

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine protease 1 (Bos taurus (bovine)) | BDBM50124947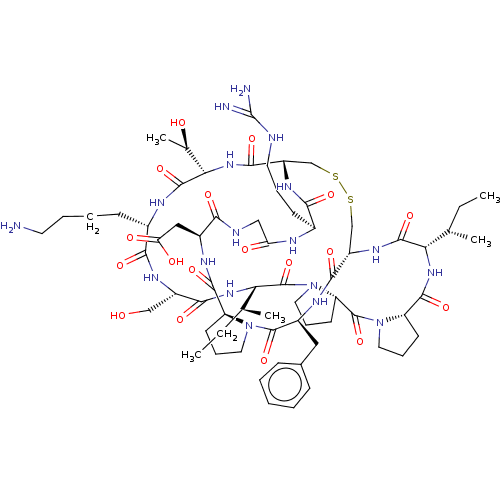 (CHEMBL453539) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanyang Technological University Curated by ChEMBL | Assay Description Inhibition of bovine beta-trypsin type-3 using L-BAPNA as substrate preincubated for 5 mins followed by substrate addition measured over 60 mins | J Med Chem 60: 504-510 (2017) Article DOI: 10.1021/acs.jmedchem.6b01011 BindingDB Entry DOI: 10.7270/Q2RJ4MRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50124947 (CHEMBL453539) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanyang Technological University Curated by ChEMBL | Assay Description Inhibition of bovine beta-trypsin using N-t-Boc Gln-Ala-Arg-AMC as substrate preincubated with enzyme followed by substrate addition by Dixon plot an... | J Med Chem 60: 504-510 (2017) Article DOI: 10.1021/acs.jmedchem.6b01011 BindingDB Entry DOI: 10.7270/Q2RJ4MRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50124947 (CHEMBL453539) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanyang Technological University Curated by ChEMBL | Assay Description Inhibition of bovine beta-trypsin using BAPNA as substrate preincubated for 15 