

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 0.000340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari Curated by ChEMBL | Assay Description Inhibition of human DHFR by spectrophotometric analysis | J Med Chem 55: 8318-29 (2012) Article DOI: 10.1021/jm300563f BindingDB Entry DOI: 10.7270/Q2R49RXF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50056769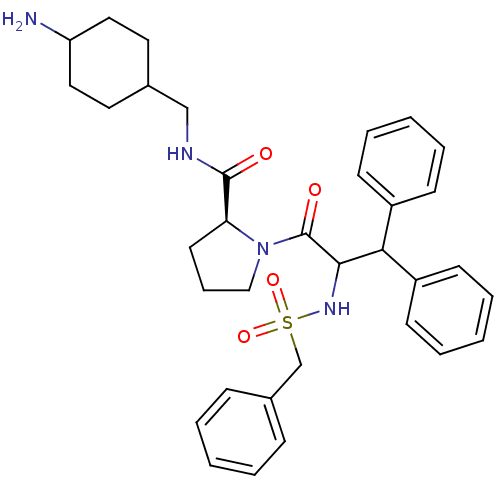 ((S)-1-(3,3-Diphenyl-2-phenylmethanesulfonylamino-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin | J Med Chem 40: 830-2 (1997) Article DOI: 10.1021/jm960762y BindingDB Entry DOI: 10.7270/Q25H7GXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Texas Curated by PDSP Ki Database | Synapse 38: 438-49 (2000) Article DOI: 10.1002/1098-2396(20001215)38:4 BindingDB Entry DOI: 10.7270/Q2SN07HX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Homo sapiens (Human)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Barrow Neurological Institute Curated by PDSP Ki Database | Mol Pharmacol 64: 1283-94 (2003) Article DOI: 10.1124/mol.64.6.1283 BindingDB Entry DOI: 10.7270/Q2GF0S2V | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50368742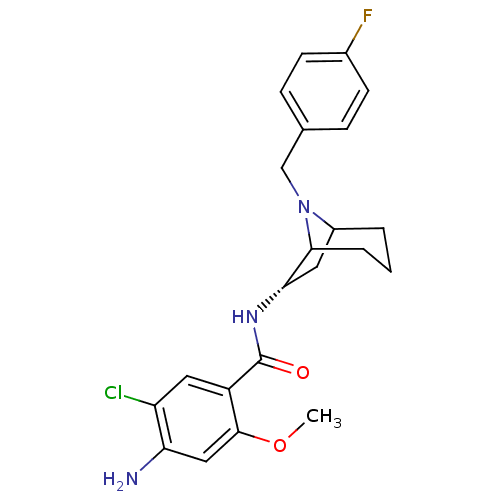 (CHEMBL1169525) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of [125I]- NCQ 298 binding to D2 receptor of rat striatal tissue | J Med Chem 36: 3707-20 (1994) BindingDB Entry DOI: 10.7270/Q2MK6DH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50042730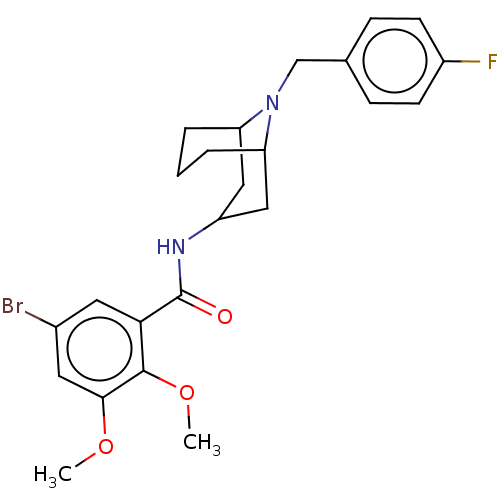 (4-Amino-5-chloro-N-[5-(4-fluoro-benzyl)-octahydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of [125I]- NCQ 298 binding to D2 receptor of rat striatal tissue | J Med Chem 36: 3707-20 (1994) BindingDB Entry DOI: 