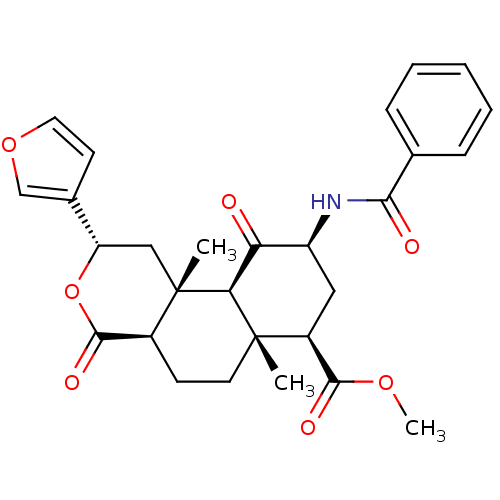
(CHEMBL259658)Show SMILES COC(=O)[C@@H]1C[C@H](NC(=O)c2ccccc2)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 Show InChI InChI=1S/C28H31NO7/c1-27-11-9-18-26(33)36-21(17-10-12-35-15-17)14-28(18,2)23(27)22(30)20(13-19(27)25(32)34-3)29-24(31)16-7-5-4-6-8-16/h4-8,10,12,15,18-21,23H,9,11,13-14H2,1-3H3,(H,29,31)/t18-,19-,20-,21-,23-,27-,28-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125I]IOXY from human recombinant mu opioid receptor expressed in CHO cells |
J Med Chem 51: 2421-31 (2008)
Article DOI: 10.1021/jm701162g
BindingDB Entry DOI: 10.7270/Q2DV1KR7 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50371092
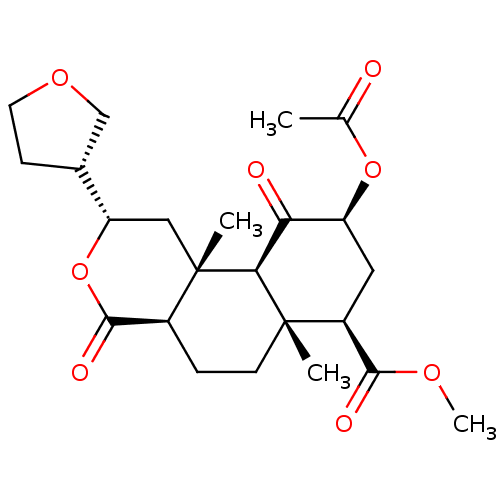
(CHEMBL427280)Show SMILES COC(=O)[C@@H]1C[C@H](OC(C)=O)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)[C@@H]1CCOC1 Show InChI InChI=1S/C23H32O8/c1-12(24)30-16-9-15(20(26)28-4)22(2)7-5-14-21(27)31-17(13-6-8-29-11-13)10-23(14,3)19(22)18(16)25/h13-17,19H,5-11H2,1-4H3/t13-,14+,15+,16+,17+,19+,22+,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125I]IOXY from human kappa opioid receptor expressed in CHO cells |
J Med Chem 50: 3596-603 (2007)
Article DOI: 10.1021/jm070393d
BindingDB Entry DOI: 10.7270/Q2ZK5HH0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50189138
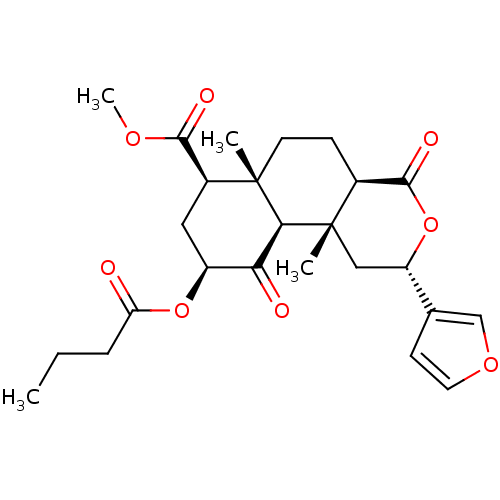
((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 9-(butyryloxy)...)Show SMILES CCCC(=O)O[C@H]1C[C@@H](C(=O)OC)[C@]2(C)CC[C@H]3C(=O)O[C@@H](C[C@]3(C)[C@H]2C1=O)c1ccoc1 |r| Show InChI InChI=1S/C25H32O8/c1-5-6-19(26)32-17-11-16(22(28)30-4)24(2)9-7-15-23(29)33-18(14-8-10-31-13-14)12-25(15,3)21(24)20(17)27/h8,10,13,15-18,21H,5-7,9,11-12H2,1-4H3/t15-,16-,17-,18-,21-,24-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125]OXY from kappa opioid receptor |
J Nat Prod 69: 914-8 (2006)
Article DOI: 10.1021/np060094b
BindingDB Entry DOI: 10.7270/Q28W3D22 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50159165

((2S,4aR,6aR,7R,9S,10aS,10bR)-9-(acetyloxy)-2-(fura...)Show SMILES COC(=O)[C@@H]1C[C@H](OC(C)=O)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 |r| Show InChI InChI=1S/C23H28O8/c1-12(24)30-16-9-15(20(26)28-4)22(2)7-5-14-21(27)31-17(13-6-8-29-11-13)10-23(14,3)19(22)18(16)25/h6,8,11,14-17,19H,5,7,9-10H2,1-4H3/t14-,15-,16-,17-,19-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from human recombinant kappa opioid receptor expressed in CHO cells after 2 hrs by liquid scintillation counting |
Bioorg Med Chem 20: 3100-10 (2012)
Article DOI: 10.1016/j.bmc.2012.02.040
BindingDB Entry DOI: 10.7270/Q27H1KK0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50159165

