

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase Yes (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human full length recombinant YES using poly(Glu,Tyr)4:1 as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometri... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90DC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK1 (Homo sapiens (Human)) | BDBM50238867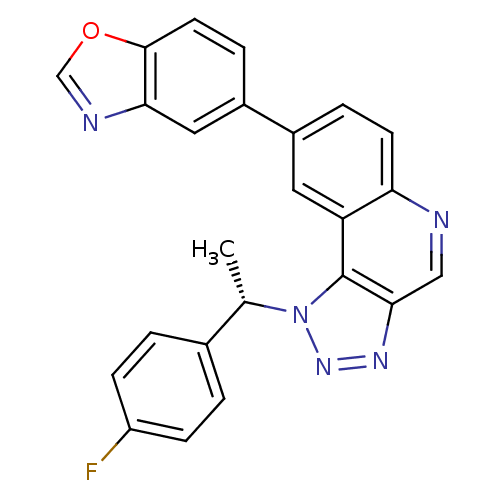 (CHEMBL4083249) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center for Biotherapy Curated by ChEMBL | Assay Description Inhibition of recombinant human CLK1 expressed in insect cells using ERMRPRKRQGSVRRRV peptide as substrate after 40 mins in presence of [gamma-33P]-A... | J Med Chem 60: 6337-6352 (2017) Article DOI: 10.1021/acs.jmedchem.7b00665 BindingDB Entry DOI: 10.7270/Q2ZW1P55 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant ABL (27 to end residues) using EAIYAAPFAKKK as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radio... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90DC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK1 (Homo sapiens (Human)) | BDBM50238867 (CHEMBL4083249) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center for Biotherapy Curated by ChEMBL | Assay Description Inhibition of recombinant human CLK1 expressed in insect cells using ERMRPRKRQGSVRRRV peptide as substrate after 40 mins in presence of [gamma-33P]-A... | J Med Chem 60: 6337-6352 (2017) Article DOI: 10.1021/acs.jmedchem.7b00665 BindingDB Entry DOI: 10.7270/Q2ZW1P55 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human full length recombinant Src using Cdc2 peptide as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometric sc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90DC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant ABL T315I mutant (27 to end residues) using EAIYAAPFAKKK as substrate incubated for 40 mins in presence of [gamma33P-... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90DC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human HCK (230 to 497 residues) using GGMEDIYFEFMGGKKK as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometric ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90DC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lyn (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human full length recombinant LYN using poly(Glu,Tyr)4:1 as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometri... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90DC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL2 (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant ARG (38 to end residues) using EAIYAAPFAKKK as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radio... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90DC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant RET (658 to end residues) using KKKSPGEYVNIEFG as substrate incubated for 40 mins in presence of [gamma33P-ATP] by ra... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90DC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human full length recombinant FYN using Cdc2 peptide as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometric sc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90DC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK1 (Homo sapiens (Human)) | BDBM50238882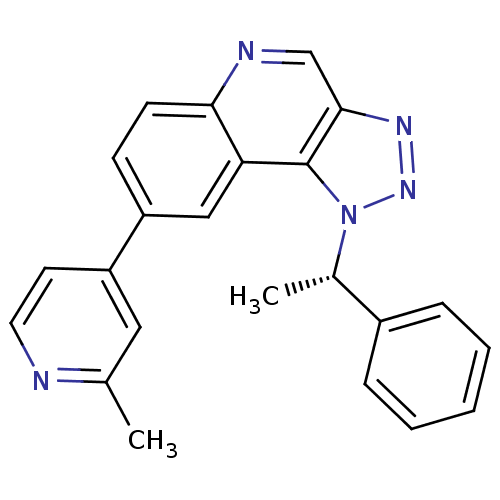 (CHEMBL4094600) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center for Biotherapy Curated by ChEMBL | Assay Description Inhibition of recombinant human CLK1 expressed in insect cells using ERMRPRKRQGSVRRRV peptide as substrate after 40 mins in presence of [gamma-33P]-A... | J Med Chem 60: 6337-6352 (2017) Article DOI: 10.1021/acs.jmedchem.7b00665 BindingDB Entry DOI: 10.7270/Q2ZW1P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK1 (Homo sapiens (Human)) | BDBM50238882 (CHEMBL4094600) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center for Biotherapy Curated by ChEMBL | Assay Description Inhibition of recombinant human CLK1 expressed in insect cells using ERMRPRKRQGSVRRRV peptide as substrate after 40 mins in presence of [gamma-33P]-A... | J Med Chem 60: 6337-6352 (2017) Article DOI: 10.1021/acs.jmedchem.7b00665 BindingDB Entry DOI: 10.7270/Q2ZW1P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase TXK (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant TXK (256 to end residues) using GEEPLYWSFPAKKK as substrate incubated for 40 mins in presence of [gamma33P-ATP] by ra... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90DC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant FGFR1 (456 to 765 residues) using KKKSPGEYVNIEFG as substrate incubated for 40 mins in presence of [gamma33P-ATP] by ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90DC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK1 (Homo sapiens (Human)) | BDBM50238893 (CHEMBL4096379) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center for Biotherapy Curated by ChEMBL | Assay Description Inhibition of recombinant human CLK1 expressed in insect cells using ERMRPRKRQGSVRRRV peptide as substrate after 40 mins in presence of [gamma-33P]-A... | J Med Chem 60: 6337-6352 (2017) Article DOI: 10.1021/acs.jmedchem.7b00665 BindingDB Entry DOI: 10.7270/Q2ZW1P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK1 (Homo sapiens (Human)) | BDBM50238893 (CHEMBL4096379) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center for Biotherapy