10.7270/Q2MK6DH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004808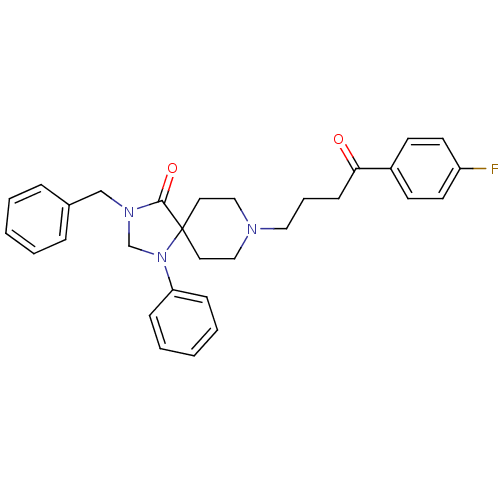 (3-Benzyl-8-[4-(4-fluoro-phenyl)-4-oxo-butyl]-1-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine receptor D2 | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004801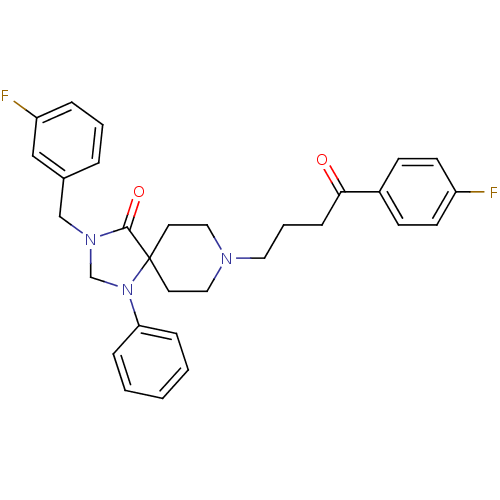 (3-(3-Fluoro-benzyl)-8-[4-(4-fluoro-phenyl)-4-oxo-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine receptor D2 | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50456207 (CHEMBL2112607) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of [125I]- NCQ 298 binding to D2 receptor of rat striatal tissue | J Med Chem 36: 3707-20 (1994) BindingDB Entry DOI: 10.7270/Q2MK6DH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004819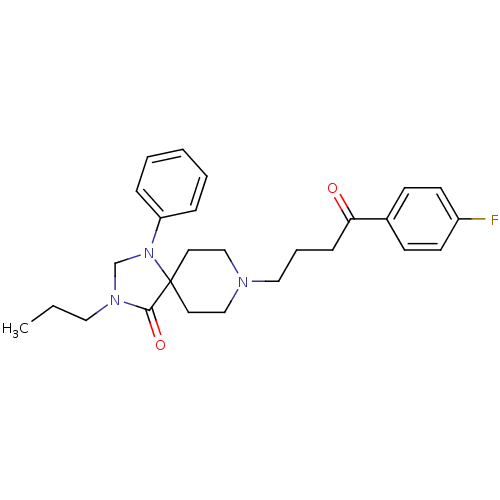 (8-[4-(4-Fluoro-phenyl)-4-oxo-butyl]-1-phenyl-3-pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine receptor D2 | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50475529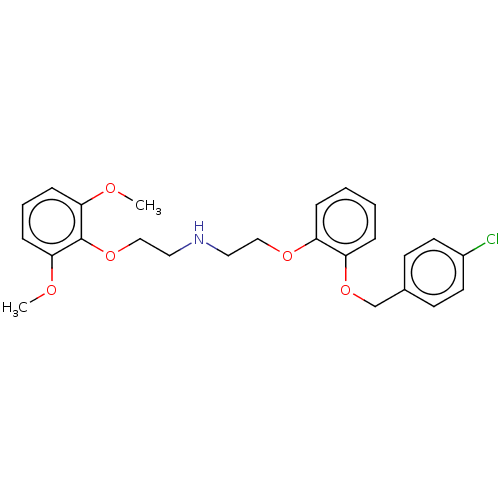 (Clopenphendioxan) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Camerino Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from cloned human ADRA1D expressed in CHO cells | J Med Chem 48: 7750-63 (2005) Article DOI: 10.1021/jm0580398 BindingDB Entry DOI: 10.7270/Q22V2JVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004817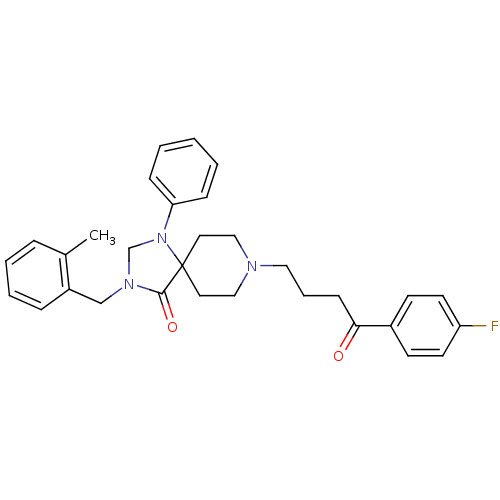 (8-[4-(4-Fluoro-phenyl)-4-oxo-butyl]-3-(2-methyl-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine receptor D2 | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50042733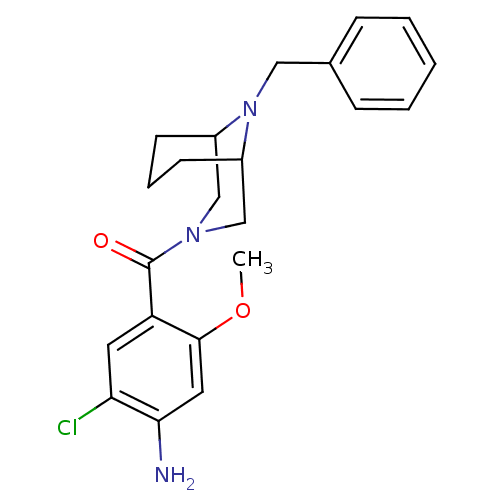 ((9-Benzyl-3,9-diaza-bicyclo[3.3.1]non-3-yl)-(5-bro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of [125I]- NCQ 298 binding