((2S,4aR,6aR,7R,9S,10aS,10bR)-9-(acetyloxy)-2-(fura...)Show SMILES COC(=O)[C@@H]1C[C@H](OC(C)=O)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 |r| Show InChI InChI=1S/C23H28O8/c1-12(24)30-16-9-15(20(26)28-4)22(2)7-5-14-21(27)31-17(13-6-8-29-11-13)10-23(14,3)19(22)18(16)25/h6,8,11,14-17,19H,5,7,9-10H2,1-4H3/t14-,15-,16-,17-,19-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from kappa opioid receptor |
J Nat Prod 74: 718-26 (2011)
Article DOI: 10.1021/np1007872
BindingDB Entry DOI: 10.7270/Q2PR7W9V |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50376842
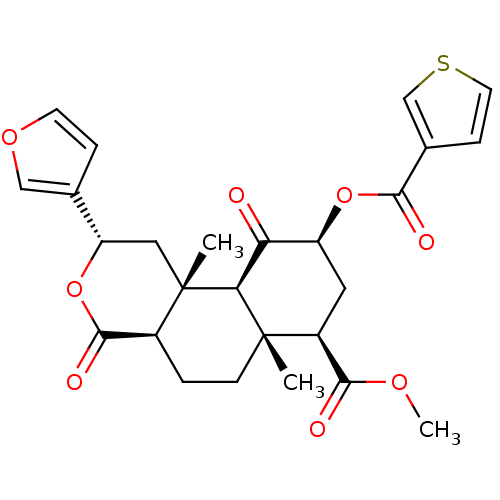
(CHEMBL410436)Show SMILES COC(=O)[C@@H]1C[C@H](OC(=O)c2ccsc2)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 Show InChI InChI=1S/C26H28O8S/c1-25-7-4-16-24(30)34-19(14-5-8-32-12-14)11-26(16,2)21(25)20(27)18(10-17(25)23(29)31-3)33-22(28)15-6-9-35-13-15/h5-6,8-9,12-13,16-19,21H,4,7,10-11H2,1-3H3/t16-,17-,18-,19-,21-,25-,26-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125I]IOXY from human recombinant mu opioid receptor expressed in CHO cells |
J Med Chem 51: 2421-31 (2008)
Article DOI: 10.1021/jm701162g
BindingDB Entry DOI: 10.7270/Q2DV1KR7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50238615
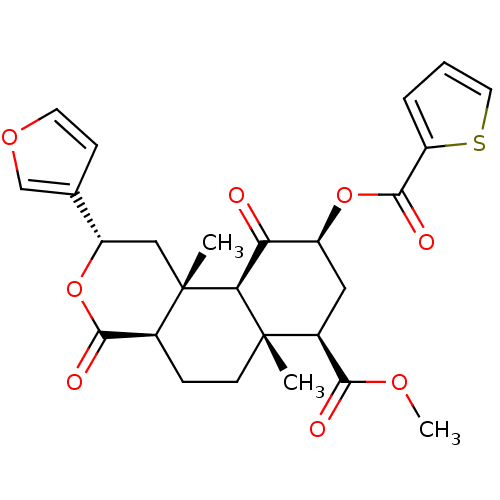
(CHEMBL264967 | Thiophene-2-carboxylic Acid (2S,4aR...)Show SMILES COC(=O)[C@@H]1C[C@H](OC(=O)c2cccs2)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 |r| Show InChI InChI=1S/C26H28O8S/c1-25-8-6-15-23(29)34-18(14-7-9-32-13-14)12-26(15,2)21(25)20(27)17(11-16(25)22(28)31-3)33-24(30)19-5-4-10-35-19/h4-5,7,9-10,13,15-18,21H,6,8,11-12H2,1-3H3/t15-,16-,17-,18-,21-,25-,26-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125]OXY from mu opioid receptor |
J Nat Prod 69: 914-8 (2006)
Article DOI: 10.1021/np060094b
BindingDB Entry DOI: 10.7270/Q28W3D22 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50376847
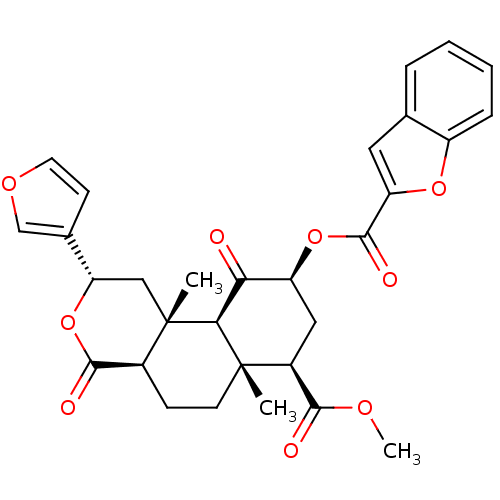
(CHEMBL260121)Show SMILES COC(=O)[C@@H]1C[C@H](OC(=O)c2cc3ccccc3o2)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 Show InChI InChI=1S/C30H30O9/c1-29-10-8-18-27(33)39-23(17-9-11-36-15-17)14-30(18,2)25(29)24(31)21(13-19(29)26(32)35-3)38-28(34)22-12-16-6-4-5-7-20(16)37-22/h4-7,9,11-12,15,18-19,21,23,25H,8,10,13-14H2,1-3H3/t18-,19-,21-,23-,25-,29-,30-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125I]IOXY from human recombinant mu opioid receptor expressed in CHO cells |
J Med Chem 51: 2421-31 (2008)
Article DOI: 10.1021/jm701162g
BindingDB Entry DOI: 10.7270/Q2DV1KR7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50238620
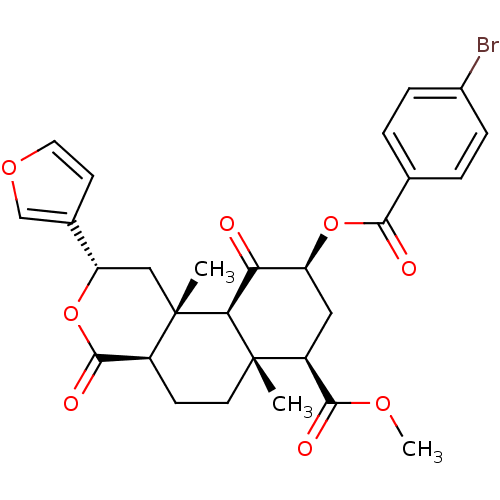
((2S,4aR,6aR,7R,9S,10aS,10bR)-9-(4-Bromobenzoyloxy)...)Show SMILES COC(=O)[C@@H]1C[C@H](OC(=O)c2ccc(Br)cc2)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 |r| Show InChI InChI=1S/C28H29BrO8/c1-27-10-8-18-26(33)37-21(16-9-11-35-14-16)13-28(18,2)23(27)22(30)20(12-19(27)25(32)34-3)36-24(31)15-4-6-17(29)7-5-15/h4-7,9,11,14,18-21,23H,8,10,12-13H2,1-3H3/t18-,19-,20-,21-,23-,27-,28-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125I]IOXY from human recombinant mu opioid receptor expressed in CHO cells |
J Med Chem 51: 2421-31 (2008)
Article DOI: 10.1021/jm701162g
BindingDB Entry DOI: 10.7270/Q2DV1KR7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50238615

(CHEMBL264967 | Thiophene-2-carboxylic Acid (2S,4aR...)Show SMILES COC(=O)[C@@H]1C[C@H](OC(=O)c2cccs2)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 |r| Show InChI InChI=1S/C26H28O8S/c1-25-8-6-15-23(29)34-18(14-7-9-32-13-14)12-26(15,2)21(25)20(27)17(11-16(25)22(28)31-3)33-24(30)19-5-4-10-35-19/h4-5,7,9-10,13,15-18,21H,6,8,11-12H2,1-3H3/t15-,16-,17-,18-,21-,25-,26-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125I]IOXY from human recombinant mu opioid receptor expressed in CHO cells |
J Med Chem 51: 2421-31 (2008)
Article DOI: 10.1021/jm701162g
BindingDB Entry DOI: 10.7270/Q2DV1KR7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50238620