mins followed by substrate addition measured over 60 mins by Dixon plo... | J Med Chem 60: 504-510 (2017) Article DOI: 10.1021/acs.jmedchem.6b01011 BindingDB Entry DOI: 10.7270/Q2RJ4MRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50233907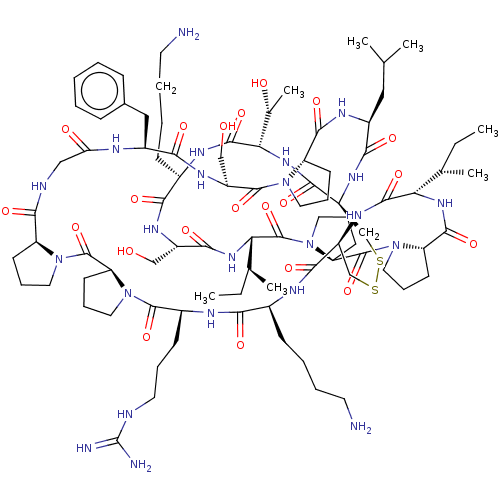 (CHEMBL4102547) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanyang Technological University Curated by ChEMBL | Assay Description Inhibition of bovine beta-trypsin using BAPNA as substrate preincubated for 15 mins followed by substrate addition measured over 60 mins by Dixon plo... | J Med Chem 60: 504-510 (2017) Article DOI: 10.1021/acs.jmedchem.6b01011 BindingDB Entry DOI: 10.7270/Q2RJ4MRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50124947 (CHEMBL453539) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanyang Technological University Curated by ChEMBL | Assay Description Inhibition of bovine beta-trypsin | J Med Chem 60: 504-510 (2017) Article DOI: 10.1021/acs.jmedchem.6b01011 BindingDB Entry DOI: 10.7270/Q2RJ4MRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Salicylate synthetase, Irp9 (Yersinia enterocolitica) | BDBM50030314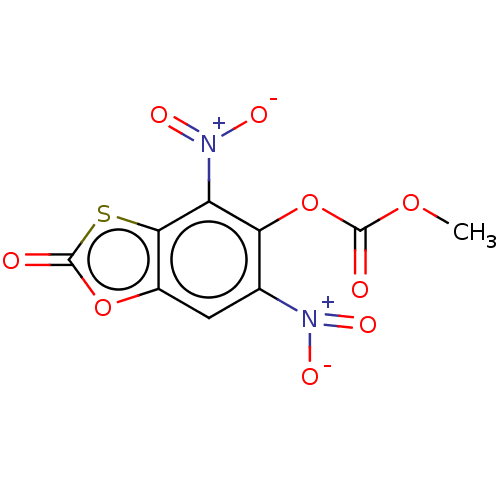 (CHEMBL3343825) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Competitive inhibition