 Found 106 hits with Last Name = 'ly' and Initial = 'tw'
Found 106 hits with Last Name = 'ly' and Initial = 'tw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine deaminase
(Bos taurus (bovine)) | BDBM50105934

(2-(6-Amino-purin-7-ylmethoxy)-ethanol | CHEMBL1260...)Show InChI InChI=1S/C8H11N5O2/c9-7-6-8(11-3-10-7)12-4-13(6)5-15-2-1-14/h3-4,14H,1-2,5H2,(H2,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry
Curated by ChEMBL
| Assay Description
Inhibitory activity against adenosine deaminase |
J Med Chem 44: 3710-20 (2001)
BindingDB Entry DOI: 10.7270/Q2G44R0Z |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Bos taurus (bovine)) | BDBM50369958
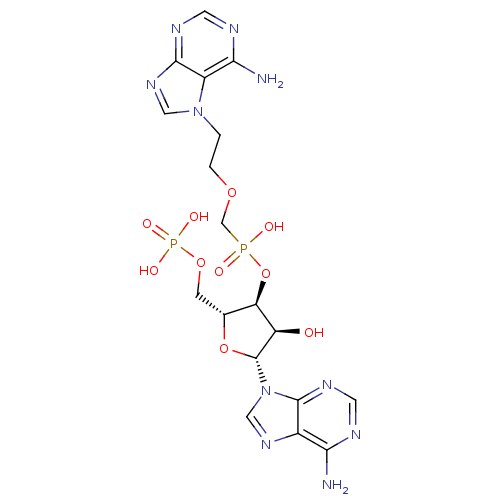
(CHEMBL1790862)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](OP(O)(=O)COCCn2cnc3ncnc(N)c23)[C@H]1O Show InChI InChI=1S/C18H24N10O10P2/c19-14-10-17(24-5-21-14)28(7-25-10)18-12(29)13(9(37-18)3-36-40(32,33)34)38-39(30,31)8-35-2-1-27-6-26-16-11(27)15(20)22-4-23-16/h4-7,9,12-13,18,29H,1-3,8H2,(H,30,31)(H2,19,21,24)(H2,20,22,23)(H2,32,33,34)/t9-,12-,13-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry
Curated by ChEMBL
| Assay Description
Inhibitory activity against adenosine deaminase |
J Med Chem 44: 3710-20 (2001)
BindingDB Entry DOI: 10.7270/Q2G44R0Z |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Bos taurus (bovine)) | BDBM50029650

(2-(6-Amino-purin-9-ylmethoxy)-ethanol | CHEMBL3775...)Show InChI InChI=1S/C8H11N5O2/c9-7-6-8(11-3-10-7)13(4-12-6)5-15-2-1-14/h3-4,14H,1-2,5H2,(H2,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry
Curated by ChEMBL
| Assay Description
Inhibitory activity against adenosine deaminase |
J Med Chem 44: 3710-20 (2001)
BindingDB Entry DOI: 10.7270/Q2G44R0Z |
More data for this
Ligand-Target Pair | |
Phosphoribosyl pyrophosphate synthase-associated protein 2
(Homo sapiens (Human)) | BDBM50369958

(CHEMBL1790862)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](OP(O)(=O)COCCn2cnc3ncnc(N)c23)[C@H]1O Show InChI InChI=1S/C18H24N10O10P2/c19-14-10-17(24-5-21-14)28(7-25-10)18-12(29)13(9(37-18)3-36-40(32,33)34)38-39(30,31)8-35-2-1-27-6-26-16-11(27)15(20)22-4-23-16/h4-7,9,12-13,18,29H,1-3,8H2,(H,30,31)(H2,19,21,24)(H2,20,22,23)(H2,32,33,34)/t9-,12-,13-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry
Curated by ChEMBL
| Assay Description
Inhibitory activity against PRPP synthetase |
J Med Chem 44: 3710-20 (2001)
BindingDB Entry DOI: 10.7270/Q2G44R0Z |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Bos taurus (bovine)) | BDBM50105931
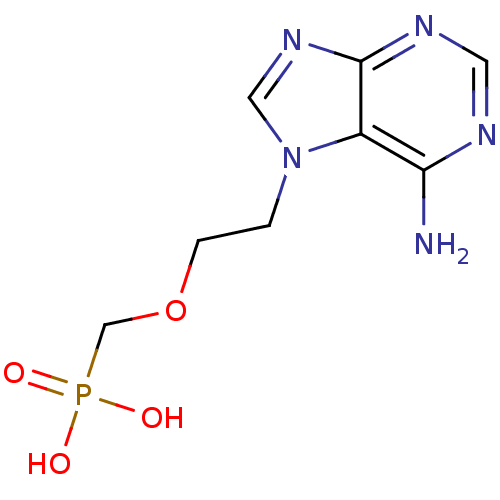
(CHEMBL123655 | [2-(6-Amino-purin-7-yl)-ethoxymethy...)Show InChI InChI=1S/C8H12N5O4P/c9-7-6-8(11-3-10-7)12-4-13(6)1-2-17-5-18(14,15)16/h3-4H,1-2,5H2,(H2,9,10,11)(H2,14,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry
Curated by ChEMBL
| Assay Description
Inhibitory activity against adenosine deaminase |
J Med Chem 44: 3710-20 (2001)
BindingDB Entry DOI: 10.7270/Q2G44R0Z |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Bos taurus (bovine)) | BDBM50369957
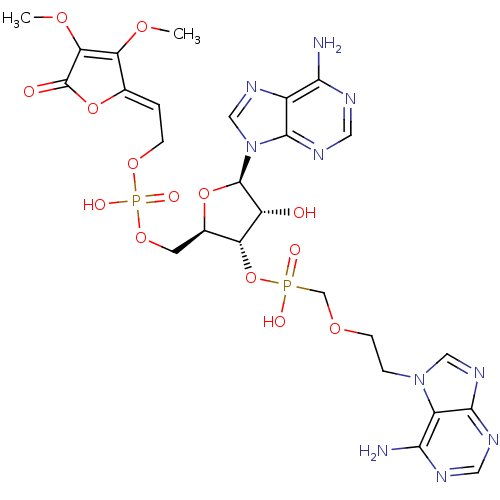
(CHEMBL1790864)Show SMILES COC1=C(OC)\C(OC1=O)=C\COP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(=O)COCCn1cnc2ncnc(N)c12)n1cnc2c(N)ncnc12 |c:2| Show InChI InChI=1S/C26H32N10O14P2/c1-43-19-13(49-26(38)20(19)44-2)3-5-46-52(41,42)47-7-14-18(17(37)25(48-14)36-11-33-15-21(27)29-9-32-24(15)36)50-51(39,40)12-45-6-4-35-10-34-23-16(35)22(28)30-8-31-23/h3,8-11,14,17-18,25,37H,4-7,12H2,1-2H3,(H,39,40)(H,41,42)(H2,27,29,32)(H2,28,30,31)/b13-3-/t14-,17-,18-,25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry
Curated by ChEMBL
| Assay Description
Inhibitory activity against adenosine deaminase |
J Med Chem 44: 3710-20 (2001)
BindingDB Entry DOI: 10.7270/Q2G44R0Z |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Bos taurus (bovine)) | BDBM50001103
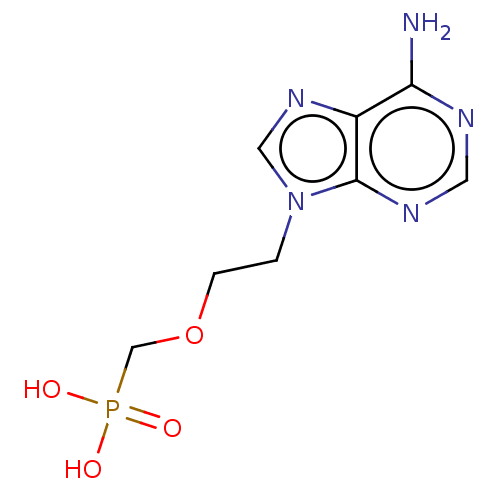
((2-(6-amino-9H-purin-9-yl)ethoxy)methylphosphonic ...)Show InChI InChI=1S/C8H12N5O4P/c9-7-6-8(11-3-10-7)13(4-12-6)1-2-17-5-18(14,15)16/h3-4H,1-2,5H2,(H2,9,10,11)(H2,14,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| >8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry
Curated by ChEMBL
| Assay Description
Inhibitory activity against adenosine deaminase |
J Med Chem 44: 3710-20 (2001)
BindingDB Entry DOI: 10.7270/Q2G44R0Z |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Bos taurus (bovine)) | BDBM50105935