((2S,4aR,6aR,7R,9S,10aS,10bR)-9-(4-Bromobenzoyloxy)...)Show SMILES COC(=O)[C@@H]1C[C@H](OC(=O)c2ccc(Br)cc2)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 |r| Show InChI InChI=1S/C28H29BrO8/c1-27-10-8-18-26(33)37-21(16-9-11-35-14-16)13-28(18,2)23(27)22(30)20(12-19(27)25(32)34-3)36-24(31)15-4-6-17(29)7-5-15/h4-7,9,11,14,18-21,23H,8,10,12-13H2,1-3H3/t18-,19-,20-,21-,23-,27-,28-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125]OXY from mu opioid receptor |
J Nat Prod 69: 914-8 (2006)
Article DOI: 10.1021/np060094b
BindingDB Entry DOI: 10.7270/Q28W3D22 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50170678
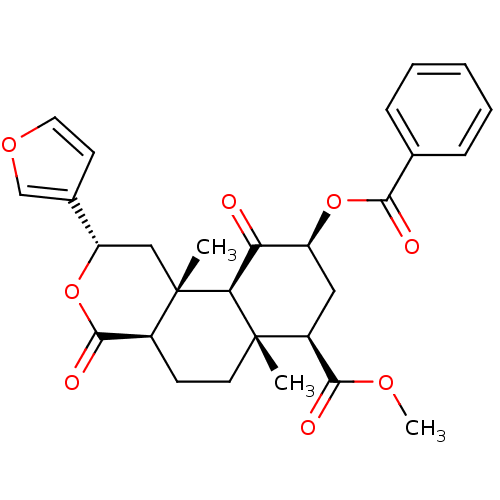
((3S,4aR,4bS,6S,8R,8aR,10aR)-6-Benzoyloxy-3-furan-3...)Show SMILES COC(=O)[C@@H]1C[C@H](OC(=O)c2ccccc2)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 |r| Show InChI InChI=1S/C28H30O8/c1-27-11-9-18-26(32)36-21(17-10-12-34-15-17)14-28(18,2)23(27)22(29)20(13-19(27)25(31)33-3)35-24(30)16-7-5-4-6-8-16/h4-8,10,12,15,18-21,23H,9,11,13-14H2,1-3H3/t18-,19-,20-,21-,23-,27-,28-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125I]IOXY from human recombinant mu opioid receptor expressed in CHO cells |
J Med Chem 51: 2421-31 (2008)
Article DOI: 10.1021/jm701162g
BindingDB Entry DOI: 10.7270/Q2DV1KR7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50170678