Curated by ChEMBL | Assay Description Inhibition of recombinant human CLK1 expressed in insect cells using ERMRPRKRQGSVRRRV peptide as substrate after 40 mins in presence of [gamma-33P]-A... | J Med Chem 60: 6337-6352 (2017) Article DOI: 10.1021/acs.jmedchem.7b00665 BindingDB Entry DOI: 10.7270/Q2ZW1P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM448306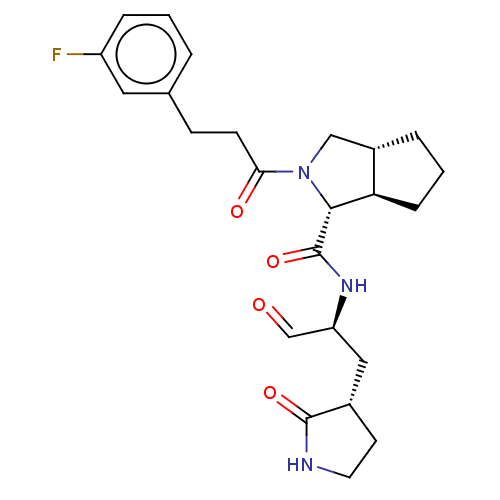 (MI-21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University | Assay Description The enzymatic inhibition assay was carried out with a final concentration of 100 nM recombinant SARS-CoV-2 Mpro and 20 μM FRET substrate. For th... | Science (2021) Article DOI: 10.1126/science.abf1611 BindingDB Entry DOI: 10.7270/Q24B34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM448308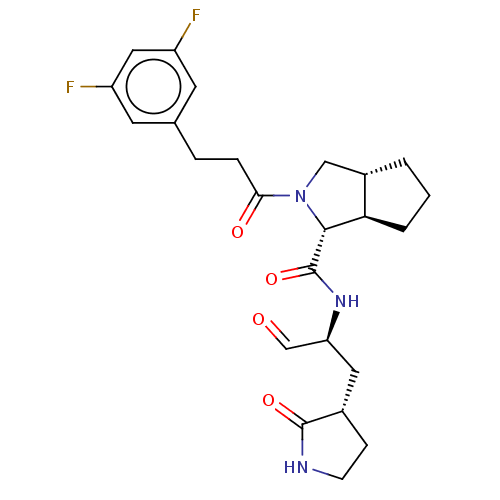 (MI-23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University | Assay Description The enzymatic inhibition assay was carried out with a final concentration of 100 nM recombinant SARS-CoV-2 Mpro and 20 μM FRET substrate. For th... | Science (2021) Article DOI: 10.1126/science.abf1611 BindingDB Entry DOI: 10.7270/Q24B34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK4 (Homo sapiens (Human)) | BDBM50238867 (CHEMBL4083249) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center for Biotherapy Curated by ChEMBL | Assay Description Inhibition of recombinant human CLK4 expressed in baculovirus infected Sf21 cells | J Med Chem 60: 6337-6352 (2017) Article DOI: 10.1021/acs.jmedchem.7b00665 BindingDB Entry DOI: 10.7270/Q2ZW1P55 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dual specificity protein kinase CLK4 (Homo sapiens (Human)) | BDBM50238867 (CHEMBL4083249) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center for Biotherapy Curated by ChEMBL | Assay Description Inhibition of recombinant human CLK4 expressed in baculovirus infected Sf21 cells | J Med Chem 60: 6337-6352 (2017) Article DOI: 10.1021/acs.jmedchem.7b00665 BindingDB Entry DOI: 10.7270/Q2ZW1P55 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase CSK (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human full length recombinant CSK using poly(Glu,Tyr)4:1 as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometri... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90DC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM448314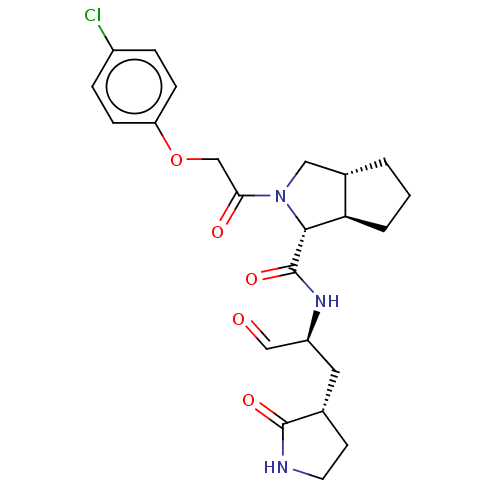 (MI-28) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University | Assay Description The enzymatic inhibition assay was carried out with a final concentration of 100 nM recombinant SARS-CoV-2 Mpro and 20 μM FRET substrate. For th... | Science (2021) Article DOI: 10.1126/science.abf1611 BindingDB Entry DOI: 