to D2 receptor of rat striatal tissue | J Med Chem 36: 3707-20 (1994) BindingDB Entry DOI: 10.7270/Q2MK6DH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004803 (8-[4-(4-Fluoro-phenyl)-4-oxo-butyl]-3-phenethyl-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine receptor D2 | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50042732 (4-Amino-N-(9-benzyl-9-aza-bicyclo[3.3.1]non-3-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of [125I]- NCQ 298 binding to D2 receptor of rat striatal tissue | J Med Chem 36: 3707-20 (1994) BindingDB Entry DOI: 10.7270/Q2MK6DH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004805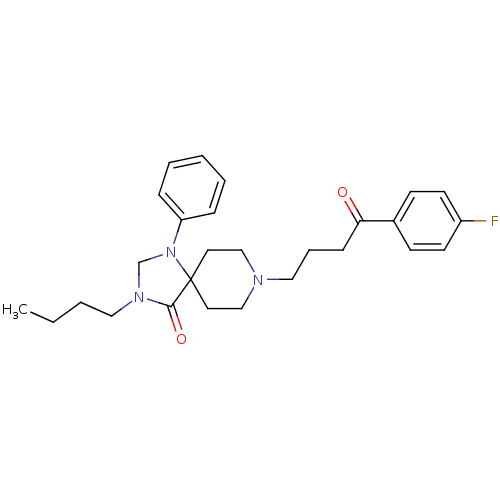 (3-Butyl-8-[4-(4-fluoro-phenyl)-4-oxo-butyl]-1-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine receptor D2 | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50456208 (CHEMBL2112605) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of [125I]- NCQ 298 binding to D2 receptor of rat striatal tissue | J Med Chem 36: 3707-20 (1994) BindingDB Entry DOI: 10.7270/Q2MK6DH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004807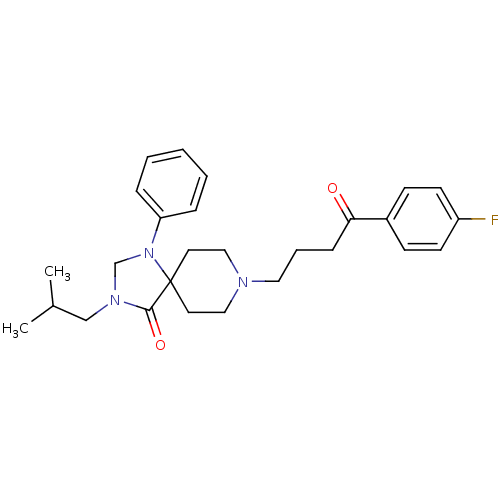 (8-[4-(4-Fluoro-phenyl)-4-oxo-butyl]-3-isobutyl-1-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine receptor D2 | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50220746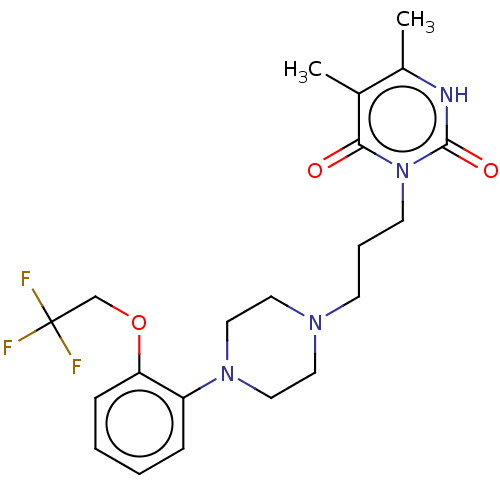 (CHEMBL292189) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description Inhibition of [3H]prazosin binding to CHO-K1 whole cells expressing human cloned Alpha-1A adrenergic receptor | Bioorg Med Chem Lett 13: 1873-8 (2003) BindingDB Entry DOI: 10.7270/Q2S46V50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50099821 (4-Bromo-1-methoxy-naphthalene-2-carboxylic acid (9...