((3S,4aR,4bS,6S,8R,8aR,10aR)-6-Benzoyloxy-3-furan-3...)Show SMILES COC(=O)[C@@H]1C[C@H](OC(=O)c2ccccc2)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 |r| Show InChI InChI=1S/C28H30O8/c1-27-11-9-18-26(32)36-21(17-10-12-34-15-17)14-28(18,2)23(27)22(29)20(13-19(27)25(31)33-3)35-24(30)16-7-5-4-6-8-16/h4-8,10,12,15,18-21,23H,9,11,13-14H2,1-3H3/t18-,19-,20-,21-,23-,27-,28-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125]OXY from mu opioid receptor |
J Nat Prod 69: 914-8 (2006)
Article DOI: 10.1021/np060094b
BindingDB Entry DOI: 10.7270/Q28W3D22 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50159168
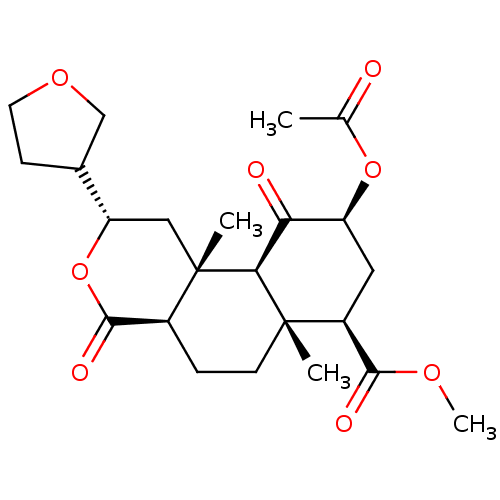
((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 9-acetoxy-6a,1...)Show SMILES COC(=O)[C@@H]1C[C@H](OC(C)=O)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)C1CCOC1 Show InChI InChI=1S/C23H32O8/c1-12(24)30-16-9-15(20(26)28-4)22(2)7-5-14-21(27)31-17(13-6-8-29-11-13)10-23(14,3)19(22)18(16)25/h13-17,19H,5-11H2,1-4H3/t13?,14-,15-,16-,17-,19-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125I]IOXY from human kappa opioid receptor expressed in CHO cells |
J Med Chem 50: 3596-603 (2007)
Article DOI: 10.1021/jm070393d
BindingDB Entry DOI: 10.7270/Q2ZK5HH0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50269283
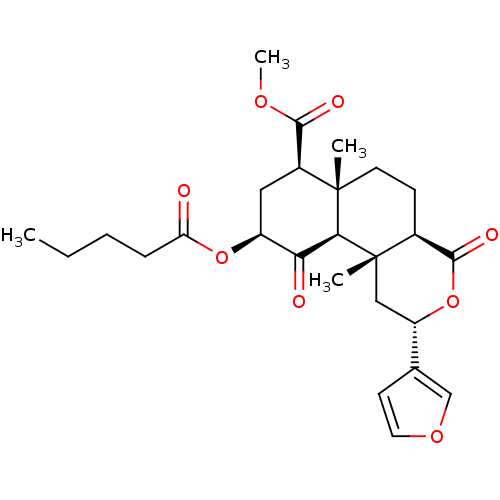
((2S,4aS,6aR,7R,9S,10aS,10bR)-9-(Pentanoyloxy)-2-(3...)Show SMILES CCCCC(=O)O[C@H]1C[C@@H](C(=O)OC)[C@]2(C)CC[C@H]3C(=O)O[C@@H](C[C@]3(C)[C@H]2C1=O)c1ccoc1 |r| Show InChI InChI=1S/C26H34O8/c1-5-6-7-20(27)33-18-12-17(23(29)31-4)25(2)10-8-16-24(30)34-19(15-9-11-32-14-15)13-26(16,3)22(25)21(18)28/h9,11,14,16-19,22H,5-8,10,12-13H2,1-4H3/t16-,17-,18-,19-,22-,25-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125]OXY from kappa opioid receptor |
J Nat Prod 69: 914-8 (2006)
Article DOI: 10.1021/np060094b
BindingDB Entry DOI: 10.7270/Q28W3D22 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50266337
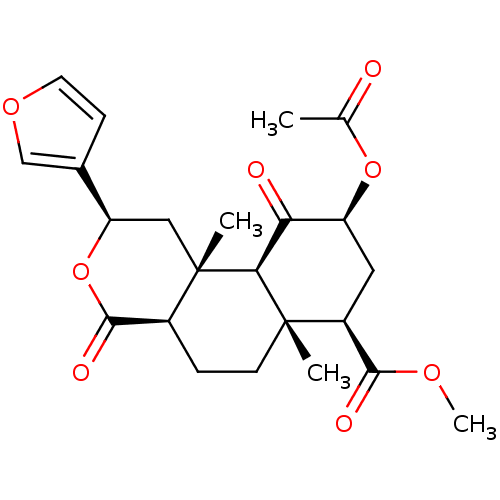
(12-epi-Salvinorin A | CHEMBL458235)Show SMILES COC(=O)[C@@H]1C[C@H](OC(C)=O)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@H](C[C@]21C)c1ccoc1 |r| Show InChI InChI=1S/C23H28O8/c1-12(24)30-16-9-15(20(26)28-4)22(2)7-5-14-21(27)31-17(13-6-8-29-11-13)10-23(14,3)19(22)18(16)25/h6,8,11,14-17,19H,5,7,9-10H2,1-4H3/t14-,15-,16-,17+,19-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from human recombinant kappa opioid receptor expressed in CHO cells after 2 hrs by liquid scintillation counting |
Bioorg Med Chem 20: 3100-10 (2012)
Article DOI: 10.1016/j.bmc.2012.02.040
BindingDB Entry DOI: 10.7270/Q27H1KK0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50216133
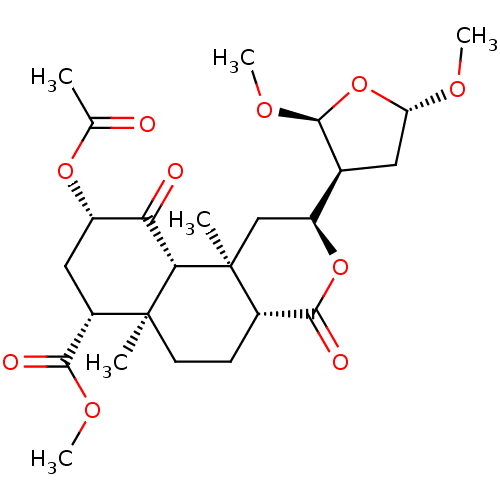
((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 9-acetoxy-2-((...)Show SMILES CO[C@@H]1C[C@H]([C@@H](OC)O1)[C@@H]1C[C@@]2(C)[C@@H](CC[C@@]3(C)[C@@H](C[C@H](OC(C)=O)C(=O)[C@H]23)C(=O)OC)C(=O)O1 Show InChI InChI=1S/C25H36O10/c1-12(26)33-16-10-15(21(28)31-5)24(2)8-7-14-22(29)34-17(11-25(14,3)20(24)19(16)27)13-9-18(30-4)35-23(13)32-6/h13-18,20,23H,7-11H2,1-6H3/t13-,14-,15-,16-,17-,18-,20-,23-,24-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125I]IOXY from human kappa opioid receptor expressed in CHO cells |
J Med Chem 50: 3596-603 (2007)
Article DOI: 10.1021/jm070393d
BindingDB Entry DOI: 10.7270/Q2ZK5HH0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50376839
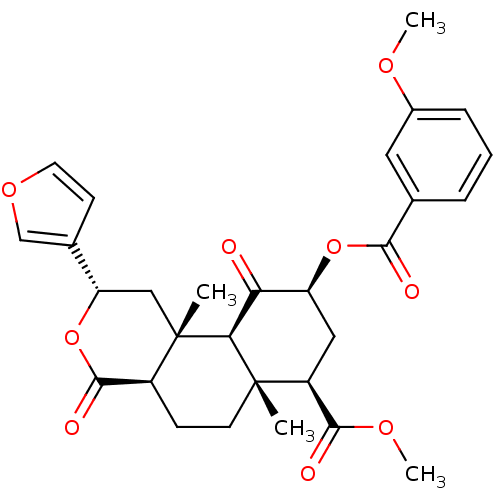
(CHEMBL260485)Show SMILES COC(=O)[C@@H]1C[C@H](OC(=O)c2cccc(OC)c2)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 Show InChI InChI=1S/C29H32O9/c1-28-10-8-19-27(33)38-22(17-9-11-36-15-17)14-29(19,2)24(28)23(30)21(13-20(28)26(32)35-4)37-25(31)16-6-5-7-18(12-16)34-3/h5-7,9,11-12,15,19-22,24H,8,10,13-14H2,1-4H3/t19-,20-,21-,22-,24-,28-,29-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125I]IOXY from human recombinant mu opioid receptor expressed in CHO cells |
J Med Chem 51: 2421-31 (2008)
Article DOI: 10.1021/jm701162g
BindingDB Entry DOI: 10.7270/Q2DV1KR7 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50381667
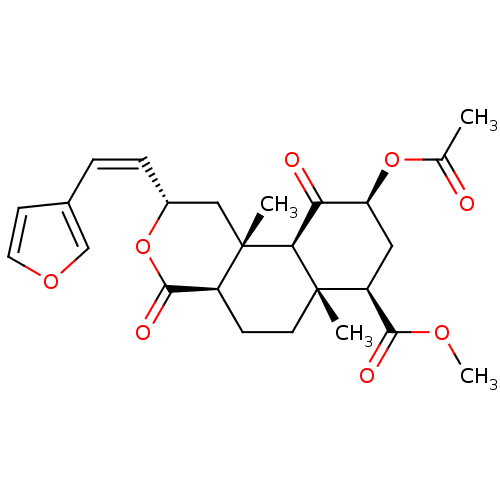
(CHEMBL2022018)Show SMILES COC(=O)[C@@H]1C[C@H](OC(C)=O)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)\C=C/c1ccoc1 |r| Show InChI InChI=1S/C25H30O8/c1-14(26)32-19-11-18(22(28)30-4)24(2)9-7-17-23(29)33-16(6-5-15-8-10-31-13-15)12-25(17,3)21(24)20(19)27/h5-6,8,10,13,16-19,21H,7,9,11-12H2,1-4H3/b6-5-/t16-,17+,18+,19+,21+,24+,25+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from human recombinant kappa opioid receptor expressed in CHO cells after 2 hrs by liquid scintillation counting |
Bioorg Med Chem 20: 3100-10 (2012)
Article DOI: 10.1016/j.bmc.2012.02.040
BindingDB Entry DOI: 10.7270/Q27H1KK0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50381666
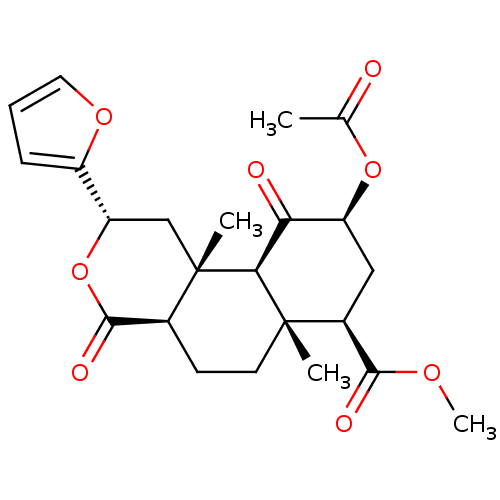
(CHEMBL2022307)Show SMILES COC(=O)[C@@H]1C[C@H](OC(C)=O)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccco1 |r| Show InChI InChI=1S/C23H28O8/c1-12(24)30-16-10-14(20(26)28-4)22(2)8-7-13-21(27)31-17(15-6-5-9-29-15)11-23(13,3)19(22)18(16)25/h5-6,9,13-14,16-17,19H,7-8,10-11H2,1-4H3/t13-,14-,16-,17-,19-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from human recombinant kappa opioid receptor expressed in CHO cells after 2 hrs by liquid scintillation counting |
Bioorg Med Chem 20: 3100-10 (2012)
Article DOI: 10.1016/j.bmc.2012.02.040
BindingDB Entry DOI: 10.7270/Q27H1KK0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50189148
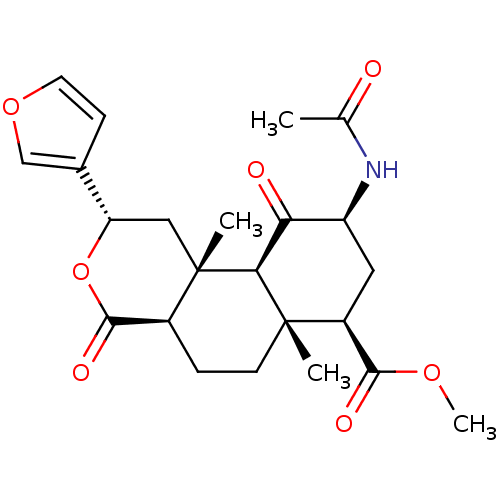
((2S,4aR,6aR,7R,9S,10aS,10bR)-9-(Acetylamino)-2-(3-...)Show SMILES COC(=O)[C@@H]1C[C@H](NC(C)=O)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 Show InChI InChI=1S/C23H29NO7/c1-12(25)24-16-9-15(20(27)29-4)22(2)7-5-14-21(28)31-17(13-6-8-30-11-13)10-23(14,3)19(22)18(16)26/h6,8,11,14-17,19H,5,7,9-10H2,1-4H3,(H,24,25)/t14-,15-,16-,17-,19-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant kappa opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay |
J Med Chem 51: 2421-31 (2008)
Article DOI: 10.1021/jm701162g
BindingDB Entry DOI: 10.7270/Q2DV1KR7 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50381662
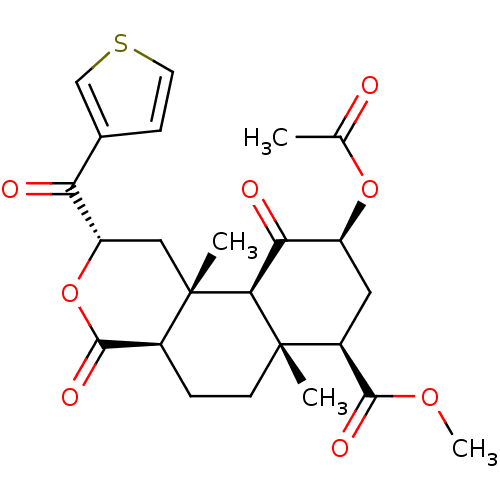
(CHEMBL2022302)Show SMILES COC(=O)[C@@H]1C[C@H](OC(C)=O)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)C(=O)c1ccsc1 |r| Show InChI InChI=1S/C24H28O8S/c1-12(25)31-16-9-15(21(28)30-4)23(2)7-5-14-22(29)32-17(18(26)13-6-8-33-11-13)10-24(14,3)20(23)19(16)27/h6,8,11,14-17,20H,5,7,9-10H2,1-4H3/t14-,15-,16-,17-,20-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from human recombinant kappa opioid receptor expressed in CHO cells after 2 hrs by liquid scintillation counting |
Bioorg Med Chem 20: 3100-10 (2012)
Article DOI: 10.1016/j.bmc.2012.02.040
BindingDB Entry DOI: 10.7270/Q27H1KK0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50266390
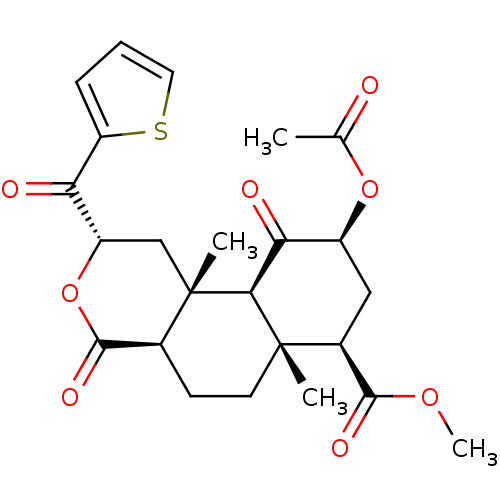
((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 9-acetoxy-6a,1...)Show SMILES COC(=O)[C@@H]1C[C@H](OC(C)=O)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)C(=O)c1cccs1 |r| Show InChI InChI=1S/C24H28O8S/c1-12(25)31-15-10-14(21(28)30-4)23(2)8-7-13-22(29)32-16(18(26)17-6-5-9-33-17)11-24(13,3)20(23)19(15)27/h5-6,9,13-16,20H,7-8,10-11H2,1-4H3/t13-,14-,15-,16-,20-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from human recombinant kappa opioid receptor expressed in CHO cells after 2 hrs by liquid scintillation counting |
Bioorg Med Chem 20: 3100-10 (2012)
Article DOI: 10.1016/j.bmc.2012.02.040
BindingDB Entry DOI: 10.7270/Q27H1KK0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50027234