(CHEMBL121723 | [2-(6-Amino-purin-9-yl)-ethoxymethy...)Show SMILES COC1=C(OC)\C(OC1=O)=C\COP(O)(=O)COCCn1cnc2c(N)ncnc12 |c:2| Show InChI InChI=1S/C16H20N5O8P/c1-25-12-10(29-16(22)13(12)26-2)3-5-28-30(23,24)9-27-6-4-21-8-20-11-14(17)18-7-19-15(11)21/h3,7-8H,4-6,9H2,1-2H3,(H,23,24)(H2,17,18,19)/b10-3- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| >8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry
Curated by ChEMBL
| Assay Description
Inhibitory activity against adenosine deaminase |
J Med Chem 44: 3710-20 (2001)
BindingDB Entry DOI: 10.7270/Q2G44R0Z |
More data for this
Ligand-Target Pair | |
Phosphoribosyl pyrophosphate synthase-associated protein 2
(Homo sapiens (Human)) | BDBM50001103

((2-(6-amino-9H-purin-9-yl)ethoxy)methylphosphonic ...)Show InChI InChI=1S/C8H12N5O4P/c9-7-6-8(11-3-10-7)13(4-12-6)1-2-17-5-18(14,15)16/h3-4H,1-2,5H2,(H2,9,10,11)(H2,14,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 3.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry
Curated by ChEMBL
| Assay Description
Inhibitory activity against PRPP synthetase |
J Med Chem 44: 3710-20 (2001)
BindingDB Entry DOI: 10.7270/Q2G44R0Z |
More data for this
Ligand-Target Pair | |
Phosphoribosyl pyrophosphate synthase-associated protein 2
(Homo sapiens (Human)) | BDBM50105931

(CHEMBL123655 | [2-(6-Amino-purin-7-yl)-ethoxymethy...)Show InChI InChI=1S/C8H12N5O4P/c9-7-6-8(11-3-10-7)12-4-13(6)1-2-17-5-18(14,15)16/h3-4H,1-2,5H2,(H2,9,10,11)(H2,14,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.70E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry
Curated by ChEMBL
| Assay Description
Inhibitory activity against PRPP synthetase |
J Med Chem 44: 3710-20 (2001)
BindingDB Entry DOI: 10.7270/Q2G44R0Z |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50561042
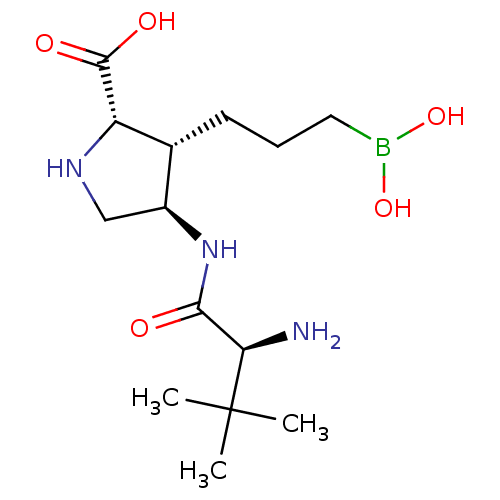
(CHEMBL4748950)Show SMILES CC(C)(C)[C@H](N)C(=O)N[C@H]1CN[C@@H]([C@@H]1CCCB(O)O)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ARG1 expressed in Escherichia coli using thioarginine as a substrate preincubated for 30 mins followed by substrate a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00195
BindingDB Entry DOI: 10.7270/Q2542SD6 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50579614

(CHEMBL4871970)Show SMILES [H][C@]12CN[C@H](C(O)=O)[C@@]1(CCCB(O)O)CCN2 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ARG1 (unknown origin) assessed as reduction in thio-ornithine production using thioarginine as substrate by fluorimetric analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00258
BindingDB Entry DOI: 10.7270/Q2QF8XQ7 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50577469
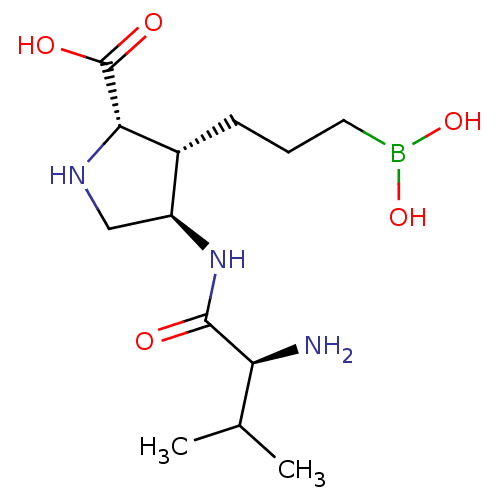
(CHEMBL4850213)Show SMILES CC(C)[C@H](N)C(=O)N[C@H]1CN[C@@H]([C@@H]1CCCB(O)O)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ARG1 expressed in Escherichia coli using thioarginine as a substrate preincubated for 30 mins followed by substrate a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00195
BindingDB Entry DOI: 10.7270/Q2542SD6 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50579610