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wake Forest University School of Medicine Curated by ChEMBL | Assay Description Binding affinity for dopamine receptor D3 expressed in Sf9 cells using [125I]-IABN the radioligand. | J Med Chem 44: 1815-26 (2001) BindingDB Entry DOI: 10.7270/Q2NK3DBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50099807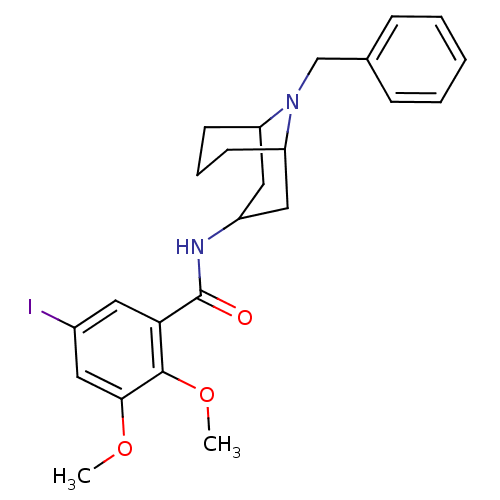 (CHEMBL54866 | N-(9-Benzyl-9-aza-bicyclo[3.3.1]non-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wake Forest University School of Medicine Curated by ChEMBL | Assay Description Binding affinity for dopamine receptor D3 expressed in Sf9 cells using [125I]-IABN the radioligand. | J Med Chem 44: 1815-26 (2001) BindingDB Entry DOI: 10.7270/Q2NK3DBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50049750 ((S)-3-(azetidin-2-ylmethoxy)pyridine | 3-((S)-1-Az...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Binding affinity to alpha4beta2 nAChR in human brain | J Med Chem 55: 9998-10009 (2012) Article DOI: 10.1021/jm301177j BindingDB Entry DOI: 10.7270/Q2CJ8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004816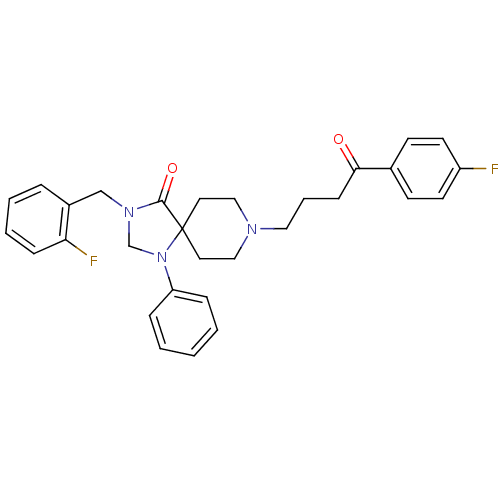 (3-(2-Fluoro-benzyl)-8-[4-(4-fluoro-phenyl)-4-oxo-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine receptor D2 | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004810 (8-[4-(4-Fluoro-phenyl)-4-oxo-butyl]-3-(2-methoxy-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 by displacing [3H]-spiperone | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50099807 (CHEMBL54866 | N-(9-Benzyl-9-aza-bicyclo[3.3.1]non-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wake Forest University School of Medicine Curated by ChEMBL | Assay