(CHEMBL2113278)Show SMILES [H][C@]1(C[C@@]2(C)[C@@]([H])(CC[C@@]3(C)[C@@H](C[C@H](OC(C)=O)C(=O)[C@]23[H])C(=O)OC)C(=O)O1)C1CC(OC)OC1OC Show InChI InChI=1S/C25H36O10/c1-12(26)33-16-10-15(21(28)31-5)24(2)8-7-14-22(29)34-17(11-25(14,3)20(24)19(16)27)13-9-18(30-4)35-23(13)32-6/h13-18,20,23H,7-11H2,1-6H3/t13?,14-,15-,16-,17-,18?,20-,23?,24-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125I]IOXY from human kappa opioid receptor expressed in CHO cells |
J Med Chem 50: 3596-603 (2007)
Article DOI: 10.1021/jm070393d
BindingDB Entry DOI: 10.7270/Q2ZK5HH0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50381656
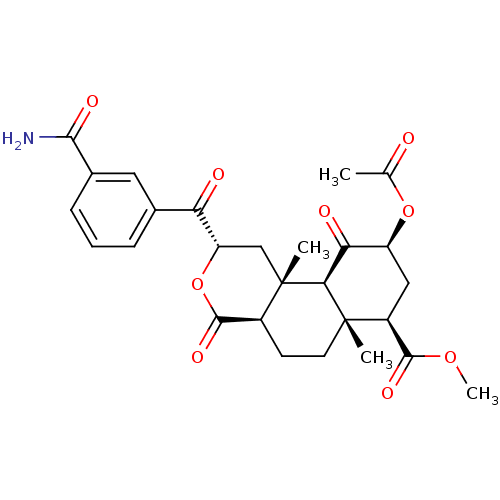
(CHEMBL2022296)Show SMILES COC(=O)[C@@H]1C[C@H](OC(C)=O)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)C(=O)c1cccc(c1)C(N)=O |r| Show InChI InChI=1S/C27H31NO9/c1-13(29)36-18-11-17(24(33)35-4)26(2)9-8-16-25(34)37-19(12-27(16,3)22(26)21(18)31)20(30)14-6-5-7-15(10-14)23(28)32/h5-7,10,16-19,22H,8-9,11-12H2,1-4H3,(H2,28,32)/t16-,17-,18-,19-,22-,26-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from human recombinant kappa opioid receptor expressed in CHO cells after 2 hrs by liquid scintillation counting |
Bioorg Med Chem 20: 3100-10 (2012)
Article DOI: 10.1016/j.bmc.2012.02.040
BindingDB Entry DOI: 10.7270/Q27H1KK0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50269313
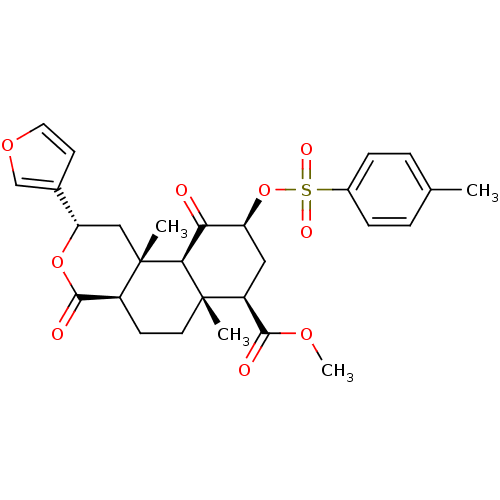
((2S,4aS,6aR,7R,9S,10aS,10bR)-9-(4-Methylbenzenesul...)Show SMILES COC(=O)[C@@H]1C[C@H](OS(=O)(=O)c2ccc(C)cc2)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 |r| Show InChI InChI=1S/C28H32O9S/c1-16-5-7-18(8-6-16)38(32,33)37-21-13-20(25(30)34-4)27(2)11-9-19-26(31)36-22(17-10-12-35-15-17)14-28(19,3)24(27)23(21)29/h5-8,10,12,15,19-22,24H,9,11,13-14H2,1-4H3/t19-,20-,21-,22-,24-,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125]OXY from kappa opioid receptor |
J Nat Prod 69: 914-8 (2006)
Article DOI: 10.1021/np060094b
BindingDB Entry DOI: 10.7270/Q28W3D22 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50216142
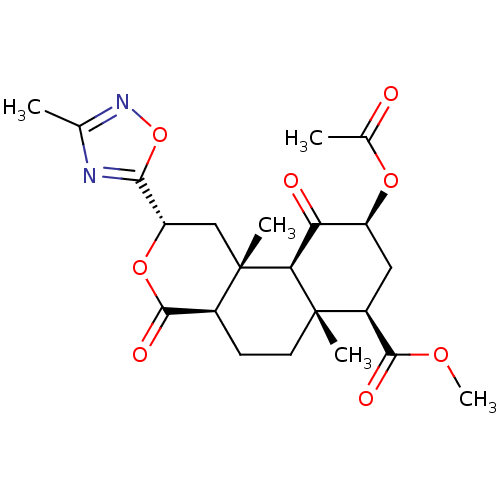
((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 9-acetoxy-6a,1...)Show SMILES COC(=O)[C@@H]1C[C@H](OC(C)=O)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1nc(C)no1 |r| Show InChI InChI=1S/C22H28N2O8/c1-10-23-18(32-24-10)15-9-22(4)12(20(28)31-15)6-7-21(3)13(19(27)29-5)8-14(30-11(2)25)16(26)17(21)22/h12-15,17H,6-9H2,1-5H3/t12-,13-,14-,15-,17-,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125I]IOXY from human kappa opioid receptor expressed in CHO cells |
J Med Chem 50: 3596-603 (2007)
Article DOI: 10.1021/jm070393d
BindingDB Entry DOI: 10.7270/Q2ZK5HH0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50343253
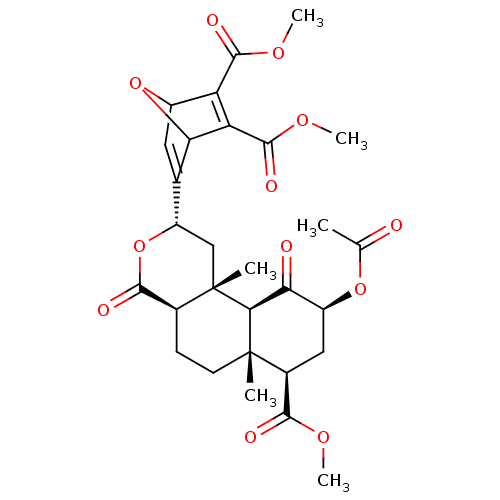
(CHEMBL1773748 | Dimethyl 5-((2S,4aR,6aR,7R,9S,10aS...)Show SMILES COC(=O)[C@@H]1C[C@H](OC(C)=O)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)C1=CC2OC1C(C(=O)OC)=C2C(=O)OC |r,c:39,t:29,TLB:32:31:26.27:29,THB:37:36:26.27:29,22:26:29:36.31| Show InChI InChI=1S/C29H34O12/c1-12(30)39-17-10-15(24(32)36-4)28(2)8-7-14-25(33)41-18(11-29(14,3)23(28)21(17)31)13-9-16-19(26(34)37-5)20(22(13)40-16)27(35)38-6/h9,14-18,22-23H,7-8,10-11H2,1-6H3/t14-,15-,16?,17-,18-,22?,23-,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from kappa opioid receptor |
J Nat Prod 74: 718-26 (2011)
Article DOI: 10.1021/np1007872
BindingDB Entry DOI: 10.7270/Q2PR7W9V |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50269312
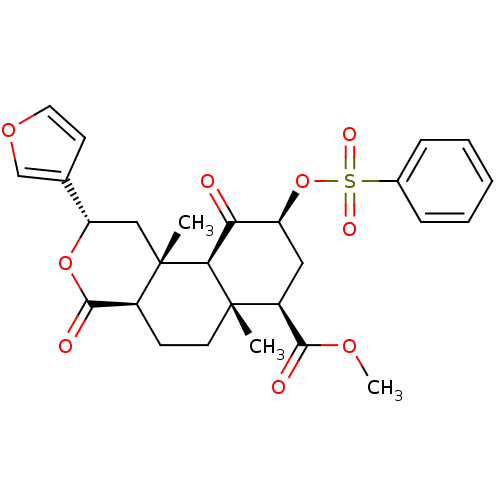
((2S,4aS,6aR,7R,9S,10aS,10bR)-9-(Benzenesulfonyloxy...)Show SMILES COC(=O)[C@@H]1C[C@H](OS(=O)(=O)c2ccccc2)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 |r| Show InChI InChI=1S/C27H30O9S/c1-26-11-9-18-25(30)35-21(16-10-12-34-15-16)14-27(18,2)23(26)22(28)20(13-19(26)24(29)33-3)36-37(31,32)17-7-5-4-6-8-17/h4-8,10,12,15,18-21,23H,9,11,13-14H2,1-3H3/t18-,19-,20-,21-,23-,26-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125]OXY from kappa opioid receptor |
J Nat Prod 69: 914-8 (2006)
Article DOI: 10.1021/np060094b
BindingDB Entry DOI: 10.7270/Q28W3D22 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50376846
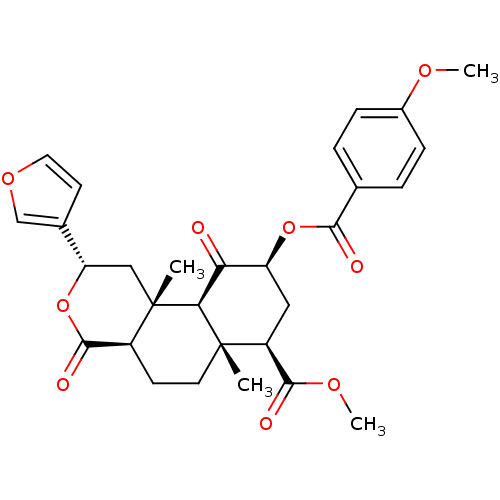
(CHEMBL260286)Show SMILES COC(=O)[C@@H]1C[C@H](OC(=O)c2ccc(OC)cc2)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 Show InChI InChI=1S/C29H32O9/c1-28-11-9-19-27(33)38-22(17-10-12-36-15-17)14-29(19,2)24(28)23(30)21(13-20(28)26(32)35-4)37-25(31)16-5-7-18(34-3)8-6-16/h5-8,10,12,15,19-22,24H,9,11,13-14H2,1-4H3/t19-,20-,21-,22-,24-,28-,29-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125I]IOXY from human recombinant mu opioid receptor expressed in CHO cells |
J Med Chem 51: 2421-31 (2008)
Article DOI: 10.1021/jm701162g
BindingDB Entry DOI: 10.7270/Q2DV1KR7 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50376847