of Yersinia enterocolitica Irp9 using chorismate as substrate | Bioorg Med Chem 22: 5961-9 (2014) Article DOI: 10.1016/j.bmc.2014.09.010 BindingDB Entry DOI: 10.7270/Q2PC340D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional chorismate mutase/prephenate dehydratase (Escherichia coli (strain K12)) | BDBM96194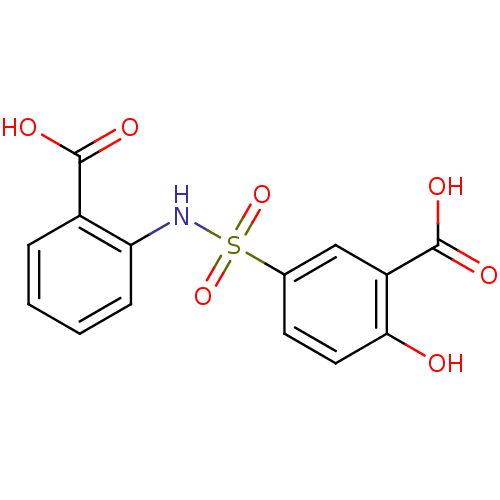 (5-[(2-carboxyphenyl)sulfamoyl]-2-hydroxy-benzoic a...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Competitive inhibition of Escherichia coli K-12 EcCM expressed in Escherichia coli BL21 Star (DE3) pLysS using chorismate as substrate | Bioorg Med Chem 22: 5961-9 (2014) Article DOI: 10.1016/j.bmc.2014.09.010 BindingDB Entry DOI: 10.7270/Q2PC340D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional chorismate mutase/prephenate dehydratase (Escherichia coli (strain K12)) | BDBM50030314 (CHEMBL3343825) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Competitive inhibition of Escherichia coli K-12 EcCM expressed in Escherichia coli BL21 Star (DE3) pLysS using chorismate as substrate | Bioorg Med Chem 22: 5961-9 (2014) Article DOI: 10.1016/j.bmc.2014.09.010 BindingDB Entry DOI: 10.7270/Q2PC340D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570745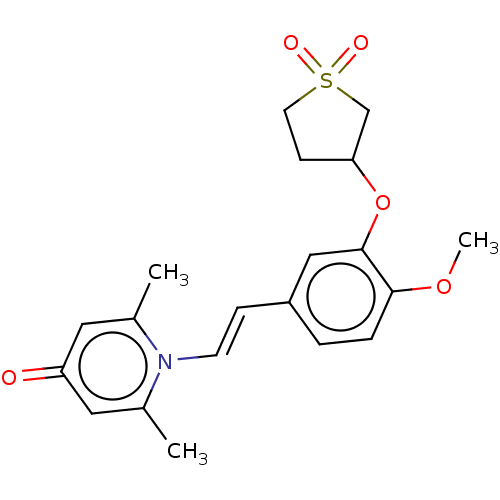 (US11440886, Example 81 | US11440886, Example 87) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570694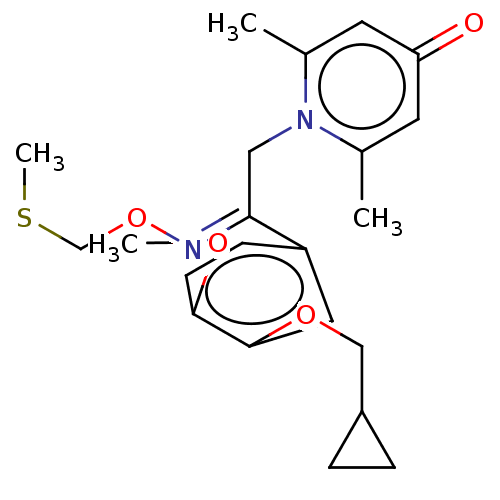 (US11440886, Example 41) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570762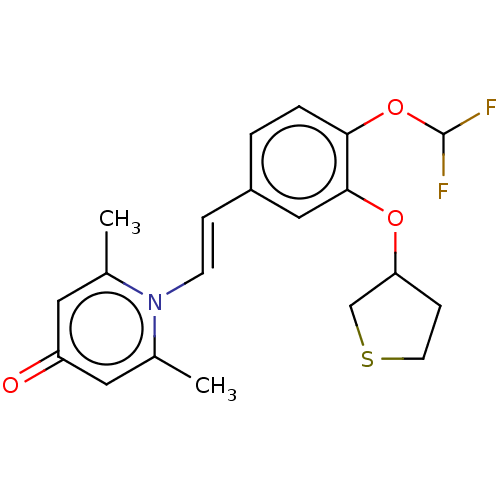 (US11440886, Example 100 | US11440886, Example 96) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570783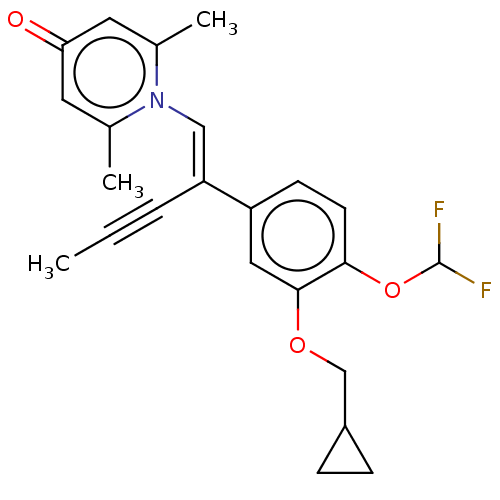 (US11440886, Example 114) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570762 (US11440886, Example 100 | US11440886, Example 96) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570775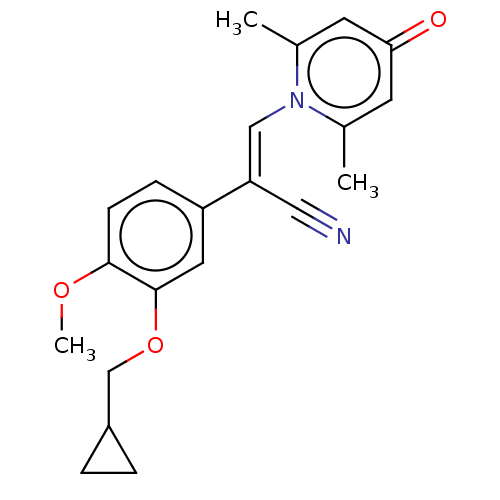 (US11440886, Example 106) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570754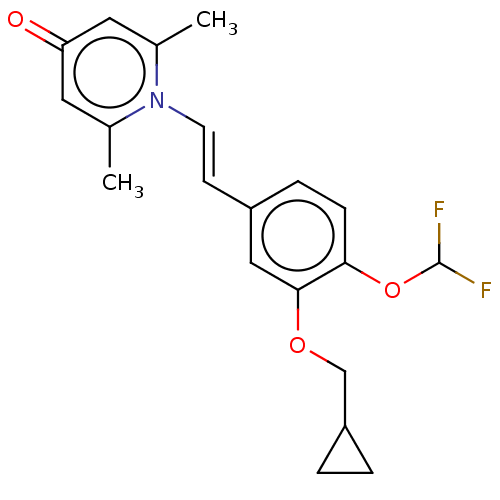 (US11440886, Example 88) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570708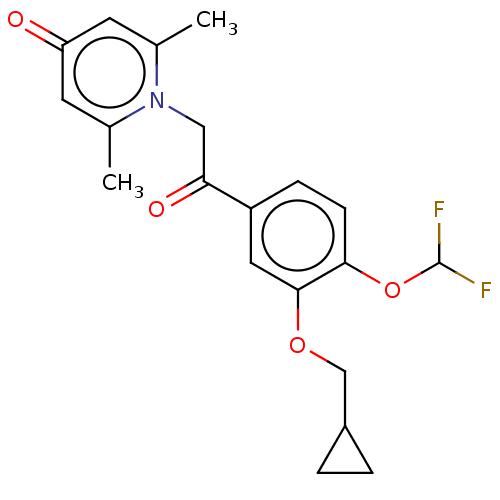 (US11440886, Example 49) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570774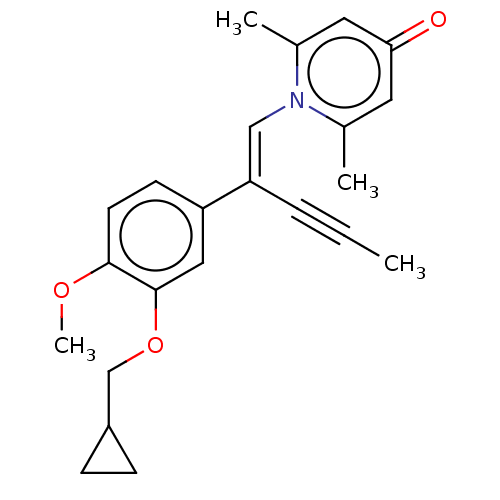 (US11440886, Example 105) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570763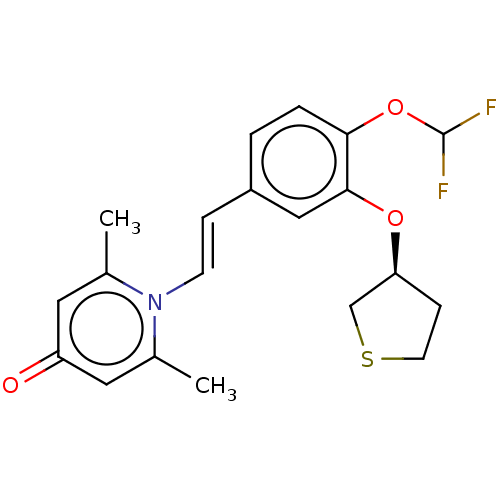 (US11440886, Example 97) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570744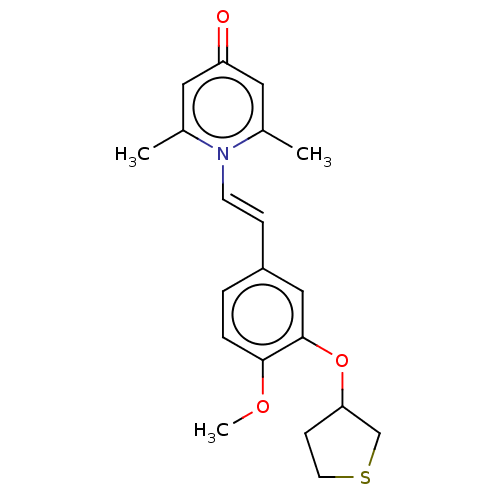 (US11440886, Example 80 | US11440886, Example 85) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4626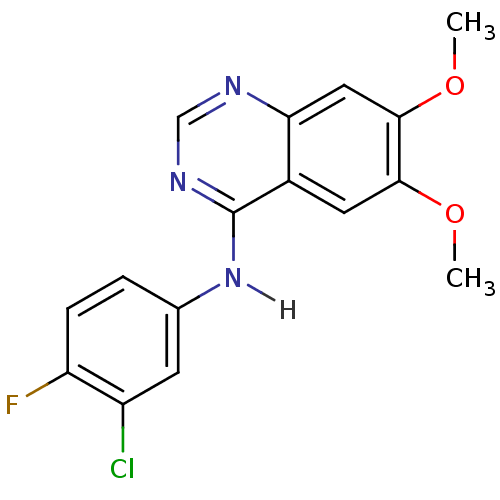 (Anilinoquinazoline deriv. 