

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin S (Homo sapiens (Human)) | BDBM122478 (US8729061, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.296 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US8729061 (2014) BindingDB Entry DOI: 10.7270/Q2MS3RF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM129694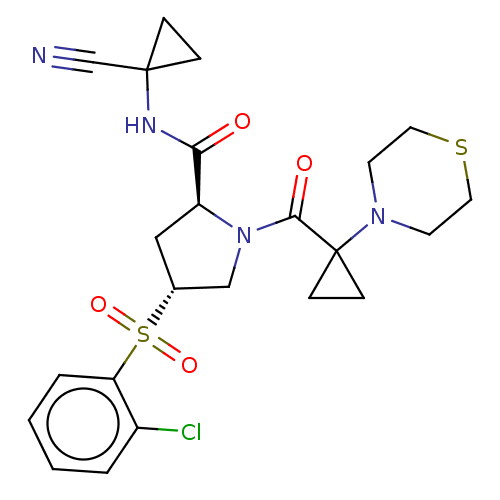 (US8802665, 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Genentech, Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US8802665 (2014) BindingDB Entry DOI: 10.7270/Q2K64GR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM129710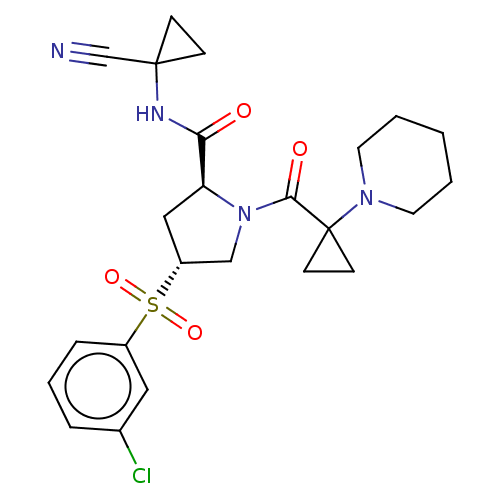 (US8802665, 43) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Genentech, Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US8802665 (2014) BindingDB Entry DOI: 10.7270/Q2K64GR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM240629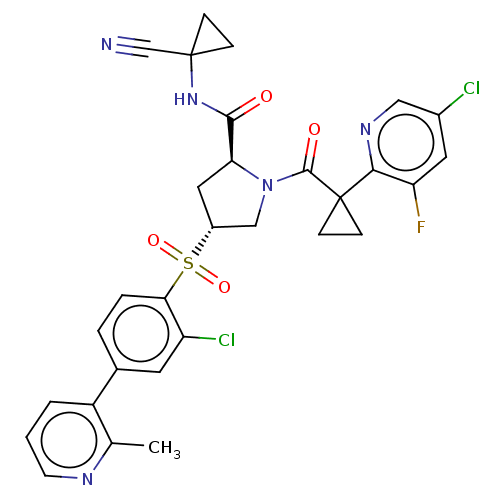 (US9409882, 34) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.322 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9409882 (2016) BindingDB Entry DOI: 10.7270/Q2B56HNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM122498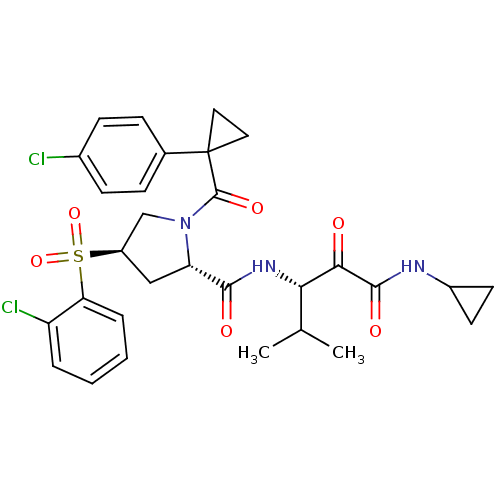 (US8729061, 28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.328 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US8729061 (2014) BindingDB Entry DOI: 10.7270/Q2MS3RF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM122508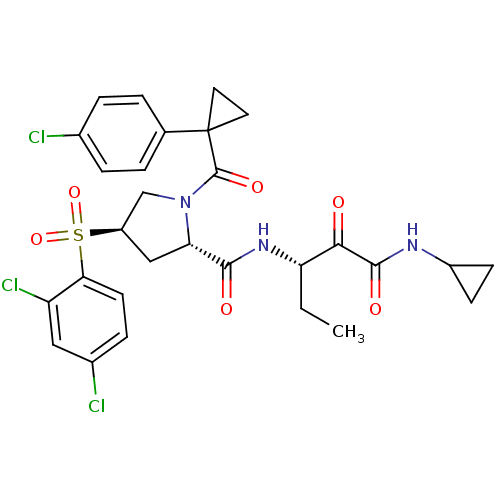 (US8729061, 38) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US8729061 (2014) BindingDB Entry DOI: 10.7270/Q2MS3RF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM447439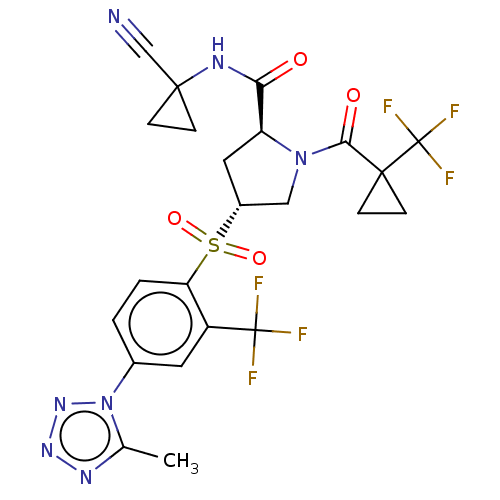 ((2S,4R)-4-[4-(5-Methyl-tetrazol-1-yl)-2-trifluorom...