(CHEMBL4873520)Show SMILES [H][C@]12CN[C@](CCN1)([C@H]2CCCB(O)O)C(O)=O |r,THB:9:8:7.6.5:3.2| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ARG1 (unknown origin) assessed as reduction in thio-ornithine production using thioarginine as substrate by fluorimetric analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00258
BindingDB Entry DOI: 10.7270/Q2QF8XQ7 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50561035
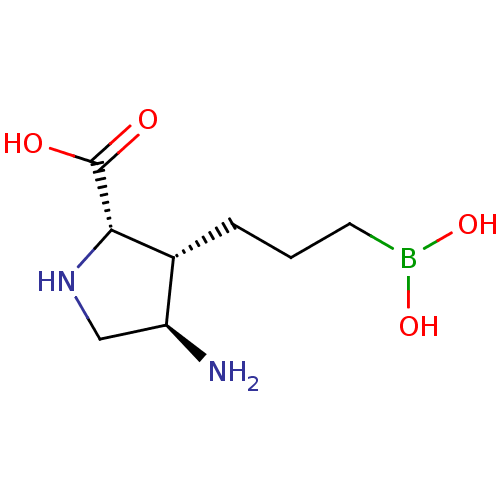
(CHEMBL4763578) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ARG1 expressed in Escherichia coli using thioarginine as a substrate preincubated for 30 mins followed by substrate a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00195
BindingDB Entry DOI: 10.7270/Q2542SD6 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50579616

(CHEMBL4845730)Show SMILES [H][C@]12CN[C@H](C(=O)OC)[C@@]1(CCCB(O)O)CCCN2 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ARG1 (unknown origin) assessed as reduction in thio-ornithine production using thioarginine as substrate by fluorimetric analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00258
BindingDB Entry DOI: 10.7270/Q2QF8XQ7 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50579611
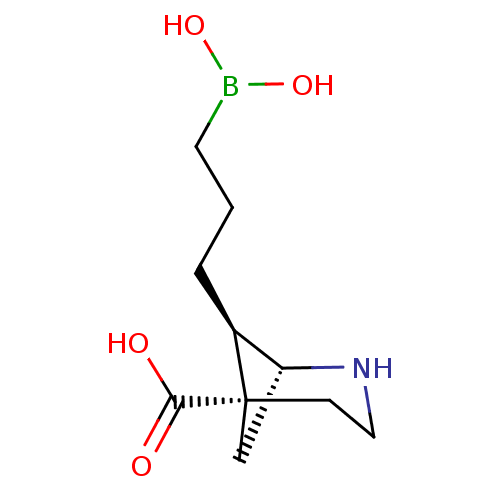
(CHEMBL4871097)Show SMILES [H][C@]12C[C@](CCN1)([C@H]2CCCB(O)O)C(O)=O |r,THB:8:7:2:4.5.6| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ARG1 (unknown origin) assessed as reduction in thio-ornithine production using thioarginine as substrate by fluorimetric analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00258
BindingDB Entry DOI: 10.7270/Q2QF8XQ7 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50561036
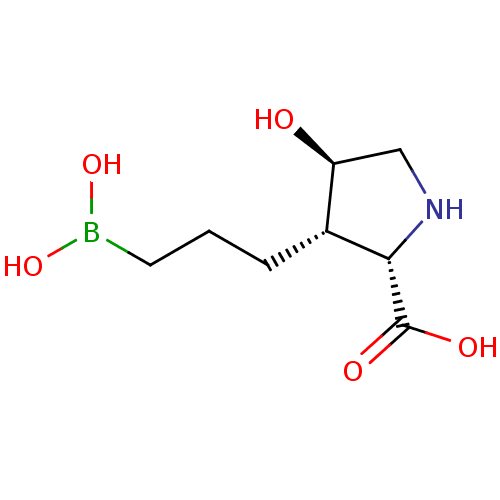
(CHEMBL4778978)Show SMILES OB(O)CCC[C@@H]1[C@@H](O)CN[C@@H]1C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ARG1 expressed in Escherichia coli using thioarginine as a substrate preincubated for 30 mins followed by substrate a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00195
BindingDB Entry DOI: 10.7270/Q2542SD6 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50538536
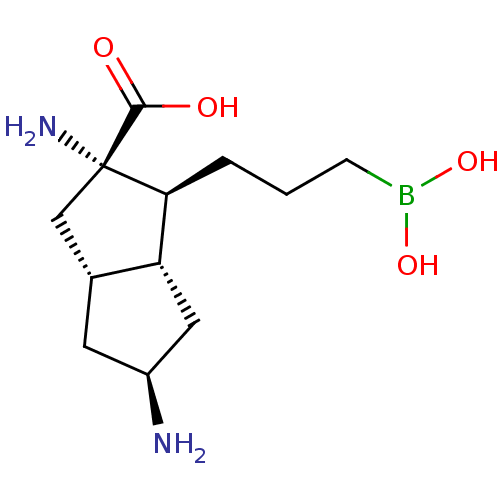
(CHEMBL4639893)Show SMILES [H][C@]12C[C@H](N)C[C@@]1([H])[C@H](CCCB(O)O)[C@@](N)(C2)C(O)=O |r| Show InChI InChI=1S/C12H23BN2O4/c14-8-4-7-6-12(15,11(16)17)10(9(7)5-8)2-1-3-13(18)19/h7-10,18-19H,1-6,14-15H2,(H,16,17)/t7-,8+,9-,10+,12+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human arginase1 in presence of DNTB by thio ornithine generation assay |
ACS Med Chem Lett 11: 582-588 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00058
BindingDB Entry DOI: 10.7270/Q26113VN |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50577476
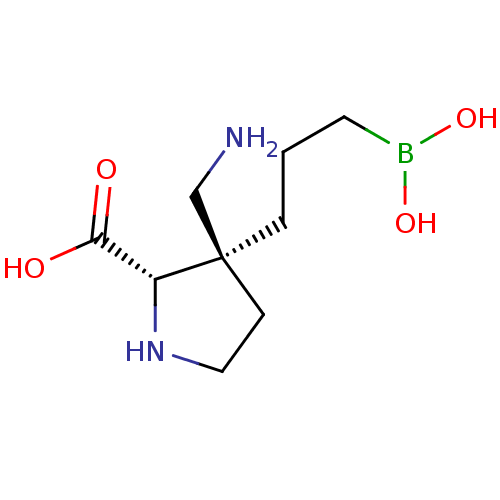
(CHEMBL4852406) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ARG1 expressed in Escherichia coli using thioarginine as a substrate preincubated for 30 mins followed by substrate a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00195
BindingDB Entry DOI: 10.7270/Q2542SD6 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50577471