9 | CHEMBL301018 | N-(3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of EGFR (unknown origin) using biotinylated-PTP1B (Tyr66) as substrate incubated for 5 mins prior to substrate addition measured after 1 h... | Eur J Med Chem 61: 84-94 (2013) Article DOI: 10.1016/j.ejmech.2012.07.036 BindingDB Entry DOI: 10.7270/Q2X068CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570756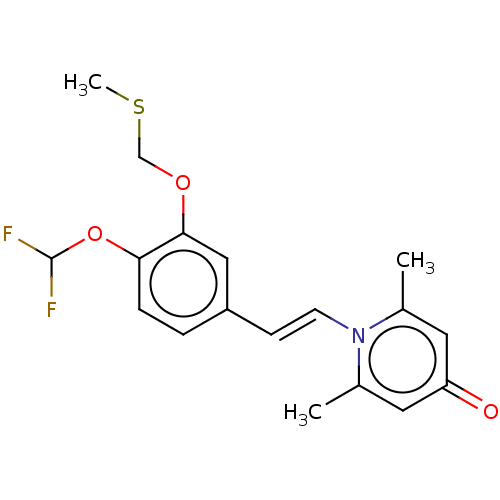 (US11440886, Example 90) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570652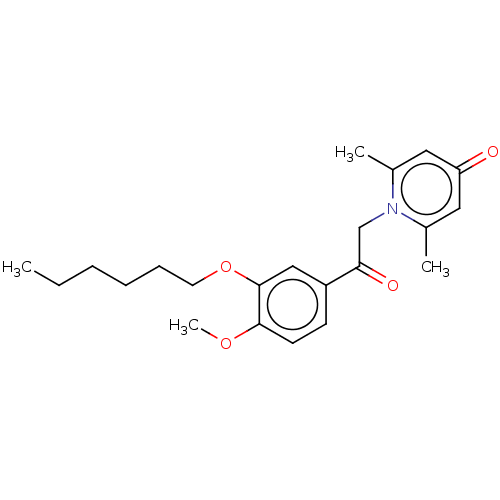 (US11440886, Example 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570655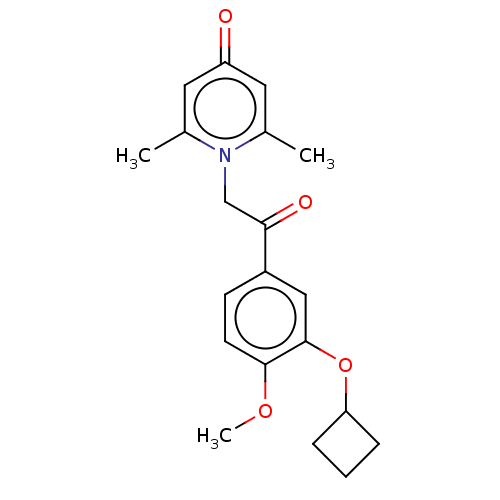 (US11440886, Example 21) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570653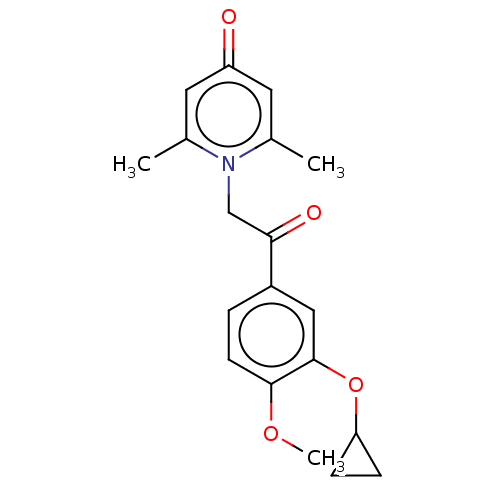 (US11440886, Example 19) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570696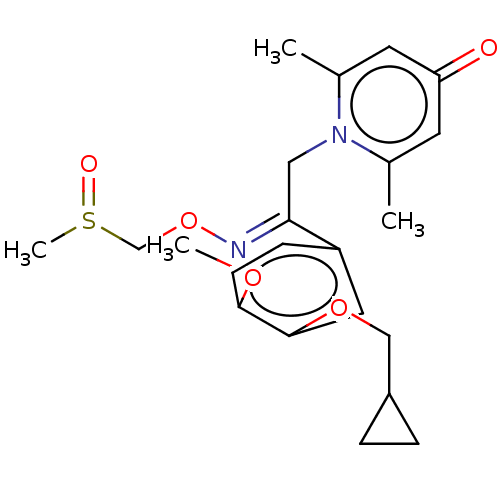 (US11440886, Example 43) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570646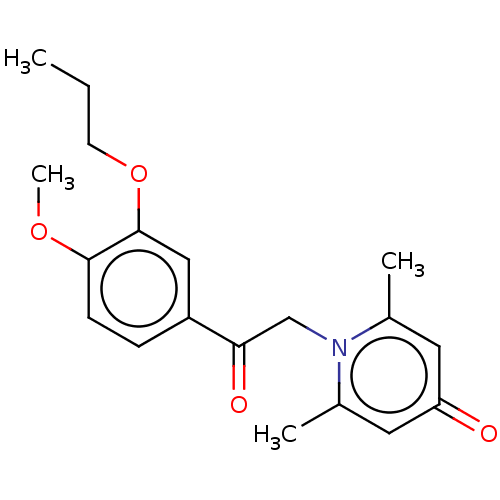 (US11440886, Example 