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.349 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US10689339 (2020) BindingDB Entry DOI: 10.7270/Q29Z97ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM122479 (US8729061, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.367 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US8729061 (2014) BindingDB Entry DOI: 10.7270/Q2MS3RF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM240628 (US9409882, 33) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.375 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9409882 (2016) BindingDB Entry DOI: 10.7270/Q2B56HNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM130419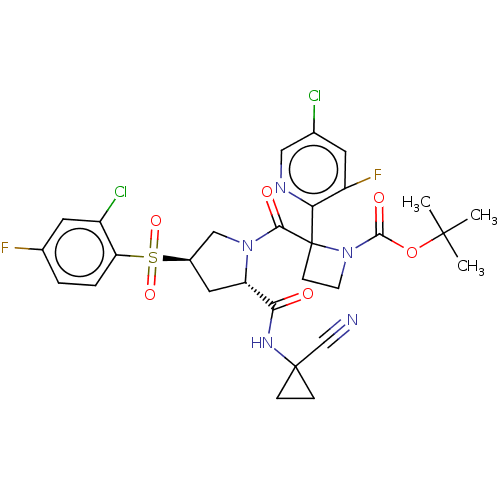 (US8822505, 21 | US8822505, 23 | US8822505, 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.377 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Genentech, Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US8822505 (2014) BindingDB Entry DOI: 10.7270/Q2CF9NTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM240631 (US9409882, 36) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.382 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9409882 (2016) BindingDB Entry DOI: 10.7270/Q2B56HNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM447446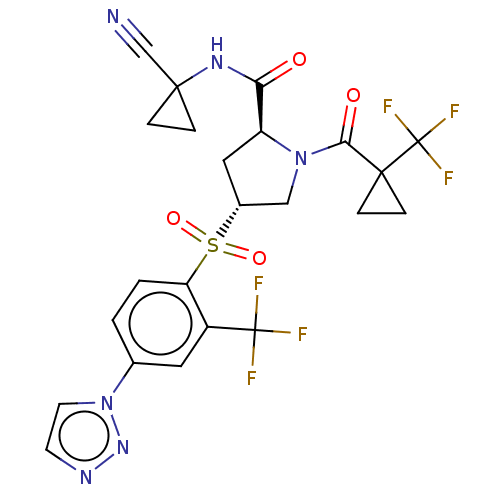 ((2S,4R)-4-(4-[1,2,3]Triazol-1-yl-2-trifluoromethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.382 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US10689339 (2020) BindingDB Entry DOI: 10.7270/Q29Z97ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM447418 ((2S,4R)-4-(4-(1H-1,2,4-triazol-1-yl)-2-(trifluorom...