10.7270/Q24B34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiopoietin-1 receptor (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant TIE2 Q939H/Q940H mutant (771 to end residues) using poly(Glu,Tyr)4:1 as substrate incubated for 40 mins in presence o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90DC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM448298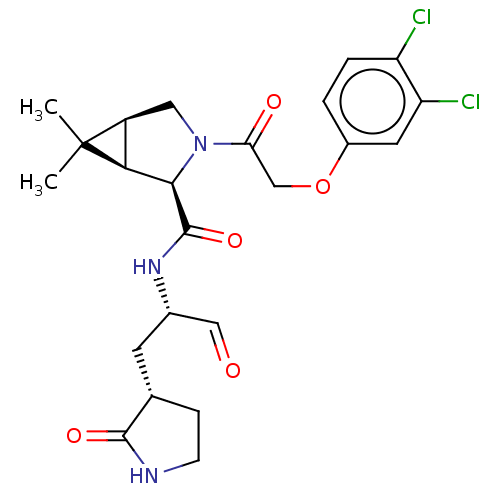 (MI-13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University | Assay Description The enzymatic inhibition assay was carried out with a final concentration of 100 nM recombinant SARS-CoV-2 Mpro and 20 μM FRET substrate. For th... | Science (2021) Article DOI: 10.1126/science.abf1611 BindingDB Entry DOI: 10.7270/Q24B34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM448259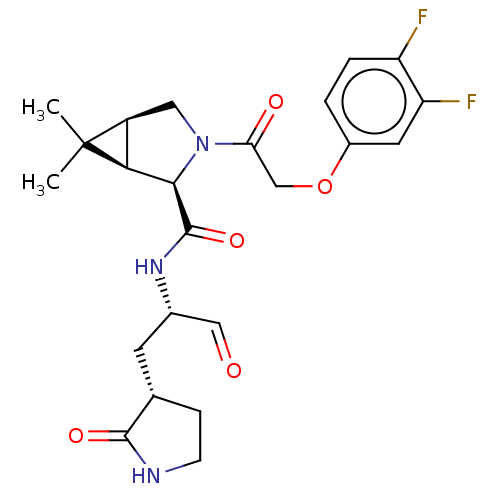 (MI-05) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University | Assay Description The enzymatic inhibition assay was carried out with a final concentration of 100 nM recombinant SARS-CoV-2 Mpro and 20 μM FRET substrate. For th... | Science (2021) Article DOI: 10.1126/science.abf1611 BindingDB Entry DOI: 10.7270/Q24B34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM448299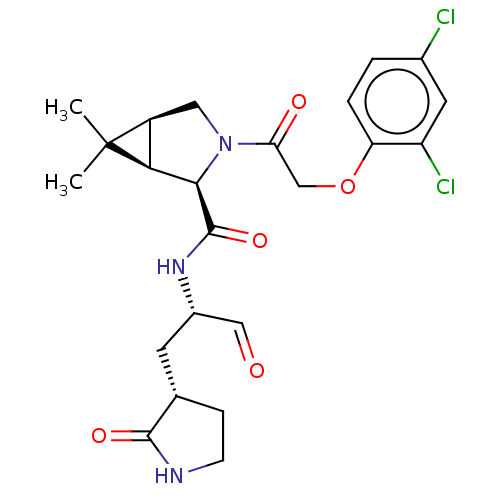 (MI-14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University | Assay Description The enzymatic inhibition assay was carried out with a final concentration of 100 nM recombinant SARS-CoV-2 Mpro and 20 μM FRET substrate. For th... | Science (2021) Article DOI: 10.1126/science.abf1611 BindingDB Entry DOI: 10.7270/Q24B34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM448296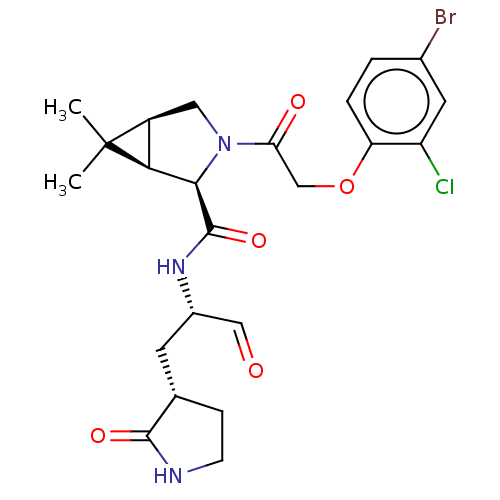 (MI-11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University | Assay Description The enzymatic inhibition assay was carried out with a final concentration of 100 nM recombinant SARS-CoV-2 Mpro and 20 μM FRET substrate. For th... | Science (2021) Article DOI: 10.1126/science.abf1611 BindingDB Entry DOI: 10.7270/Q24B34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human full length recombinant LCK using KVEKIGEGTYGVVYK as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometric... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90DC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant FLT1 (783 to end residues) using KKKSPGEYVNIEFG as substrate incubated for 40 mins in presence of [gamma33P-ATP] by r... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90DC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM448272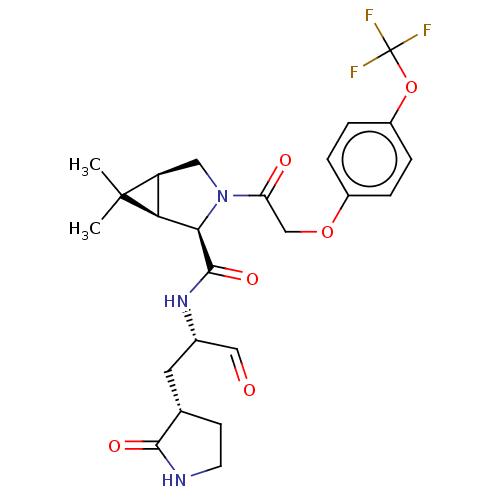 (MI-09) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University | Assay Description The enzymatic inhibition assay was carried out with a final concentration of 100 nM recombinant SARS-CoV-2 Mpro and 20 μM FRET substrate. For th... | Science (2021) Article DOI: 10.1126/science.abf1611 BindingDB Entry DOI: 10.7270/Q24B34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM448267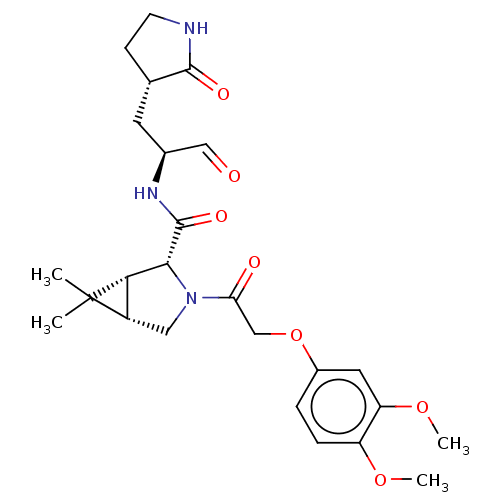 (MI-06) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University | Assay Description The enzymatic inhibition assay was carried out with a final concentration of 100 nM recombinant SARS-CoV-2 Mpro and 20 μM FRET substrate. For th... | Science (2021) Article DOI: 10.1126/science.abf1611 BindingDB Entry DOI: 10.7270/Q24B34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Blk (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human full length recombinant BLK M287V mutant using poly(Glu,Tyr)4:1 as substrate incubated for 40 mins in presence of [gamma33P-ATP] ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90DC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK1 (Homo sapiens (Human)) | BDBM50026622 (CHEMBL408982) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center for Biotherapy Curated by ChEMBL | Assay Description Inhibition of recombinant human CLK1 expressed in insect cells using ERMRPRKRQGSVRRRV peptide as substrate after 40 mins in presence of [gamma-33P]-A... | J Med Chem 60: 6337-6352 (2017) Article DOI: 10.1021/acs.jmedchem.7b00665 BindingDB Entry DOI: 10.7270/Q2ZW1P55 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dual specificity protein kinase CLK1 (Homo sapiens (Human)) | BDBM50026622 (CHEMBL408982) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center for Biotherapy Curated by ChEMBL | Assay Description Inhibition of recombinant human CLK1 expressed in insect cells using ERMRPRKRQGSVRRRV peptide as substrate after 40 mins in presence of [gamma-33P]-A... | J Med Chem 60: 6337-6352 (2017) Article DOI: 10.1021/acs.jmedchem.7b00665 BindingDB Entry DOI: 10.7270/Q2ZW1P55 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM448316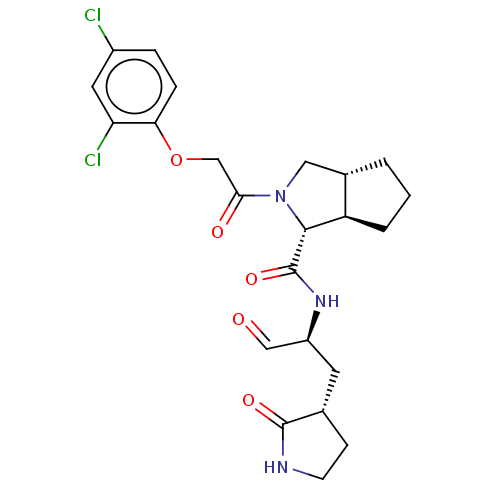 (MI-30) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University | Assay Description The enzymatic inhibition assay was carried out with a final concentration of 100 nM recombinant SARS-CoV-2 Mpro and 20 μM FRET substrate. For th... | Science (2021) Article DOI: 10.1126/science.abf1611 BindingDB Entry DOI: 10.7270/Q24B34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM448307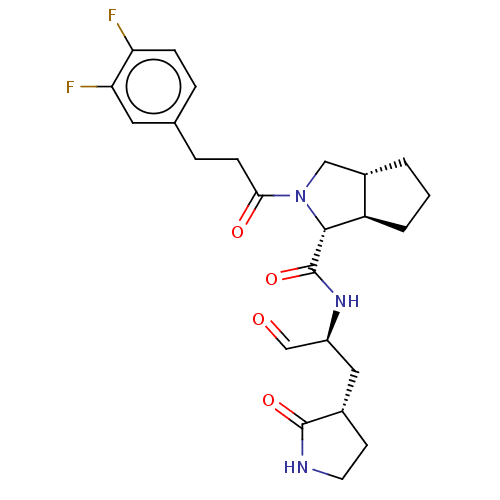 (MI-22) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University | Assay Description The enzymatic inhibition assay was carried out with a final concentration of 100 nM recombinant SARS-CoV-2 Mpro and 20 μM FRET substrate. For th... | Science (2021) Article DOI: 