(CHEMBL260121)Show SMILES COC(=O)[C@@H]1C[C@H](OC(=O)c2cc3ccccc3o2)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 Show InChI InChI=1S/C30H30O9/c1-29-10-8-18-27(33)39-23(17-9-11-36-15-17)14-30(18,2)25(29)24(31)21(13-19(29)26(32)35-3)38-28(34)22-12-16-6-4-5-7-20(16)37-22/h4-7,9,11-12,15,18-19,21,23,25H,8,10,13-14H2,1-3H3/t18-,19-,21-,23-,25-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125I]IOXY from human recombinant kappa opioid receptor expressed in CHO cells |
J Med Chem 51: 2421-31 (2008)
Article DOI: 10.1021/jm701162g
BindingDB Entry DOI: 10.7270/Q2DV1KR7 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50269284
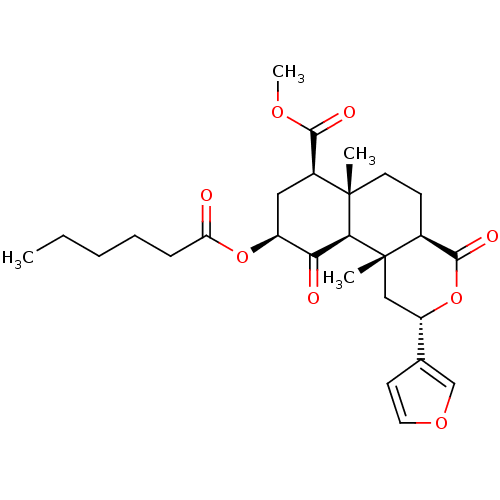
((2S,4aS,6aR,7R,9S,10aS,10bR)-9-(Hexanoyloxy)-2-(3-...)Show SMILES CCCCCC(=O)O[C@H]1C[C@@H](C(=O)OC)[C@]2(C)CC[C@H]3C(=O)O[C@@H](C[C@]3(C)[C@H]2C1=O)c1ccoc1 |r| Show InChI InChI=1S/C27H36O8/c1-5-6-7-8-21(28)34-19-13-18(24(30)32-4)26(2)11-9-17-25(31)35-20(16-10-12-33-15-16)14-27(17,3)23(26)22(19)29/h10,12,15,17-20,23H,5-9,11,13-14H2,1-4H3/t17-,18-,19-,20-,23-,26-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125]OXY from kappa opioid receptor |
J Nat Prod 69: 914-8 (2006)
Article DOI: 10.1021/np060094b
BindingDB Entry DOI: 10.7270/Q28W3D22 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50238624
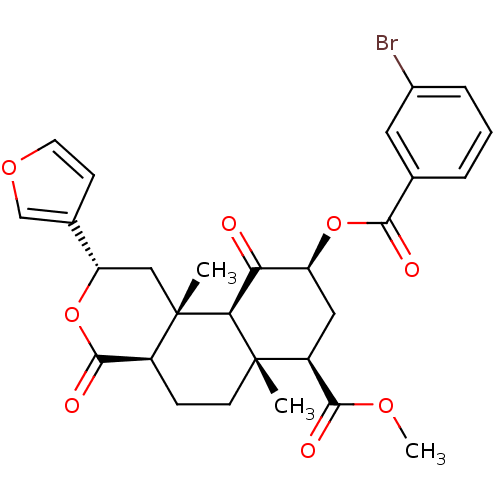
((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 9-(3-bromobenz...)Show SMILES COC(=O)[C@@H]1C[C@H](OC(=O)c2cccc(Br)c2)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 |r| Show InChI InChI=1S/C28H29BrO8/c1-27-9-7-18-26(33)37-21(16-8-10-35-14-16)13-28(18,2)23(27)22(30)20(12-19(27)25(32)34-3)36-24(31)15-5-4-6-17(29)11-15/h4-6,8,10-11,14,18-21,23H,7,9,12-13H2,1-3H3/t18-,19-,20-,21-,23-,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125]OXY from kappa opioid receptor |
J Nat Prod 69: 914-8 (2006)
Article DOI: 10.1021/np060094b
BindingDB Entry DOI: 10.7270/Q28W3D22 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50381668
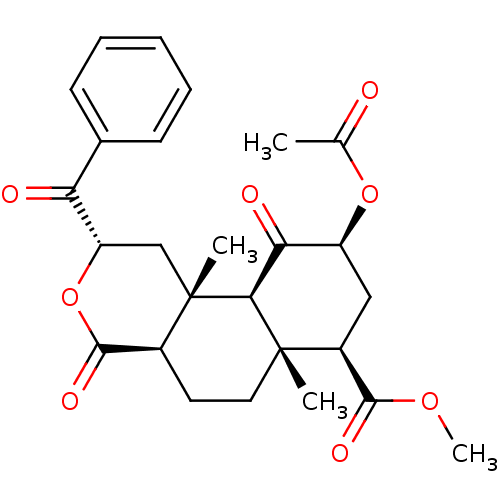
(CHEMBL2022019)Show SMILES COC(=O)[C@@H]1C[C@H](OC(C)=O)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C26H30O8/c1-14(27)33-18-12-17(23(30)32-4)25(2)11-10-16-24(31)34-19(13-26(16,3)22(25)21(18)29)20(28)15-8-6-5-7-9-15/h5-9,16-19,22H,10-13H2,1-4H3/t16-,17-,18-,19-,22-,25-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from human recombinant kappa opioid receptor expressed in CHO cells after 2 hrs by liquid scintillation counting |
Bioorg Med Chem 20: 3100-10 (2012)
Article DOI: 10.1016/j.bmc.2012.02.040
BindingDB Entry DOI: 10.7270/Q27H1KK0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50238624