(CHEMBL4864502)Show SMILES CN[C@@H](C(C)C)C(=O)N[C@H]1CN[C@@H]([C@@H]1CCCB(O)O)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ARG1 expressed in Escherichia coli using thioarginine as a substrate preincubated for 30 mins followed by substrate a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00195
BindingDB Entry DOI: 10.7270/Q2542SD6 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50538548
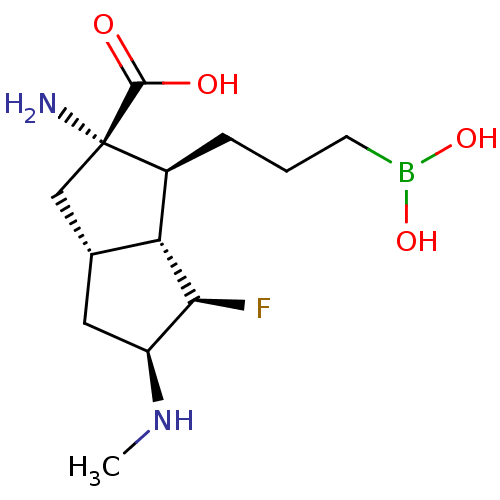
(CHEMBL4640681)Show SMILES [H][C@]12C[C@H](NC)[C@H](F)[C@@]1([H])[C@H](CCCB(O)O)[C@@](N)(C2)C(O)=O |r| Show InChI InChI=1S/C13H24BFN2O4/c1-17-9-5-7-6-13(16,12(18)19)8(10(7)11(9)15)3-2-4-14(20)21/h7-11,17,20-21H,2-6,16H2,1H3,(H,18,19)/t7-,8+,9+,10-,11+,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human arginase1 in presence of DNTB by thio ornithine generation assay |
ACS Med Chem Lett 11: 582-588 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00058
BindingDB Entry DOI: 10.7270/Q26113VN |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50579615

(CHEMBL4865209)Show SMILES [H][C@]12CN[C@H](C(O)=O)[C@@]1(CCCB(O)O)COC2 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ARG1 (unknown origin) assessed as reduction in thio-ornithine production using thioarginine as substrate by fluorimetric analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00258
BindingDB Entry DOI: 10.7270/Q2QF8XQ7 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50538547
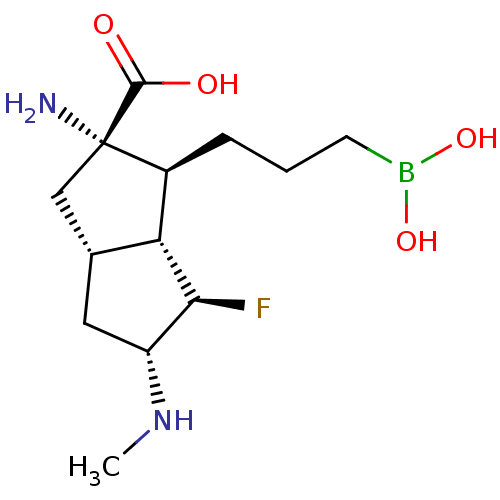
(CHEMBL4648504)Show SMILES [H][C@]12C[C@@H](NC)[C@H](F)[C@@]1([H])[C@H](CCCB(O)O)[C@@](N)(C2)C(O)=O |r| Show InChI InChI=1S/C13H24BFN2O4/c1-17-9-5-7-6-13(16,12(18)19)8(10(7)11(9)15)3-2-4-14(20)21/h7-11,17,20-21H,2-6,16H2,1H3,(H,18,19)/t7-,8+,9-,10-,11+,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human arginase1 in presence of DNTB by thio ornithine generation assay |
ACS Med Chem Lett 11: 582-588 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00058
BindingDB Entry DOI: 10.7270/Q26113VN |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50577477
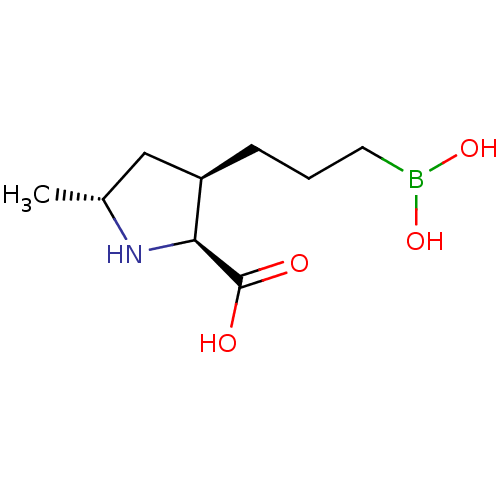
(CHEMBL4868283)Show SMILES C[C@@H]1C[C@@H](CCCB(O)O)[C@H](N1)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ARG1 expressed in Escherichia coli using thioarginine as a substrate preincubated for 30 mins followed by substrate a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00195
BindingDB Entry DOI: 10.7270/Q2542SD6 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50577478