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.391 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US10689339 (2020) BindingDB Entry DOI: 10.7270/Q29Z97ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM240599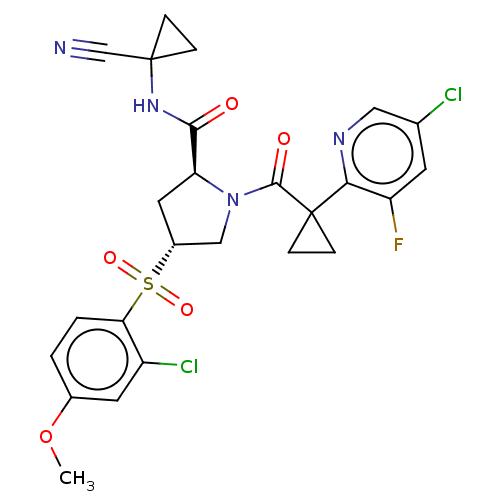 (US9409882, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.393 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9409882 (2016) BindingDB Entry DOI: 10.7270/Q2B56HNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM240600 (US9409882, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9409882 (2016) BindingDB Entry DOI: 10.7270/Q2B56HNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM129709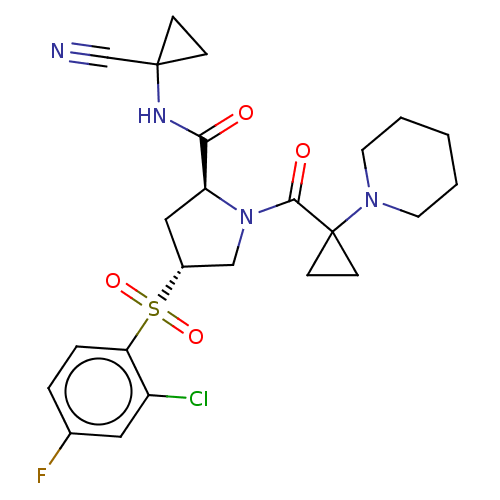 (US8802665, 42) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Genentech, Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US8802665 (2014) BindingDB Entry DOI: 10.7270/Q2K64GR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM129711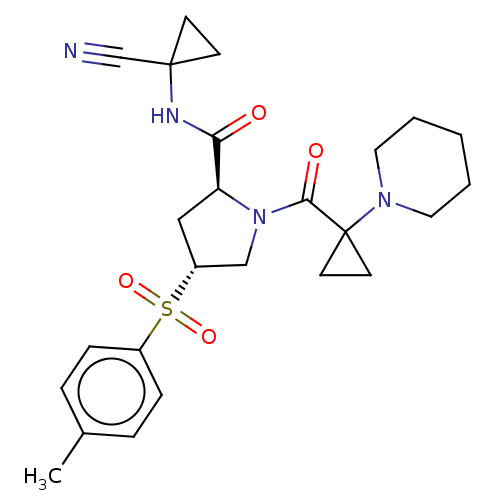 (US8802665, 44) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Genentech, Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US8802665 (2014) BindingDB Entry DOI: 10.7270/Q2K64GR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM129713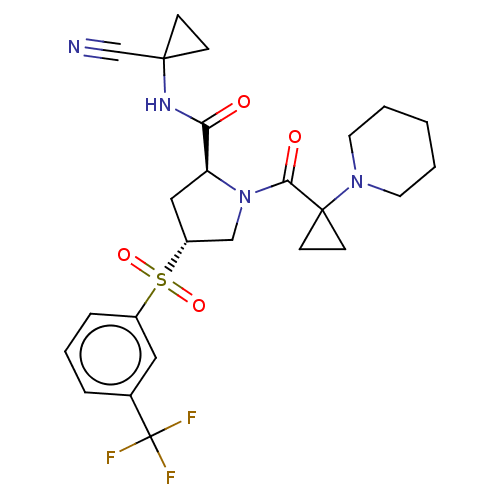 (US8802665, 46) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Genentech, Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US8802665 (2014) BindingDB Entry DOI: 10.7270/Q2K64GR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM129693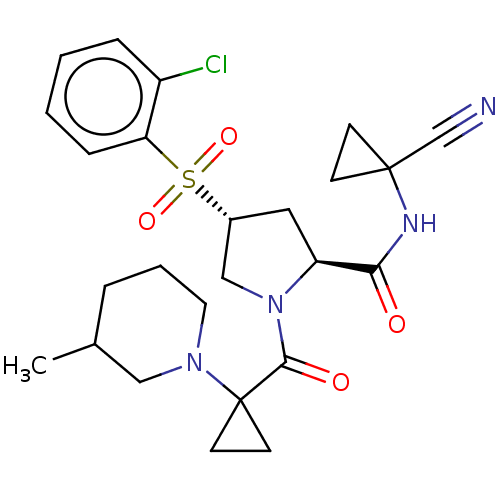 (US8802665, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Genentech, Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US8802665 (2014) BindingDB Entry DOI: 10.7270/Q2K64GR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM122495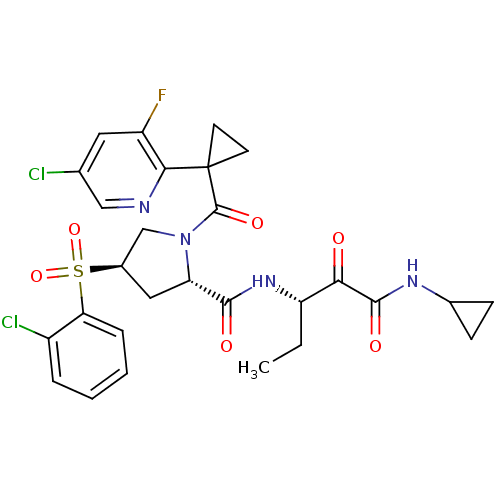 (US8729061, 25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.403 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US8729061 (2014) BindingDB Entry DOI: 10.7270/Q2MS3RF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM240603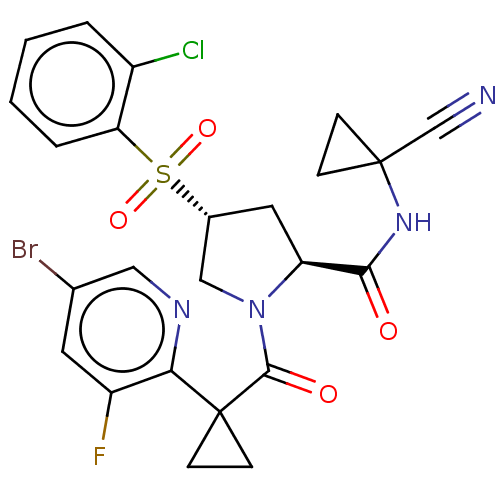 (US9409882, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.438 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9409882 (2016) BindingDB Entry DOI: 10.7270/Q2B56HNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM122486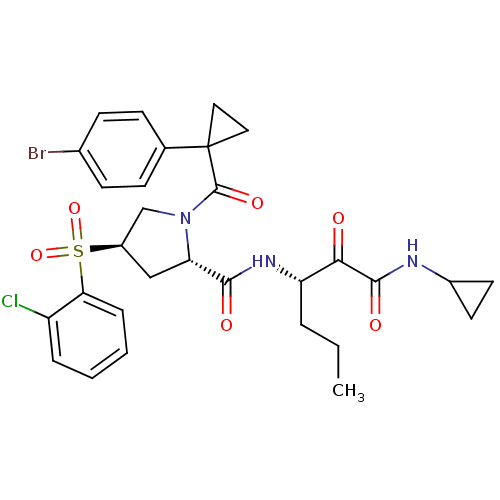 (US8729061, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.442 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US8729061 (2014) BindingDB Entry DOI: 10.7270/Q2MS3RF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM240625 (US9409882, 30) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.474 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9409882 (2016) BindingDB Entry DOI: 10.7270/Q2B56HNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM122528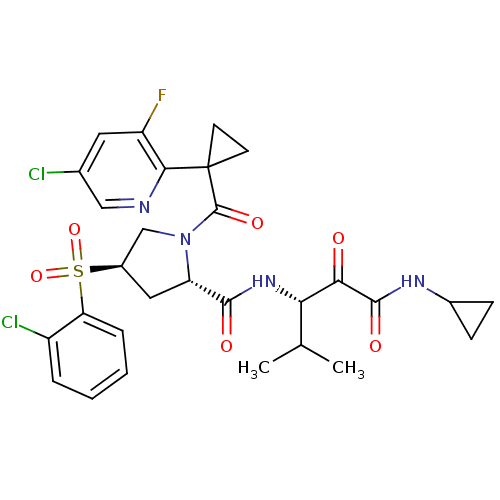 (US8729061, 58) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.478 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US8729061 (2014) BindingDB Entry DOI: 10.7270/Q2MS3RF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM447431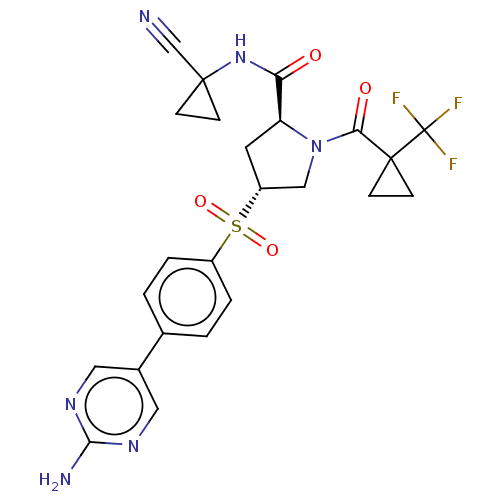 ((2S,4R)-4-(4-(2-aminopyrimidin-5-yl)phenylsulfonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.483 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US10689339 (2020) BindingDB Entry DOI: 10.7270/Q29Z97ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM447444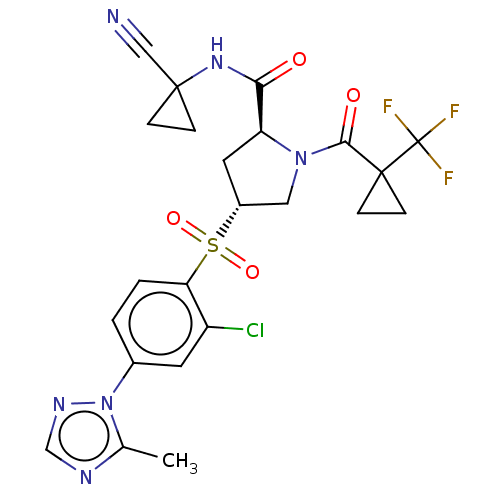 ((2S,4R)-4-(2-chloro-4-(5-methyl-1H-1,2,4-triazol-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.486 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US10689339 (2020) BindingDB Entry DOI: 10.7270/Q29Z97ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM240597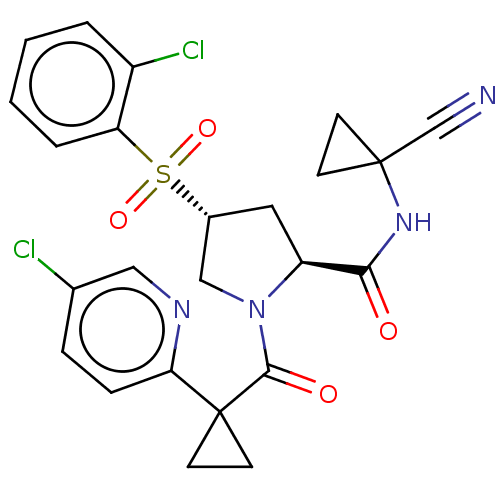 (US9409882, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.492 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9409882 (2016) BindingDB Entry DOI: 10.7270/Q2B56HNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM130420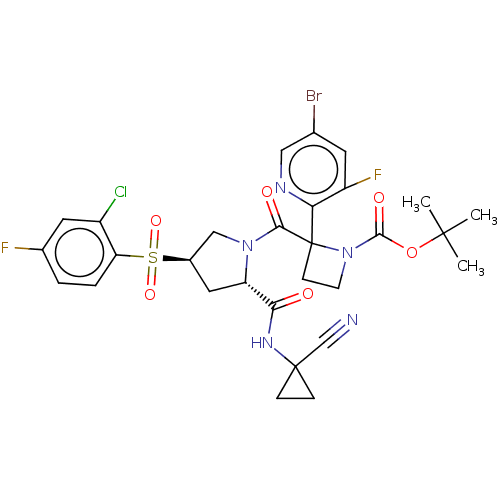 (US8822505, 22 | US8822505, 25 | US8822505, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.492 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Genentech, Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US8822505 (2014) BindingDB Entry DOI: 10.7270/Q2CF9NTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50309502 (CHEMBL591279 | rac-3-(3-(fluoromethyl)phenyl)-9,10...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of DPP4 | Bioorg Med Chem Lett 20: 1106-8 (2010) Article DOI: 10.1016/j.bmcl.2009.12.025 BindingDB Entry DOI: 10.7270/Q2SJ1KQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM129692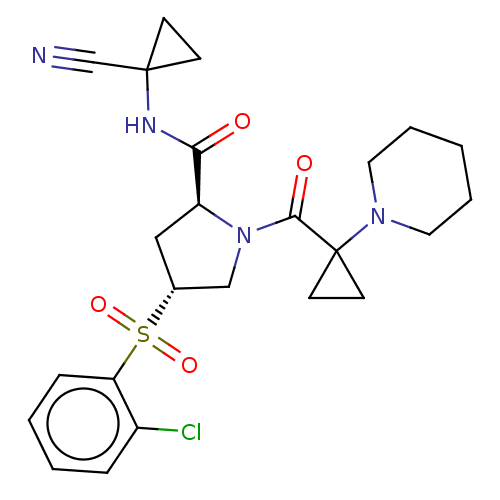 (US8802665, 25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Genentech, Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US8802665 (2014) BindingDB Entry DOI: 10.7270/Q2K64GR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM129707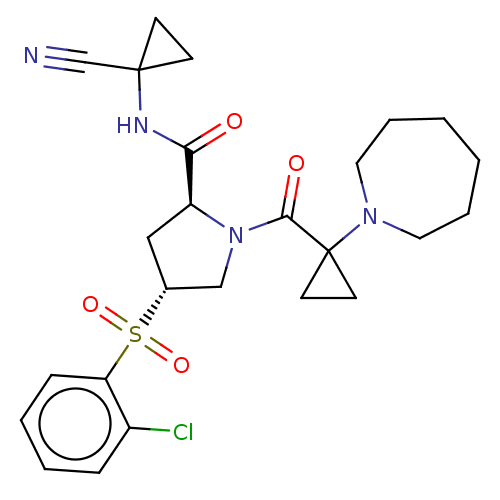 (US8802665, 40) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Genentech, Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US8802665 (2014) BindingDB Entry DOI: 10.7270/Q2K64GR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM129705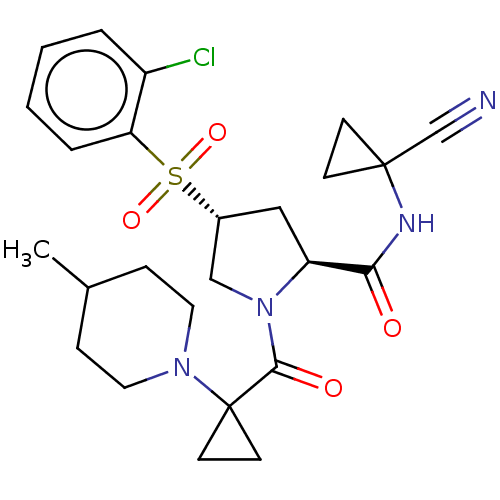 (US8802665, 38) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Genentech, Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US8802665 (2014) BindingDB Entry DOI: 10.7270/Q2K64GR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM240626 (US9409882, 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.505 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9409882 (2016) BindingDB Entry DOI: 10.7270/Q2B56HNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM447445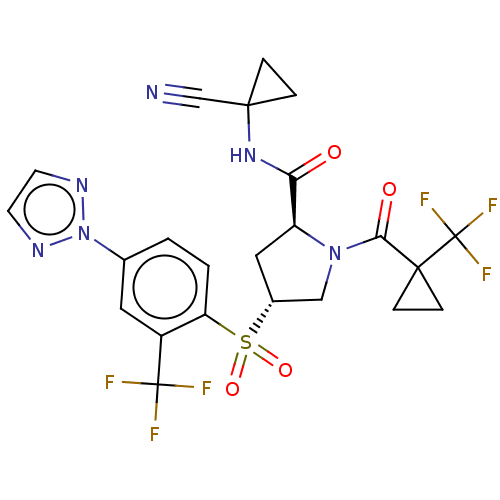 ((2S,4R)-4-(4-[1,2,3]Triazol-2-yl-2-trifluoromethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.506 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US10689339 (2020) BindingDB Entry DOI: 10.7270/Q29Z97ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM122513 (US8729061, 43) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.508 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US8729061 (2014) BindingDB Entry DOI: 10.7270/Q2MS3RF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM447436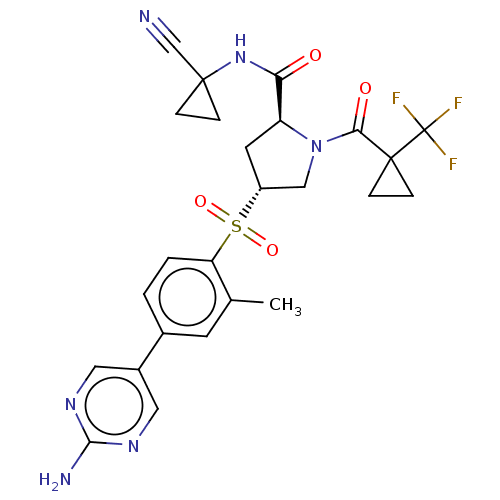 ((2S,4R)-4-(4-(2-aminopyrimidin-5-yl)-2-methylpheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US10689339 (2020) BindingDB Entry DOI: 10.7270/Q29Z97ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM122485 (US8729061, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US8729061 (2014) BindingDB Entry DOI: 10.7270/Q2MS3RF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM130414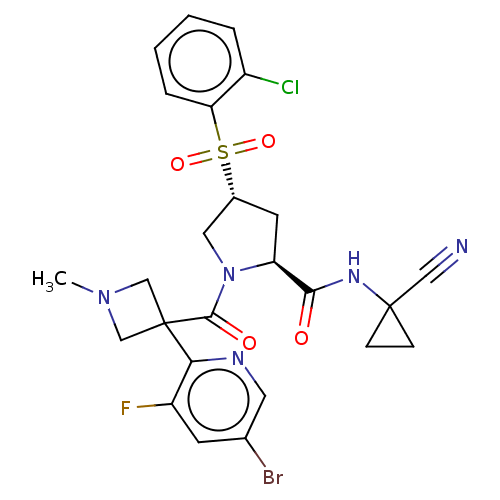 (US8822505, 14 | US8822505, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.525 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Genentech, Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US8822505 (2014) BindingDB Entry DOI: 10.7270/Q2CF9NTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM447448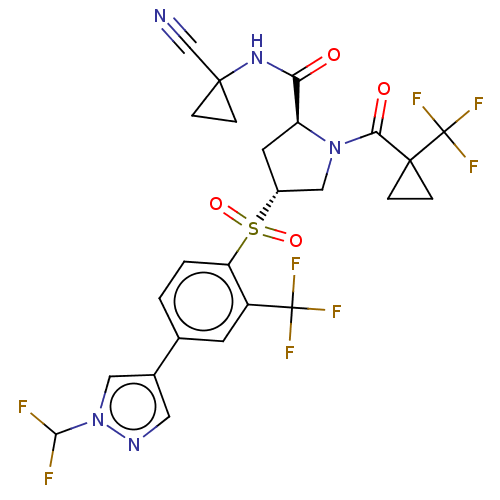 ((2S,4R)-4-[4-(1-Difluoromethyl-1Hpyrazol-4-yl)-2-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.526 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US10689339 (2020) BindingDB Entry DOI: 10.7270/Q29Z97ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM122518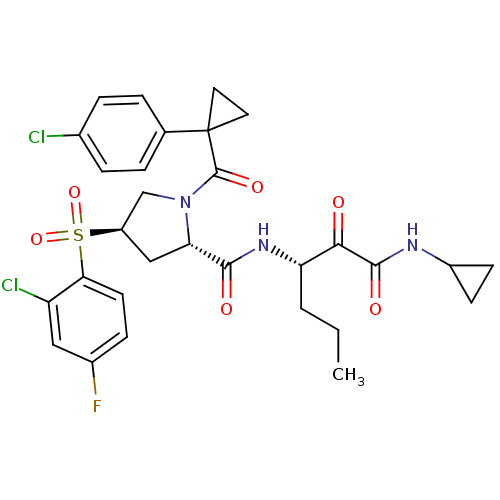 (US8729061, 48) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.527 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US8729061 (2014) BindingDB Entry DOI: 10.7270/Q2MS3RF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM447360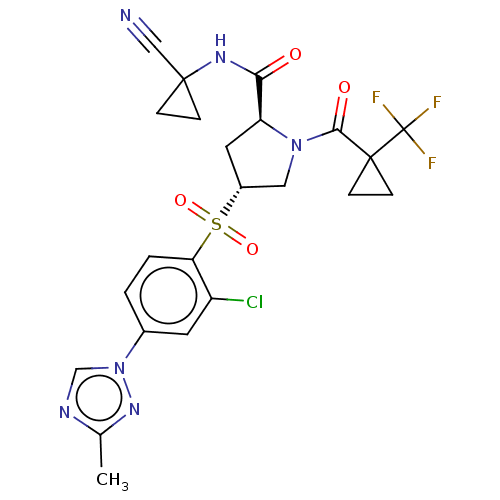 ((2S,4R)-4-(2-chloro-4-(3-methyl-1H-1,2,4-triazol-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.529 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US10689339 (2020) BindingDB Entry DOI: 10.7270/Q29Z97ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM447313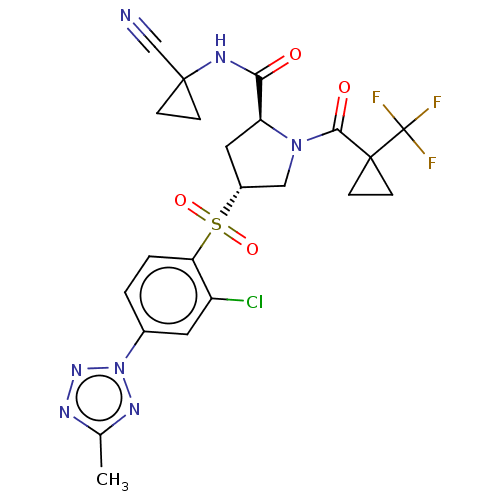 ((2S,4R)-4-(2-chloro-4-(5-methyl-2H-tetrazol-2-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.533 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US10689339 (2020) BindingDB Entry DOI: 10.7270/Q29Z97ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM447423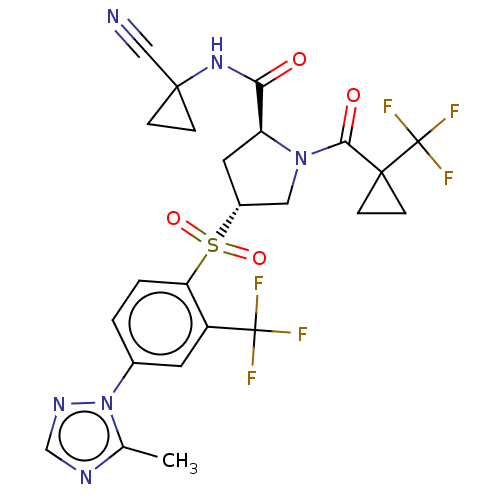 ((2S,4R)óN-(1-cyanocyclopropyl)-4-(4-(5-methyl-1H-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US10689339 (2020) BindingDB Entry DOI: 10.7270/Q29Z97ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM240623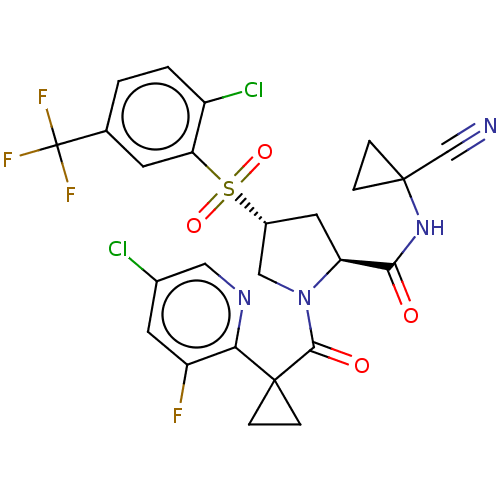 (US9409882, 28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.543 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9409882 (2016) BindingDB Entry DOI: 10.7270/Q2B56HNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM122494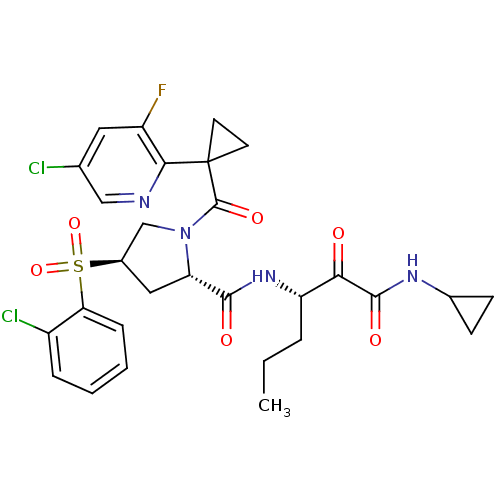 (US8729061, 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US8729061 (2014) BindingDB Entry DOI: 10.7270/Q2MS3RF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM447419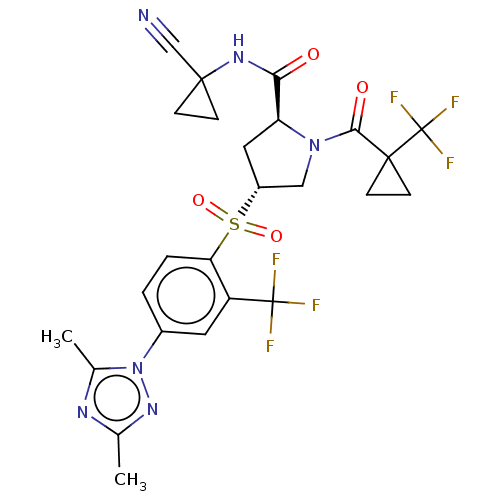 ((2S,4R)-4-[4-(3,5-Dimethyl-[1,2,4]triazol-1-yl)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.558 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US10689339 (2020) BindingDB Entry DOI: 10.7270/Q29Z97ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM122509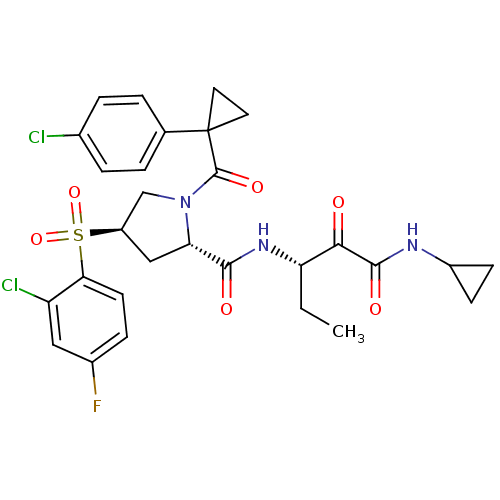 (US8729061, 39) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.558 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US8729061 (2014) BindingDB Entry DOI: 10.7270/Q2MS3RF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM122490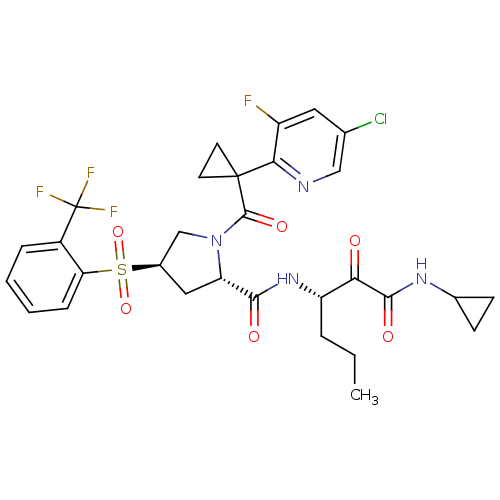 (US8729061, 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US8729061 (2014) BindingDB Entry DOI: 10.7270/Q2MS3RF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM447340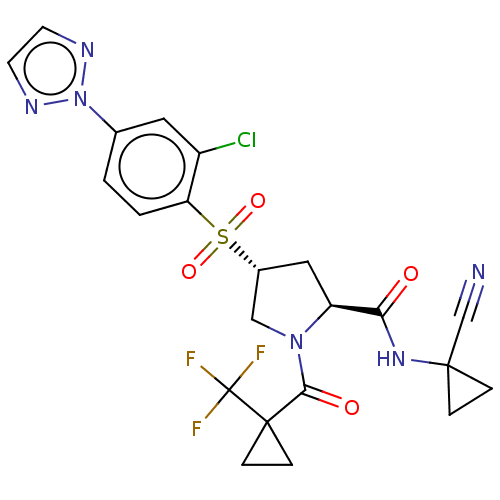 ((2S,4R)-4-(2-chloro-4-(2H-1,2,3-triazol-2-yl)pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US10689339 (2020) BindingDB Entry DOI: 10.7270/Q29Z97ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM447408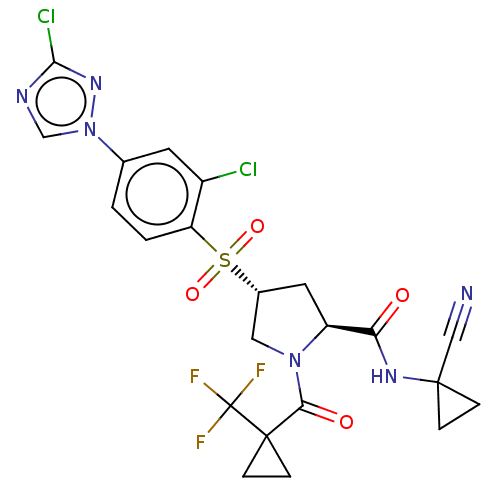 ((2S,4R)-4-(2-chloro-4-(3-chloro-1H-1,2,4-triazol-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.563 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US10689339 (2020) BindingDB Entry DOI: 10.7270/Q29Z97ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 850 total ) | Next | Last >> |