((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 9-(3-bromobenz...)Show SMILES COC(=O)[C@@H]1C[C@H](OC(=O)c2cccc(Br)c2)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 |r| Show InChI InChI=1S/C28H29BrO8/c1-27-9-7-18-26(33)37-21(16-8-10-35-14-16)13-28(18,2)23(27)22(30)20(12-19(27)25(32)34-3)36-24(31)15-5-4-6-17(29)11-15/h4-6,8,10-11,14,18-21,23H,7,9,12-13H2,1-3H3/t18-,19-,20-,21-,23-,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant kappa opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay |
J Med Chem 51: 2421-31 (2008)
Article DOI: 10.1021/jm701162g
BindingDB Entry DOI: 10.7270/Q2DV1KR7 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50376842

(CHEMBL410436)Show SMILES COC(=O)[C@@H]1C[C@H](OC(=O)c2ccsc2)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 Show InChI InChI=1S/C26H28O8S/c1-25-7-4-16-24(30)34-19(14-5-8-32-12-14)11-26(16,2)21(25)20(27)18(10-17(25)23(29)31-3)33-22(28)15-6-9-35-13-15/h5-6,8-9,12-13,16-19,21H,4,7,10-11H2,1-3H3/t16-,17-,18-,19-,21-,25-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125I]IOXY from human recombinant kappa opioid receptor expressed in CHO cells |
J Med Chem 51: 2421-31 (2008)
Article DOI: 10.1021/jm701162g
BindingDB Entry DOI: 10.7270/Q2DV1KR7 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50381660

(CHEMBL2022300)Show SMILES COC(=O)[C@@H]1C[C@H](OC(C)=O)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)C(=O)c1ccco1 |r| Show InChI InChI=1S/C24H28O9/c1-12(25)32-16-10-14(21(28)30-4)23(2)8-7-13-22(29)33-17(18(26)15-6-5-9-31-15)11-24(13,3)20(23)19(16)27/h5-6,9,13-14,16-17,20H,7-8,10-11H2,1-4H3/t13-,14-,16-,17-,20-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from human recombinant kappa opioid receptor expressed in CHO cells after 2 hrs by liquid scintillation counting |
Bioorg Med Chem 20: 3100-10 (2012)
Article DOI: 10.1016/j.bmc.2012.02.040
BindingDB Entry DOI: 10.7270/Q27H1KK0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50170678