(CHEMBL4872801)Show SMILES OC[C@@H]1C[C@@H](CCCB(O)O)[C@H](N1)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ARG1 expressed in Escherichia coli using thioarginine as a substrate preincubated for 30 mins followed by substrate a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00195
BindingDB Entry DOI: 10.7270/Q2542SD6 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50577468
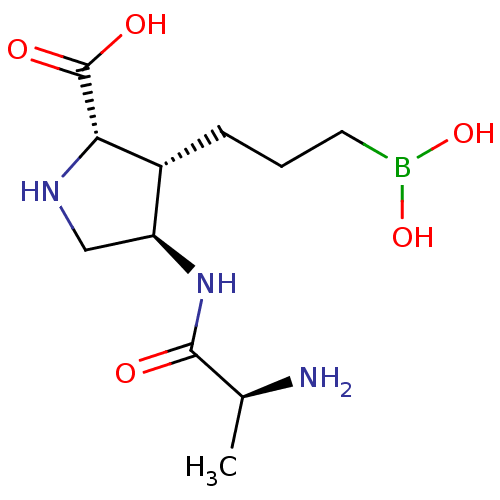
(CHEMBL4875641)Show SMILES C[C@H](N)C(=O)N[C@H]1CN[C@@H]([C@@H]1CCCB(O)O)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ARG1 expressed in Escherichia coli using thioarginine as a substrate preincubated for 30 mins followed by substrate a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00195
BindingDB Entry DOI: 10.7270/Q2542SD6 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50538522
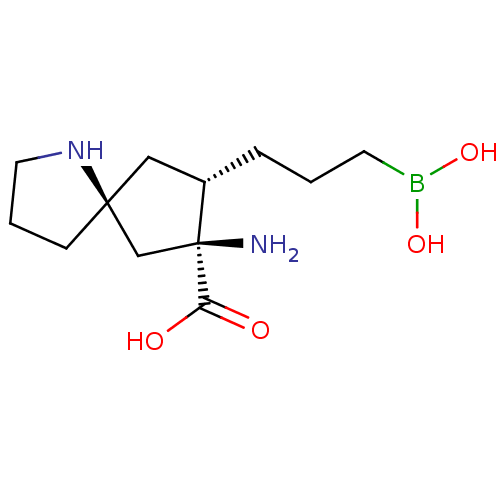
(CHEMBL4633222)Show SMILES N[C@]1(C[C@]2(CCCN2)C[C@@H]1CCCB(O)O)C(O)=O |r| Show InChI InChI=1S/C12H23BN2O4/c14-12(10(16)17)8-11(4-2-6-15-11)7-9(12)3-1-5-13(18)19/h9,15,18-19H,1-8,14H2,(H,16,17)/t9-,11-,12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human arginase1 in presence of DNTB by thio ornithine generation assay |
ACS Med Chem Lett 11: 582-588 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00058
BindingDB Entry DOI: 10.7270/Q26113VN |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50538523
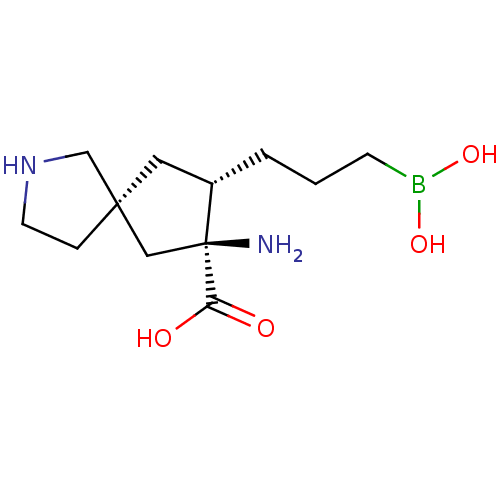
(CHEMBL4646013)Show SMILES N[C@]1(C[C@]2(CCNC2)C[C@@H]1CCCB(O)O)C(O)=O |r| Show InChI InChI=1S/C12H23BN2O4/c14-12(10(16)17)7-11(3-5-15-8-11)6-9(12)2-1-4-13(18)19/h9,15,18-19H,1-8,14H2,(H,16,17)/t9-,11-,12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human arginase1 in presence of DNTB by thio ornithine generation assay |
ACS Med Chem Lett 11: 582-588 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00058
BindingDB Entry DOI: 10.7270/Q26113VN |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50538541
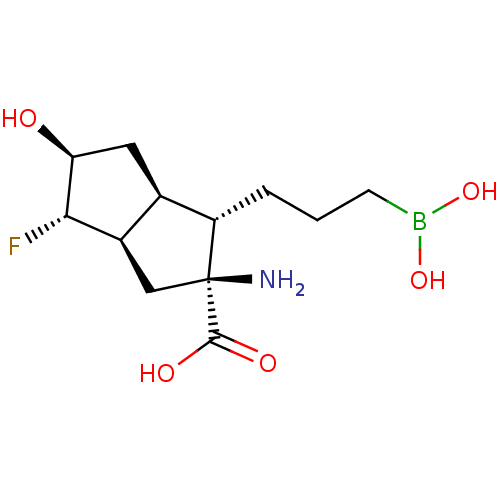
(CHEMBL4633123)Show SMILES [H][C@@]12C[C@](N)([C@@H](CCCB(O)O)[C@]1([H])C[C@H](O)[C@H]2F)C(O)=O |r| Show InChI InChI=1S/C12H21BFNO5/c14-10-7-5-12(15,11(17)18)8(2-1-3-13(19)20)6(7)4-9(10)16/h6-10,16,19-20H,1-5,15H2,(H,17,18)/t6-,7-,8+,9+,10+,12+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human arginase1 in presence of DNTB by thio ornithine generation assay |
ACS Med Chem Lett 11: 582-588 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00058
BindingDB Entry DOI: 10.7270/Q26113VN |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM290365
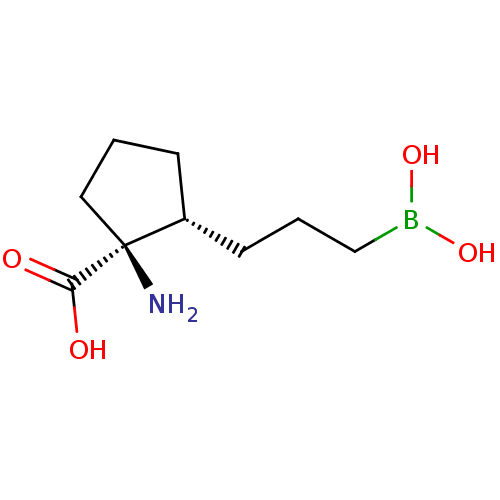
((1S,2S)-1-amino-2-(3-boronopropyl)cyclopentanecarb...)Show InChI InChI=1S/C9H18BNO4/c11-9(8(12)13)5-1-3-7(9)4-2-6-10(14)15/h7,14-15H,1-6,11H2,(H,12,13)/t7-,9+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human arginase1 in presence of DNTB by thio ornithine generation assay |
ACS Med Chem Lett 11: 582-588 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00058
BindingDB Entry DOI: 10.7270/Q26113VN |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50538543
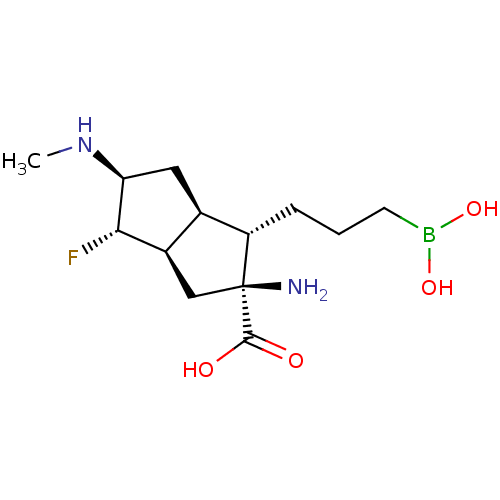
(CHEMBL4632493)Show SMILES [H][C@@]12C[C@](N)([C@@H](CCCB(O)O)[C@]1([H])C[C@H](NC)[C@H]2F)C(O)=O |r| Show InChI InChI=1S/C13H24BFN2O4/c1-17-10-5-7-8(11(10)15)6-13(16,12(18)19)9(7)3-2-4-14(20)21/h7-11,17,20-21H,2-6,16H2,1H3,(H,18,19)/t7-,8-,9+,10+,11+,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human arginase1 in presence of DNTB by thio ornithine generation assay |
ACS Med Chem Lett 11: 582-588 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00058
BindingDB Entry DOI: 10.7270/Q26113VN |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50577458
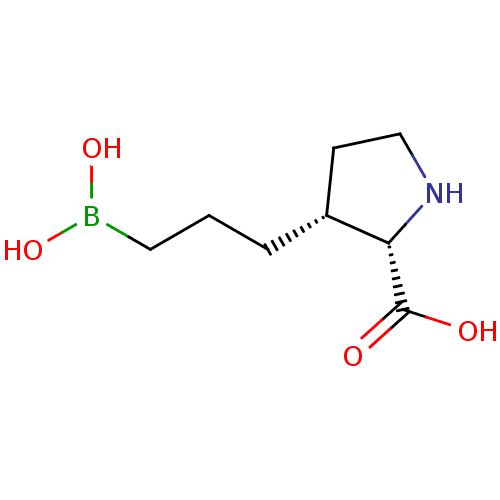
(CHEMBL4858437) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ARG1 expressed in Escherichia coli using thioarginine as a substrate preincubated for 30 mins followed by substrate a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00195
BindingDB Entry DOI: 10.7270/Q2542SD6 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50577458