10.1126/science.abf1611 BindingDB Entry DOI: 10.7270/Q24B34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM448244 (MI-03) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University | Assay Description The enzymatic inhibition assay was carried out with a final concentration of 100 nM recombinant SARS-CoV-2 Mpro and 20 μM FRET substrate. For th... | Science (2021) Article DOI: 10.1126/science.abf1611 BindingDB Entry DOI: 10.7270/Q24B34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK1 (Homo sapiens (Human)) | BDBM50238868 (CHEMBL4072795) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center for Biotherapy Curated by ChEMBL | Assay Description Inhibition of recombinant human CLK1 expressed in insect cells using ERMRPRKRQGSVRRRV peptide as substrate after 40 mins in presence of [gamma-33P]-A... | J Med Chem 60: 6337-6352 (2017) Article DOI: 10.1021/acs.jmedchem.7b00665 BindingDB Entry DOI: 10.7270/Q2ZW1P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK1 (Homo sapiens (Human)) | BDBM50238868 (CHEMBL4072795) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center for Biotherapy Curated by ChEMBL | Assay Description Inhibition of recombinant human CLK1 expressed in insect cells using ERMRPRKRQGSVRRRV peptide as substrate after 40 mins in presence of [gamma-33P]-A... | J Med Chem 60: 6337-6352 (2017) Article DOI: 10.1021/acs.jmedchem.7b00665 BindingDB Entry DOI: 10.7270/Q2ZW1P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase FRK (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant PTK5 (218 to end residues) using GGEEEEYFELVKKKK as substrate incubated for 40 mins in presence of [gamma33P-ATP] by ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90DC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM448297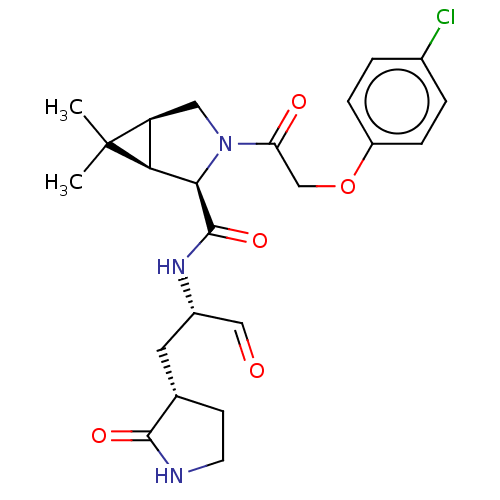 (MI-12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University | Assay Description The enzymatic inhibition assay was carried out with a final concentration of 100 nM recombinant SARS-CoV-2 Mpro and 20 μM FRET substrate. For th... | Science (2021) Article DOI: 10.1126/science.abf1611 BindingDB Entry DOI: 10.7270/Q24B34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM448257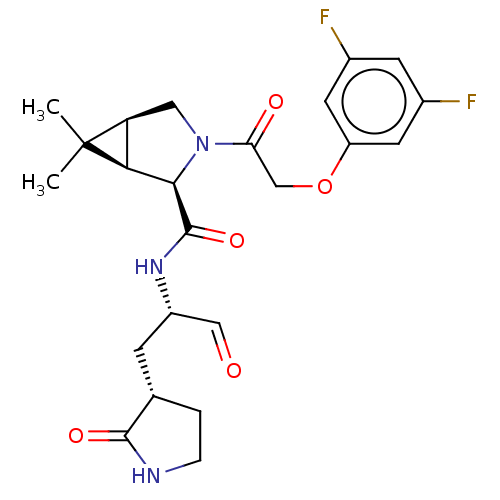 (MI-04) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University | Assay Description The enzymatic inhibition assay was carried out with a final concentration of 100 nM recombinant SARS-CoV-2 Mpro and 20 μM FRET substrate. For th... | Science (2021) Article DOI: 10.1126/science.abf1611 BindingDB Entry DOI: 10.7270/Q24B34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant EGFR T790M mutant (696 to end residues) using GGMEDIYFEFMGGKKK as substrate incubated for 40 mins in presence of [gam... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90DC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM448318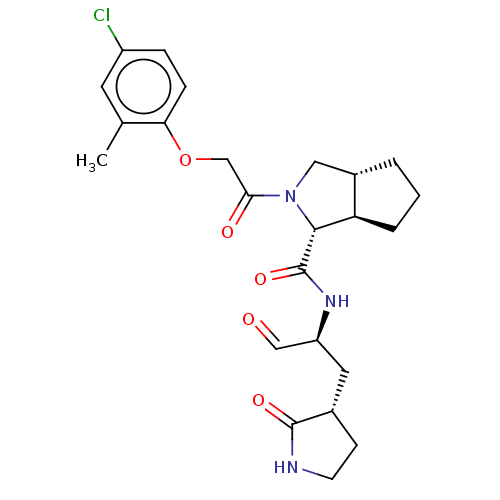 (MI-32) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University | Assay Description The enzymatic inhibition assay was carried out with a final concentration of 100 nM recombinant SARS-CoV-2 Mpro and 20 μM FRET substrate. For th... | Science (2021) Article DOI: 10.1126/science.abf1611 BindingDB Entry DOI: 10.7270/Q24B34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytoplasmic tyrosine-protein kinase BMX (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human full length recombinant BMX using poly (Glu,Tyr)4:1 as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometr... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90DC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK1 (Homo sapiens (Human)) | BDBM50238881 (CHEMBL4067143) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center for Biotherapy Curated by ChEMBL | Assay Description Inhibition of recombinant human CLK1 expressed in insect cells using ERMRPRKRQGSVRRRV peptide as substrate after 40 mins in presence of [gamma-33P]-A... | J Med Chem 60: 6337-6352 (2017) Article DOI: 10.1021/acs.jmedchem.7b00665 BindingDB Entry DOI: 10.7270/Q2ZW1P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK1 (Homo sapiens (Human)) | BDBM50238881 (CHEMBL4067143) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University and Collaborative Innovation Center for Biotherapy Curated by ChEMBL | Assay Description Inhibition of recombinant human CLK1 expressed in insect cells using ERMRPRKRQGSVRRRV peptide as substrate after 40 mins in presence of [gamma-33P]-A... | J Med Chem 60: 6337-6352 (2017) Article DOI: 10.1021/acs.jmedchem.7b00665 BindingDB Entry DOI: 10.7270/Q2ZW1P55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM448230 (11b | MI-02) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University | Assay Description The enzymatic inhibition assay was carried out with a final concentration of 100 nM recombinant SARS-CoV-2 Mpro and 20 μM FRET substrate. For th... | Science (2021) Article DOI: 10.1126/science.abf1611 BindingDB Entry DOI: 10.7270/Q24B34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50319612 ((R)-(+)-4-[4,4-Dimethyl-1-(4-methylsulfanyl-benzyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Antagonist activity at androgen receptor in human MDA-MB-453 cells coexpressing MMTV-ARE reporter gene assessed as inhibition of DHT-induced response | J Med Chem 53: 4422-7 (2010) Article DOI: 10.1021/jm9018004 BindingDB Entry DOI: 10.7270/Q2DV1K1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 276 total ) | Next | Last >> |