((3S,4aR,4bS,6S,8R,8aR,10aR)-6-Benzoyloxy-3-furan-3...)Show SMILES COC(=O)[C@@H]1C[C@H](OC(=O)c2ccccc2)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 |r| Show InChI InChI=1S/C28H30O8/c1-27-11-9-18-26(32)36-21(17-10-12-34-15-17)14-28(18,2)23(27)22(29)20(13-19(27)25(31)33-3)35-24(30)16-7-5-4-6-8-16/h4-8,10,12,15,18-21,23H,9,11,13-14H2,1-3H3/t18-,19-,20-,21-,23-,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125]OXY from kappa opioid receptor |
J Nat Prod 69: 914-8 (2006)
Article DOI: 10.1021/np060094b
BindingDB Entry DOI: 10.7270/Q28W3D22 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50269285
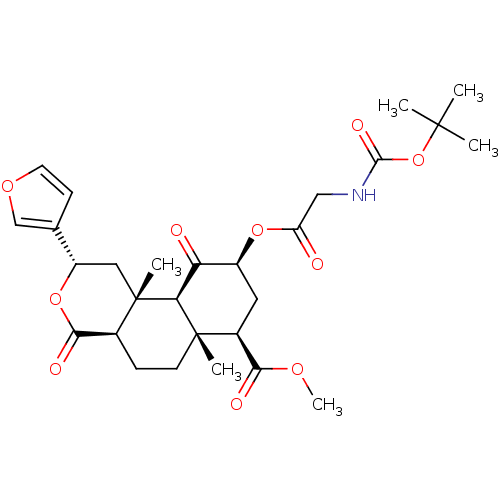
((2S,4aS,6aR,7R,9S,10aS,10bR)-9-(2-tert-Butoxycarbo...)Show SMILES COC(=O)[C@@H]1C[C@H](OC(=O)CNC(=O)OC(C)(C)C)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 |r| Show InChI InChI=1S/C28H37NO10/c1-26(2,3)39-25(34)29-13-20(30)37-18-11-17(23(32)35-6)27(4)9-7-16-24(33)38-19(15-8-10-36-14-15)12-28(16,5)22(27)21(18)31/h8,10,14,16-19,22H,7,9,11-13H2,1-6H3,(H,29,34)/t16-,17-,18-,19-,22-,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125]OXY from kappa opioid receptor |
J Nat Prod 69: 914-8 (2006)
Article DOI: 10.1021/np060094b
BindingDB Entry DOI: 10.7270/Q28W3D22 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50170678

((3S,4aR,4bS,6S,8R,8aR,10aR)-6-Benzoyloxy-3-furan-3...)Show SMILES COC(=O)[C@@H]1C[C@H](OC(=O)c2ccccc2)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 |r| Show InChI InChI=1S/C28H30O8/c1-27-11-9-18-26(32)36-21(17-10-12-34-15-17)14-28(18,2)23(27)22(29)20(13-19(27)25(31)33-3)35-24(30)16-7-5-4-6-8-16/h4-8,10,12,15,18-21,23H,9,11,13-14H2,1-3H3/t18-,19-,20-,21-,23-,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant kappa opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay |
J Med Chem 51: 2421-31 (2008)
Article DOI: 10.1021/jm701162g
BindingDB Entry DOI: 10.7270/Q2DV1KR7 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50238628
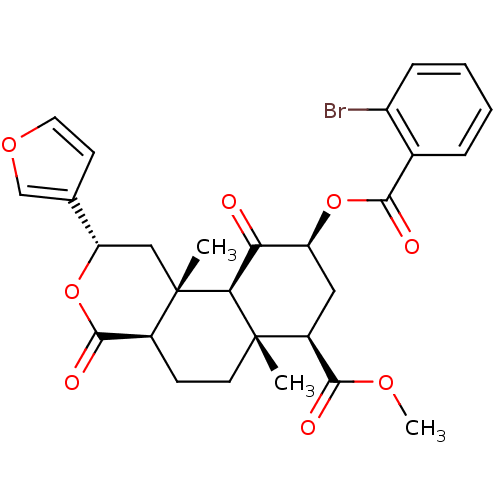
((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 9-(2-bromobenz...)Show SMILES COC(=O)[C@@H]1C[C@H](OC(=O)c2ccccc2Br)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 |r| Show InChI InChI=1S/C28H29BrO8/c1-27-10-8-17-26(33)37-21(15-9-11-35-14-15)13-28(17,2)23(27)22(30)20(12-18(27)25(32)34-3)36-24(31)16-6-4-5-7-19(16)29/h4-7,9,11,14,17-18,20-21,23H,8,10,12-13H2,1-3H3/t17-,18-,20-,21-,23-,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125]OXY from kappa opioid receptor |
J Nat Prod 69: 914-8 (2006)
Article DOI: 10.1021/np060094b
BindingDB Entry DOI: 10.7270/Q28W3D22 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50238628

((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 9-(2-bromobenz...)Show SMILES COC(=O)[C@@H]1C[C@H](OC(=O)c2ccccc2Br)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 |r| Show InChI InChI=1S/C28H29BrO8/c1-27-10-8-17-26(33)37-21(15-9-11-35-14-15)13-28(17,2)23(27)22(30)20(12-18(27)25(32)34-3)36-24(31)16-6-4-5-7-19(16)29/h4-7,9,11,14,17-18,20-21,23H,8,10,12-13H2,1-3H3/t17-,18-,20-,21-,23-,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant kappa opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay |
J Med Chem 51: 2421-31 (2008)
Article DOI: 10.1021/jm701162g
BindingDB Entry DOI: 10.7270/Q2DV1KR7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50238624

((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 9-(3-bromobenz...)Show SMILES COC(=O)[C@@H]1C[C@H](OC(=O)c2cccc(Br)c2)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 |r| Show InChI InChI=1S/C28H29BrO8/c1-27-9-7-18-26(33)37-21(16-8-10-35-14-16)13-28(18,2)23(27)22(30)20(12-19(27)25(32)34-3)36-24(31)15-5-4-6-17(29)11-15/h4-6,8,10-11,14,18-21,23H,7,9,12-13H2,1-3H3/t18-,19-,20-,21-,23-,27-,28-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125I]IOXY from human recombinant mu opioid receptor expressed in CHO cells |
J Med Chem 51: 2421-31 (2008)
Article DOI: 10.1021/jm701162g
BindingDB Entry DOI: 10.7270/Q2DV1KR7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50238628

((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 9-(2-bromobenz...)Show SMILES COC(=O)[C@@H]1C[C@H](OC(=O)c2ccccc2Br)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 |r| Show InChI InChI=1S/C28H29BrO8/c1-27-10-8-17-26(33)37-21(15-9-11-35-14-15)13-28(17,2)23(27)22(30)20(12-18(27)25(32)34-3)36-24(31)16-6-4-5-7-19(16)29/h4-7,9,11,14,17-18,20-21,23H,8,10,12-13H2,1-3H3/t17-,18-,20-,21-,23-,27-,28-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125I]IOXY from human recombinant mu opioid receptor expressed in CHO cells |
J Med Chem 51: 2421-31 (2008)
Article DOI: 10.1021/jm701162g
BindingDB Entry DOI: 10.7270/Q2DV1KR7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data