(CHEMBL4858437) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ARG1 (unknown origin) assessed as reduction in thio-ornithine production using thioarginine as substrate by fluorimetric analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00258
BindingDB Entry DOI: 10.7270/Q2QF8XQ7 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM130381
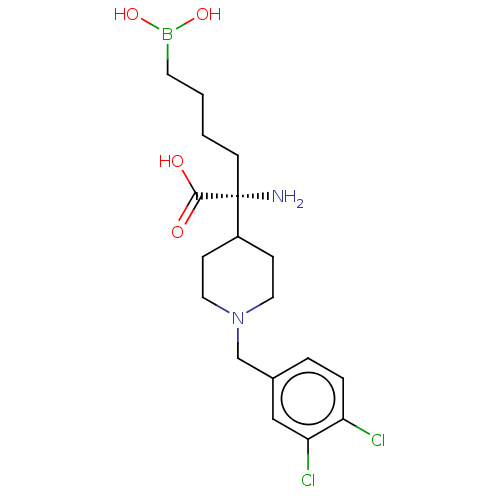
((R)-2-amino-6-borono-2-[1-(3,4-dichlorobenzyl)pipe...)Show SMILES N[C@](CCCCB(O)O)(C1CCN(Cc2ccc(Cl)c(Cl)c2)CC1)C(O)=O Show InChI InChI=1S/C18H27BCl2N2O4/c20-15-4-3-13(11-16(15)21)12-23-9-5-14(6-10-23)18(22,17(24)25)7-1-2-8-19(26)27/h3-4,11,14,26-27H,1-2,5-10,12,22H2,(H,24,25)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human arginase1 in presence of DNTB by thio ornithine generation assay |
ACS Med Chem Lett 11: 582-588 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00058
BindingDB Entry DOI: 10.7270/Q26113VN |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50577475
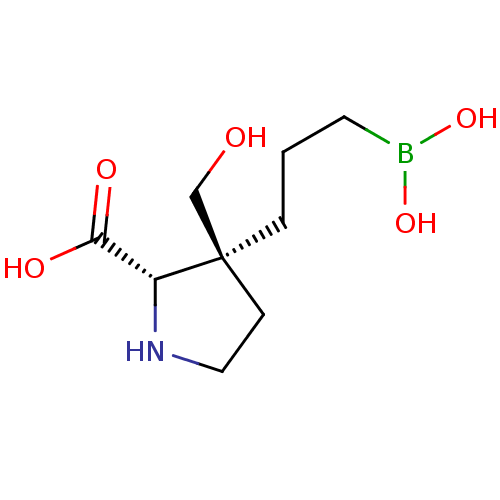
(CHEMBL4855923) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ARG1 expressed in Escherichia coli using thioarginine as a substrate preincubated for 30 mins followed by substrate a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00195
BindingDB Entry DOI: 10.7270/Q2542SD6 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50538538
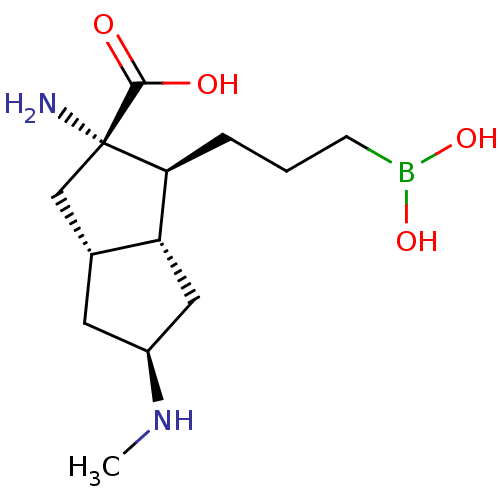
(CHEMBL4647529)Show SMILES [H][C@]12C[C@@H](C[C@@]1([H])[C@H](CCCB(O)O)[C@@](N)(C2)C(O)=O)NC |r| Show InChI InChI=1S/C13H25BN2O4/c1-16-9-5-8-7-13(15,12(17)18)11(10(8)6-9)3-2-4-14(19)20/h8-11,16,19-20H,2-7,15H2,1H3,(H,17,18)/t8-,9+,10-,11+,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human arginase1 in presence of DNTB by thio ornithine generation assay |
ACS Med Chem Lett 11: 582-588 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00058
BindingDB Entry DOI: 10.7270/Q26113VN |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50577464
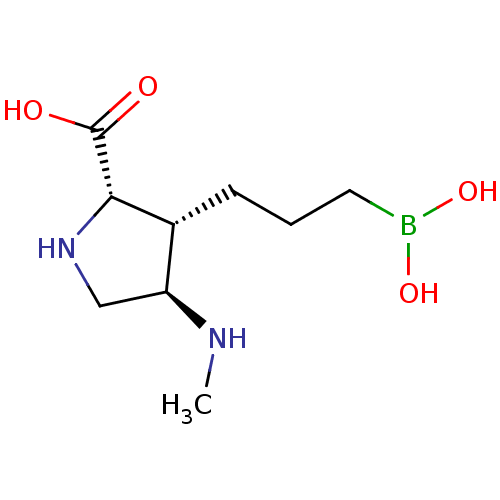
(CHEMBL4863489)Show SMILES CN[C@H]1CN[C@@H]([C@@H]1CCCB(O)O)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ARG1 expressed in Escherichia coli using thioarginine as a substrate preincubated for 30 mins followed by substrate a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00195
BindingDB Entry DOI: 10.7270/Q2542SD6 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50538520