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570657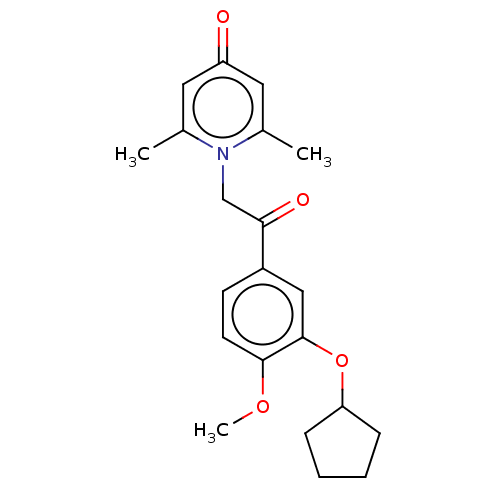 (US11440886, Example 23) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570639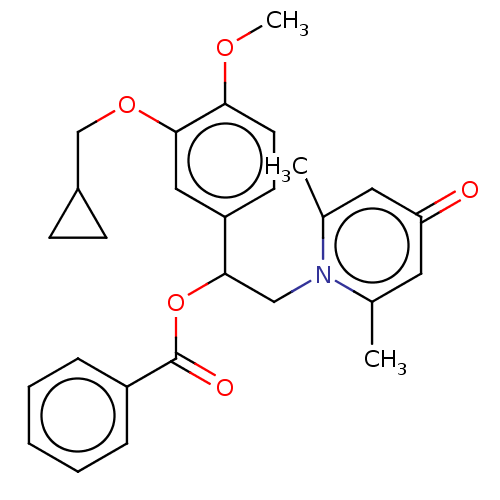 (US11440886, Example 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570722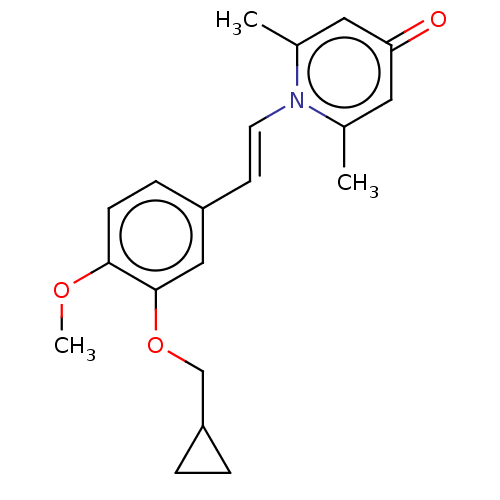 (US11440886, Example 59) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570721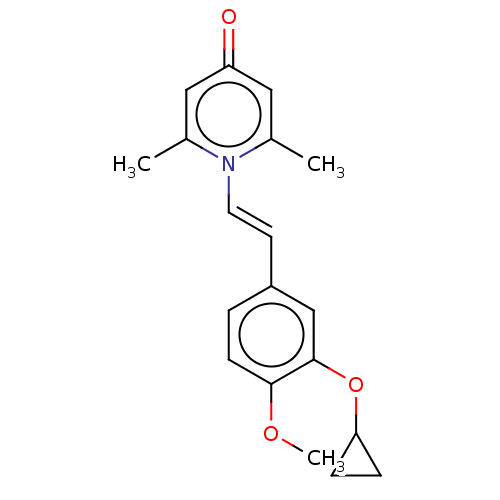 (US11440886, Example 58) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570641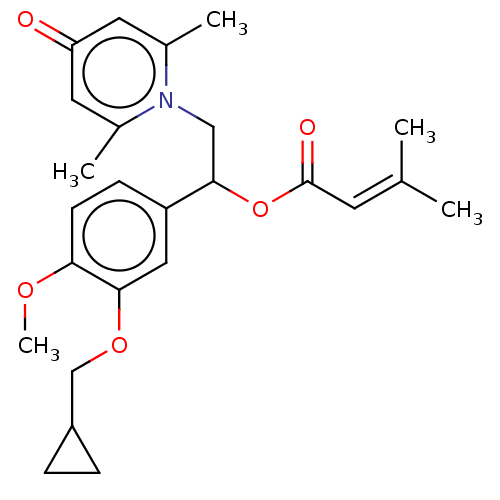 (US11440886, Example 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570725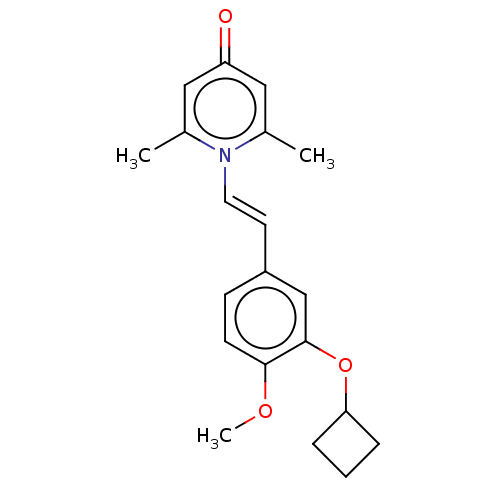 (US11440886, Example 61) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570693 (US11440886, Example 40) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570755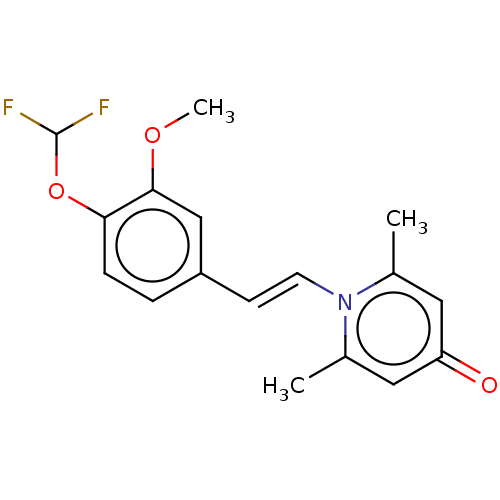 (US11440886, Example 89) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570744 (US11440886, Example 80 | US11440886, Example 85) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570706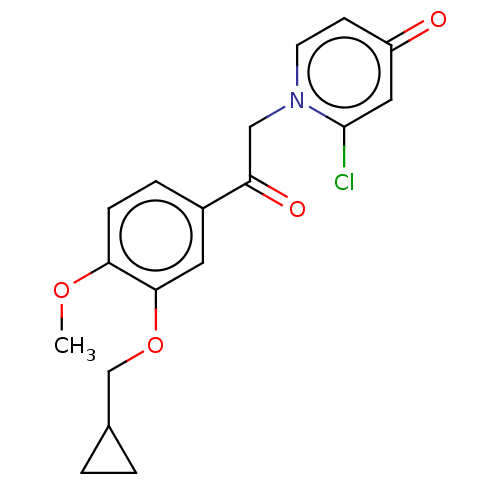 (US11440886, Example 47) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570746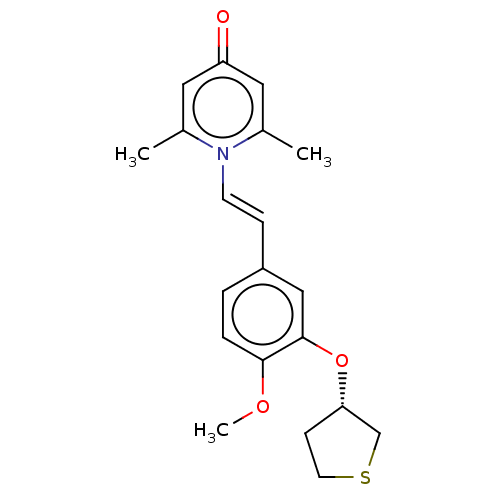 (US11440886, Example 82) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570734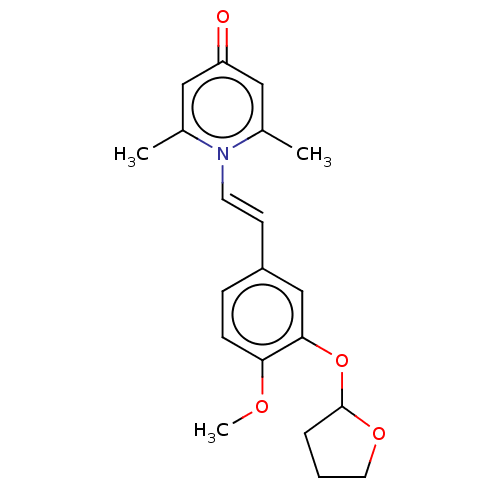 (US11440886, Example 70) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570695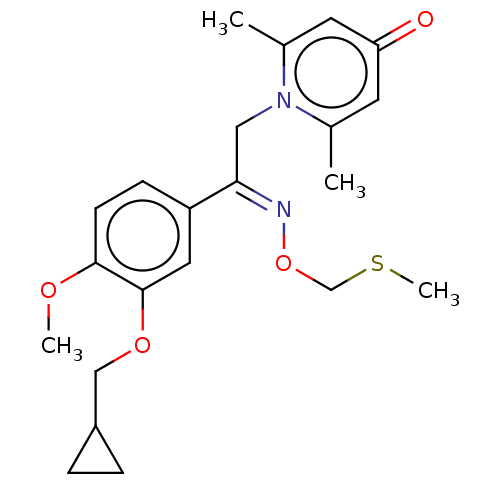 (US11440886, Example 42) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4626 (Anilinoquinazoline deriv. 9 | CHEMBL301018 | N-(3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of EGFR in human A431 cells | Eur J Med Chem 61: 84-94 (2013) Article DOI: 10.1016/j.ejmech.2012.07.036 BindingDB Entry DOI: 10.7270/Q2X068CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570784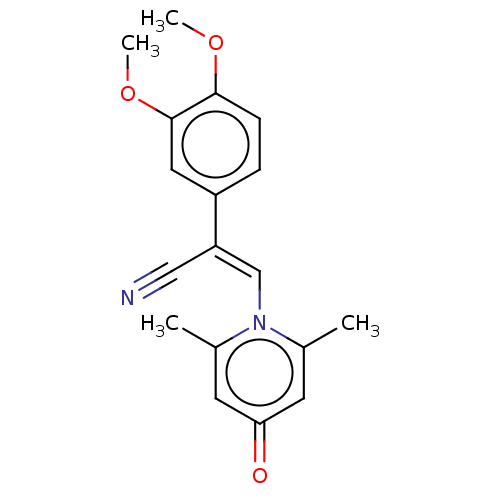 (US11440886, Example 115) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570726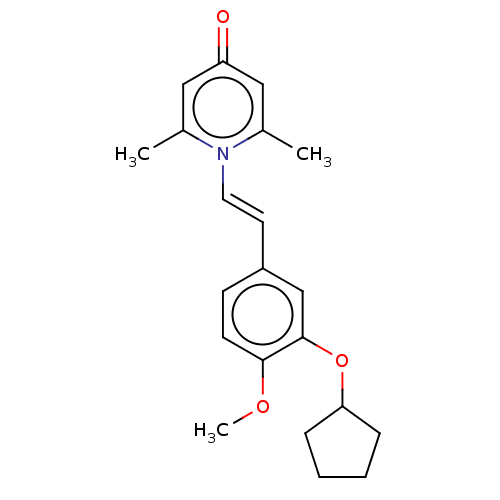 (US11440886, Example 62) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570649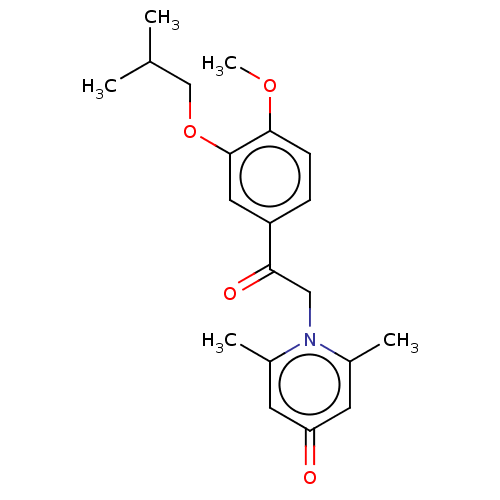 (US11440886, Example 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10.3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570727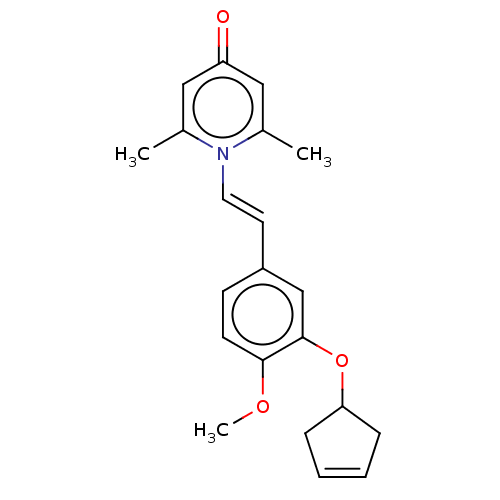 (US11440886, Example 63) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11.1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570659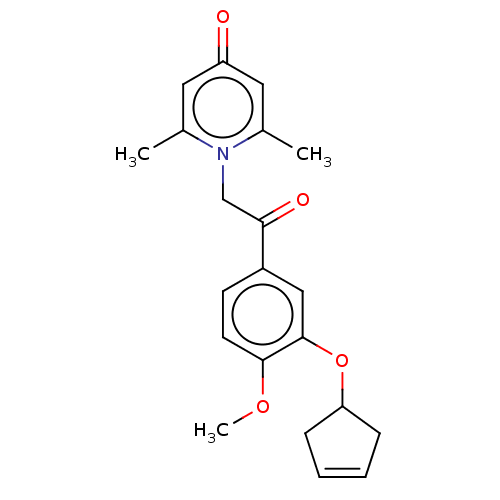 (US11440886, Example 