Description Binding affinity for dopamine receptor D2 long expressed in Sf9 cells using [125I]-IABN radioligand. | J Med Chem 44: 1815-26 (2001) BindingDB Entry DOI: 10.7270/Q2NK3DBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50008735 ((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Texas Curated by PDSP Ki Database | Synapse 38: 438-49 (2000) Article DOI: 10.1002/1098-2396(20001215)38:4 BindingDB Entry DOI: 10.7270/Q2SN07HX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50475534 (CHEMBL198860) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Camerino Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from cloned human ADRA1D expressed in CHO cells | J Med Chem 48: 7750-63 (2005) Article DOI: 10.1021/jm0580398 BindingDB Entry DOI: 10.7270/Q22V2JVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50475537 (CHEMBL196817) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Camerino Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from cloned human ADRA1D expressed in CHO cells | J Med Chem 48: 7750-63 (2005) Article DOI: 10.1021/jm0580398 BindingDB Entry DOI: 10.7270/Q22V2JVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004818 (3-Ethyl-8-[4-(4-fluoro-phenyl)-4-oxo-butyl]-1-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 by displacing [3H]-spiperone | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine receptor D2 | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Displacement of [125I]ABN from human dopamine D2 long receptor expressed in HEK293 cell membranes after 60 mins by scintillation counting method | J Med Chem 60: 9905-9910 (2017) Article DOI: 10.1021/acs.jmedchem.7b01248 BindingDB Entry DOI: 10.7270/Q21838X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Displacement of [125I]IABN from recombinant human D2 long receptor stably expressed in HEK293 cell membranes measured after 60 mins by scintillation ... | J Med Chem 62: 5132-5147 (2019) Article DOI: 10.1021/acs.jmedchem.9b00412 BindingDB Entry DOI: 10.7270/Q2Z03CM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004802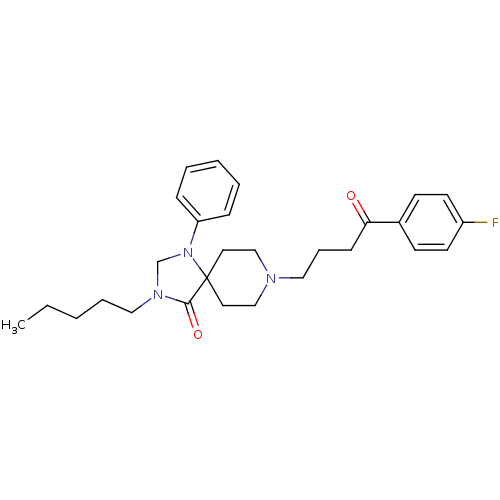 (8-[4-(4-Fluoro-phenyl)-4-oxo-butyl]-3-pentyl-1-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine receptor D2 | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004804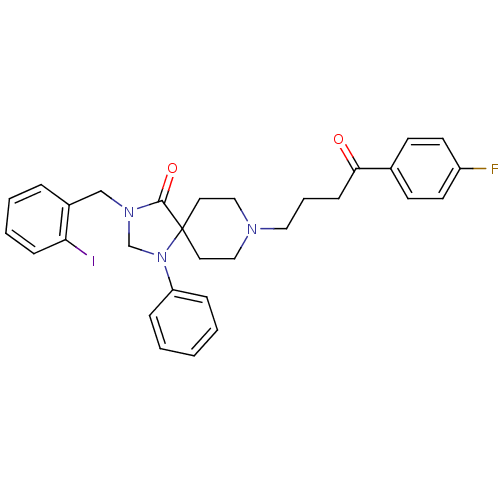 (8-[4-(4-Fluoro-phenyl)-4-oxo-butyl]-3-(2-iodo-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 by displacing [3H]-spiperone | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004815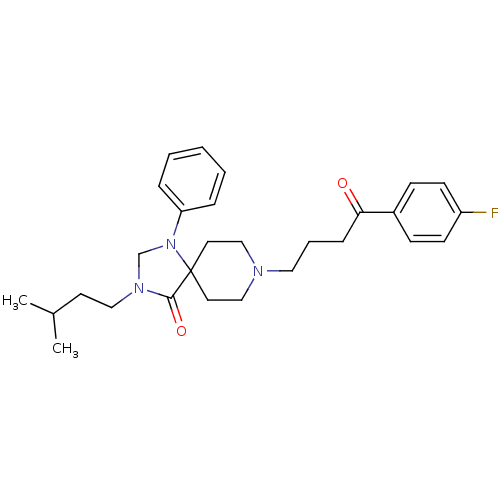 (8-[4-(4-Fluoro-phenyl)-4-oxo-butyl]-3-(3-methyl-bu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine receptor D2 | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50220747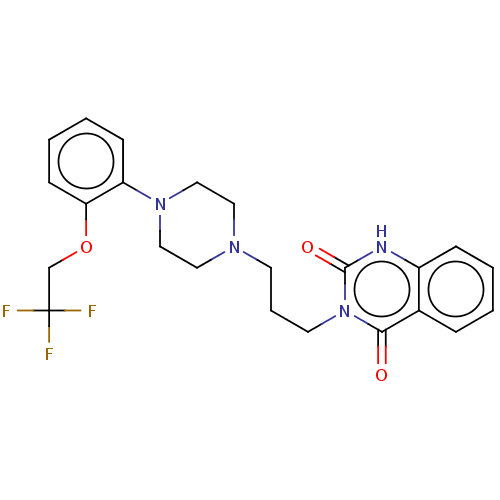 (CHEMBL56863) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description Inhibition of [3H]prazosin binding to CHO-K1 whole cells expressing human cloned Alpha-1A adrenergic receptor | Bioorg Med Chem Lett 13: 1873-8 (2003) BindingDB Entry DOI: 10.7270/Q2S46V50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50475532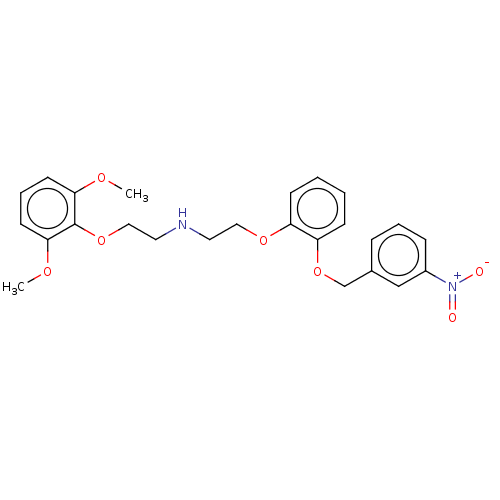 (CHEMBL197442) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Camerino Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from cloned human ADRA1D expressed in CHO cells | J Med Chem 48: 7750-63 (2005) Article DOI: 10.1021/jm0580398 BindingDB Entry DOI: 10.7270/Q22V2JVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50475538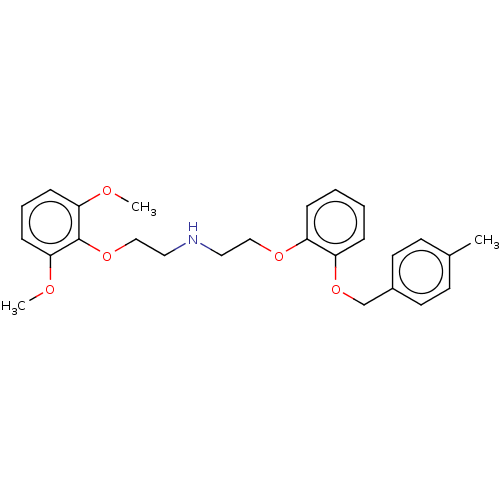 (CHEMBL200366) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Camerino Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from cloned human ADRA1D expressed in CHO cells | J Med Chem 48: 7750-63 (2005) Article DOI: 10.1021/jm0580398 BindingDB Entry DOI: 10.7270/Q22V2JVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004806 (8-[4-(4-Fluoro-phenyl)-4-oxo-butyl]-3-(4-methyl-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 by displacing [3H]-spiperone | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50416853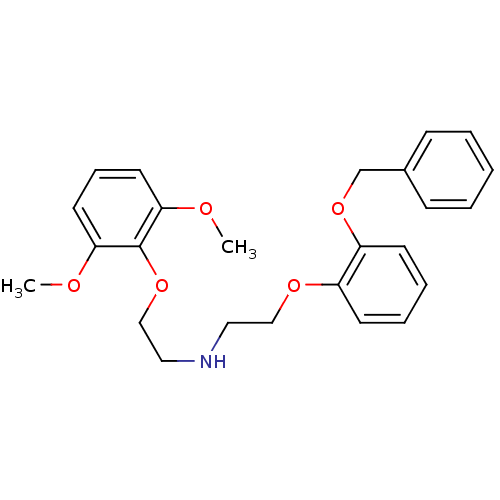 (CHEMBL43116) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Camerino Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from cloned human ADRA1D expressed in CHO cells | J Med Chem 48: 7750-63 (2005) Article DOI: 10.1021/jm0580398 BindingDB Entry DOI: 10.7270/Q22V2JVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004800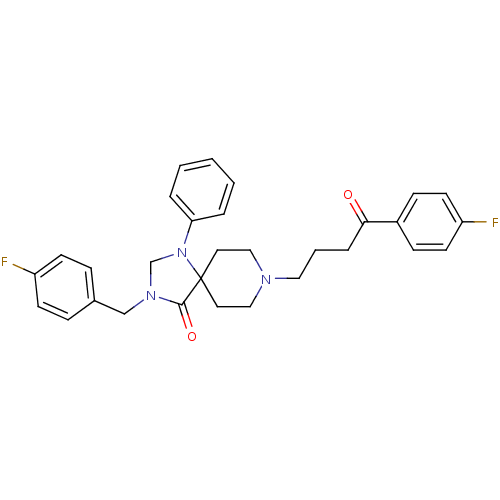 (3-(4-Fluoro-benzyl)-8-[4-(4-fluoro-phenyl)-4-oxo-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine receptor D2 | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc. Curated by PDSP Ki Database | J Pharmacol Exp Ther 312: 619-26 (2005) Article DOI: 10.1124/jpet.104.075069 BindingDB Entry DOI: 10.7270/Q2BK19XS | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50349871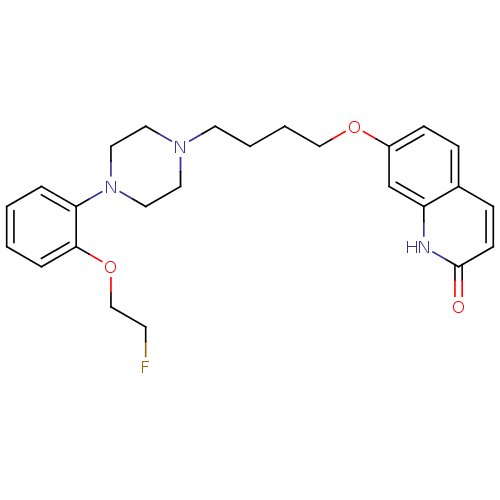 (CHEMBL1813595) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Displacement of [125I]ABN from human recombinant D2L receptor expressed in HEK cells after 60 mins by gamma counter | Bioorg Med Chem 19: 3502-11 (2011) Article DOI: 10.1016/j.bmc.2011.04.021 BindingDB Entry DOI: 10.7270/Q25X2990 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50042731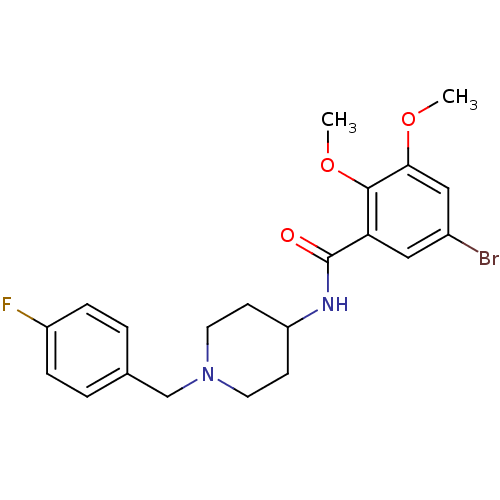 (5-Bromo-N-[1-(4-fluoro-benzyl)-piperidin-4-yl]-2,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of [125I]- NCQ 298 binding to D2 receptor of rat striatal tissue | J Med Chem 36: 3707-20 (1994) BindingDB Entry DOI: 10.7270/Q2MK6DH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50408198 (CHEMBL91278) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50512416 (CHEMBL4447162) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human P2Y14 receptor expressed in CHO cells by fluorescence assay | Eur J Med Chem 175: 34-39 (2019) Article DOI: 10.1016/j.ejmech.2019.04.068 BindingDB Entry DOI: 10.7270/Q29C71R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004812 (8-[4-(4-Fluoro-phenyl)-4-oxo-butyl]-3-(4-methoxy-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 by displacing [3H]-spiperone | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50454822 (CHEMBL2062141 | L-370518) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin | J Med Chem 40: 830-2 (1997) Article DOI: 10.1021/jm960762y BindingDB Entry DOI: 10.7270/Q25H7GXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50004819 (8-[4-(4-Fluoro-phenyl)-4-oxo-butyl]-1-phenyl-3-pro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description The compound was tested in vitro for binding affinity towards 5-hydroxytryptamine 2 receptor by displacing [125]I-LSD radioligand | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-5 (RAT) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc. Curated by PDSP Ki Database | J Pharmacol Exp Ther 312: 619-26 (2005) Article DOI: 10.1124/jpet.104.075069 BindingDB Entry DOI: 10.7270/Q2BK19XS | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 15795 total ) | Next | Last >> |