(CHEMBL4648095)Show SMILES [H][C@@]12C[C@H](CCCB(O)O)[C@](N)(C(O)=O)[C@]1([H])CCN2 |r| Show InChI InChI=1S/C11H21BN2O4/c13-11(10(15)16)7(2-1-4-12(17)18)6-9-8(11)3-5-14-9/h7-9,14,17-18H,1-6,13H2,(H,15,16)/t7-,8+,9+,11+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human arginase1 in presence of DNTB by thio ornithine generation assay |
ACS Med Chem Lett 11: 582-588 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00058
BindingDB Entry DOI: 10.7270/Q26113VN |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50538544

(CHEMBL4632821)Show SMILES [H][C@@]12C[C@](N)([C@@H](CCCB(O)O)[C@]1([H])C[C@@H](NC)[C@H]2F)C(O)=O |r| Show InChI InChI=1S/C13H24BFN2O4/c1-17-10-5-7-8(11(10)15)6-13(16,12(18)19)9(7)3-2-4-14(20)21/h7-11,17,20-21H,2-6,16H2,1H3,(H,18,19)/t7-,8-,9+,10-,11+,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human arginase1 in presence of DNTB by thio ornithine generation assay |
ACS Med Chem Lett 11: 582-588 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00058
BindingDB Entry DOI: 10.7270/Q26113VN |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50579613
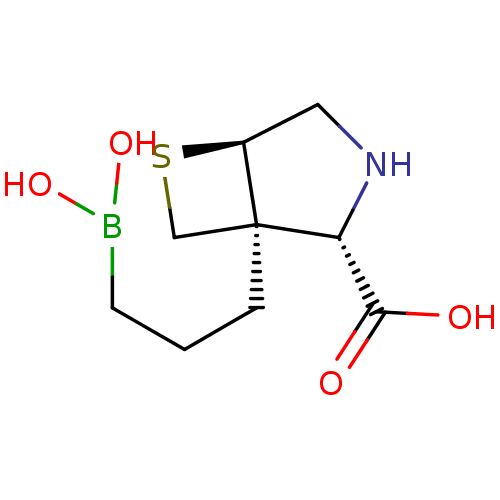
(CHEMBL4850423)Show SMILES [H][C@]12CN[C@H](C(O)=O)[C@@]1(CCCB(O)O)CS2 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ARG1 (unknown origin) assessed as reduction in thio-ornithine production using thioarginine as substrate by fluorimetric analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00258
BindingDB Entry DOI: 10.7270/Q2QF8XQ7 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50538518

(CHEMBL4639162)Show SMILES [H][C@]12CNC[C@@]1([H])[C@H](CCCB(O)O)[C@@](N)(C2)C(O)=O |r| Show InChI InChI=1S/C11H21BN2O4/c13-11(10(15)16)4-7-5-14-6-8(7)9(11)2-1-3-12(17)18/h7-9,14,17-18H,1-6,13H2,(H,15,16)/t7-,8+,9-,11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human arginase1 in presence of DNTB by thio ornithine generation assay |
ACS Med Chem Lett 11: 582-588 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00058
BindingDB Entry DOI: 10.7270/Q26113VN |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50577472
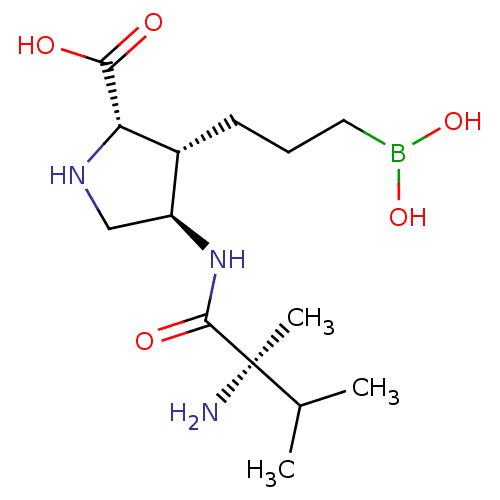
(CHEMBL4845882)Show SMILES CC(C)[C@@](C)(N)C(=O)N[C@H]1CN[C@@H]([C@@H]1CCCB(O)O)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ARG1 expressed in Escherichia coli using thioarginine as a substrate preincubated for 30 mins followed by substrate a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00195
BindingDB Entry DOI: 10.7270/Q2542SD6 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50577479
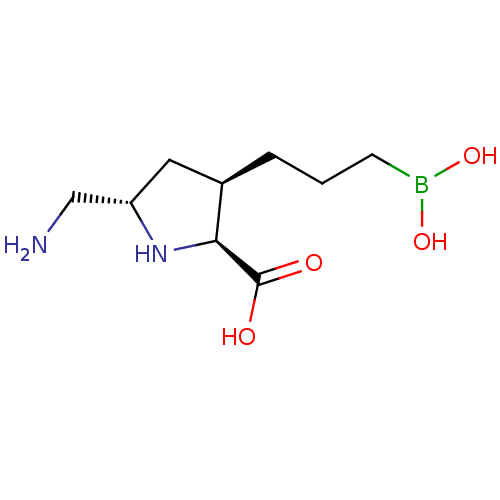
(CHEMBL4862990)Show SMILES NC[C@@H]1C[C@@H](CCCB(O)O)[C@H](N1)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ARG1 expressed in Escherichia coli using thioarginine as a substrate preincubated for 30 mins followed by substrate a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00195
BindingDB Entry DOI: 10.7270/Q2542SD6 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50538540