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50428416 (CHEMBL2334002) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of EGFR (unknown origin) using biotinylated-PTP1B (Tyr66) as substrate incubated for 5 mins prior to substrate addition measured after 1 h... | Eur J Med Chem 61: 84-94 (2013) Article DOI: 10.1016/j.ejmech.2012.07.036 BindingDB Entry DOI: 10.7270/Q2X068CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570765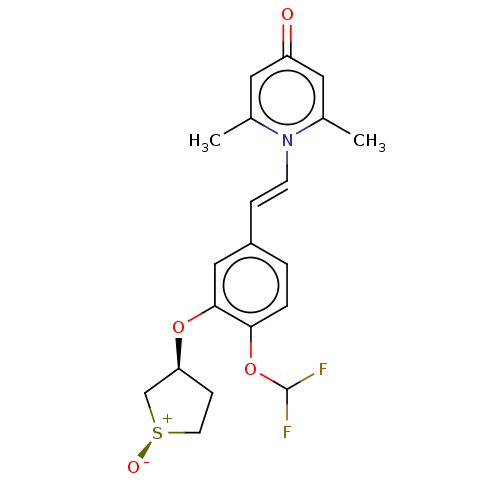 (US11440886, Example 101-2 | US11440886, Example 98...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570759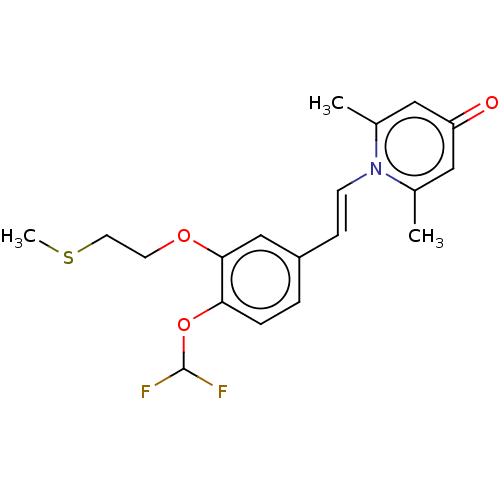 (US11440886, Example 93) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12.9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570654 (US11440886, Example 20) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM570667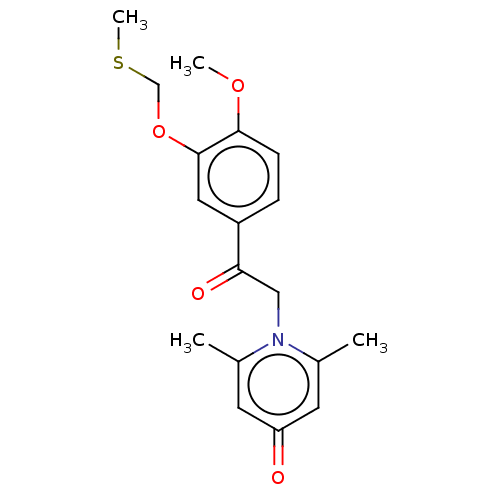 (US11440886, Example 32) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13.9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory effect of the compounds on human PDE4B1 enzyme activity was determined by quantifying the 5′-adenosine monophosphate (5′-A... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TM7FBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 330 total ) | Next | Last >> |