(CHEMBL4638209)Show SMILES [H][C@]12C[C@@H](C[C@@]1([H])[C@H](CCCB(O)O)[C@@](N)(C2)C(O)=O)NC(=O)[C@@H](N)C(C)C |r| Show InChI InChI=1S/C17H32BN3O5/c1-9(2)14(19)15(22)21-11-6-10-8-17(20,16(23)24)13(12(10)7-11)4-3-5-18(25)26/h9-14,25-26H,3-8,19-20H2,1-2H3,(H,21,22)(H,23,24)/t10-,11+,12-,13+,14+,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of arginase1 in human cancer patient serum |
ACS Med Chem Lett 11: 582-588 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00058
BindingDB Entry DOI: 10.7270/Q26113VN |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50577474
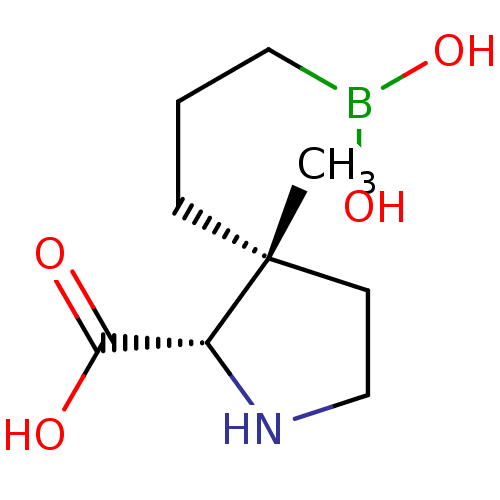
(CHEMBL4876150) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ARG1 expressed in Escherichia coli using thioarginine as a substrate preincubated for 30 mins followed by substrate a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00195
BindingDB Entry DOI: 10.7270/Q2542SD6 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50538537
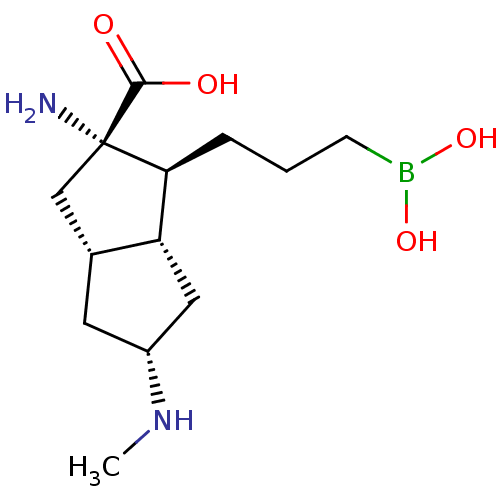
(CHEMBL4639122)Show SMILES [H][C@]12C[C@H](C[C@@]1([H])[C@H](CCCB(O)O)[C@@](N)(C2)C(O)=O)NC |r| Show InChI InChI=1S/C13H25BN2O4/c1-16-9-5-8-7-13(15,12(17)18)11(10(8)6-9)3-2-4-14(19)20/h8-11,16,19-20H,2-7,15H2,1H3,(H,17,18)/t8-,9-,10-,11+,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human arginase1 in presence of DNTB by thio ornithine generation assay |
ACS Med Chem Lett 11: 582-588 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00058
BindingDB Entry DOI: 10.7270/Q26113VN |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50577470
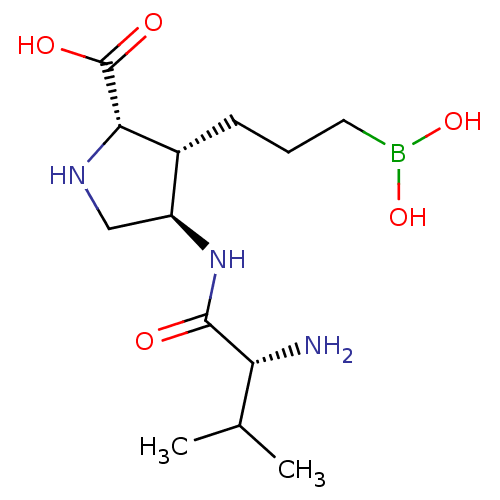
(CHEMBL4856276)Show SMILES CC(C)[C@@H](N)C(=O)N[C@H]1CN[C@@H]([C@@H]1CCCB(O)O)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ARG1 expressed in Escherichia coli using thioarginine as a substrate preincubated for 30 mins followed by substrate a... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00195
BindingDB Entry DOI: 10.7270/Q2542SD6 |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50538545
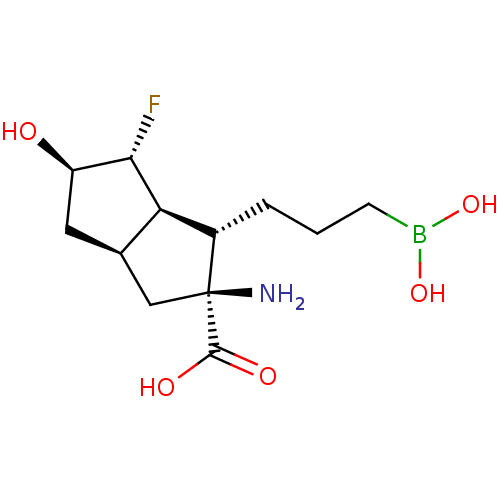
(CHEMBL4639443)Show SMILES [H][C@]12C[C@@H](O)[C@H](F)[C@@]1([H])[C@H](CCCB(O)O)[C@@](N)(C2)C(O)=O |r| Show InChI InChI=1S/C12H21BFNO5/c14-10-8(16)4-6-5-12(15,11(17)18)7(9(6)10)2-1-3-13(19)20/h6-10,16,19-20H,1-5,15H2,(H,17,18)/t6-,7+,8-,9-,10+,12+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human arginase1 in presence of DNTB by thio ornithine generation assay |
ACS Med Chem Lett 11: 582-588 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00058
BindingDB Entry DOI: 10.7270/Q26113VN |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50538539

(CHEMBL4636066)Show SMILES [H][C@]12C[C@@H](C[C@@]1([H])[C@H](CCCB(O)O)[C@@](N)(C2)C(O)=O)NC(=O)[C@H](C)N |r| Show InChI InChI=1S/C15H28BN3O5/c1-8(17)13(20)19-10-5-9-7-15(18,14(21)22)12(11(9)6-10)3-2-4-16(23)24/h8-12,23-24H,2-7,17-18H2,1H3,(H,19,20)(H,21,22)/t8-,9+,10-,11+,12-,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of arginase1 in human cancer patient serum |
ACS Med Chem Lett 11: 582-588 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00058
BindingDB Entry DOI: 10.7270/Q26113VN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data