

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25457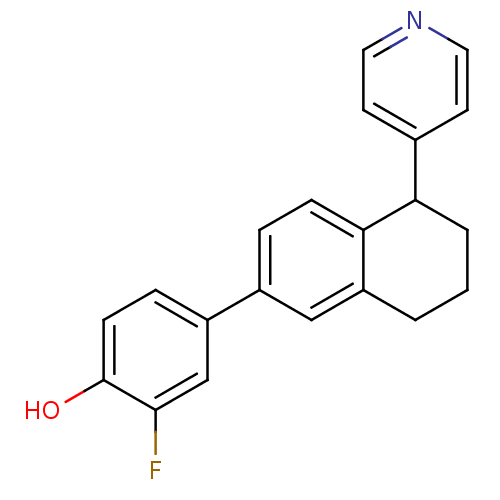 (2-fluoro-4-[5-(pyridin-4-yl)-5,6,7,8-tetrahydronap...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description To measure CYP3A4 activity, the product of testosterone 6-hydroxylation, 6beta-hydroxytestosterone was determined. After incubation, the reaction was... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25458 ((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM25448 (4-[6-(4-fluorophenyl)-3,4-dihydronaphthalen-1-yl]p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25456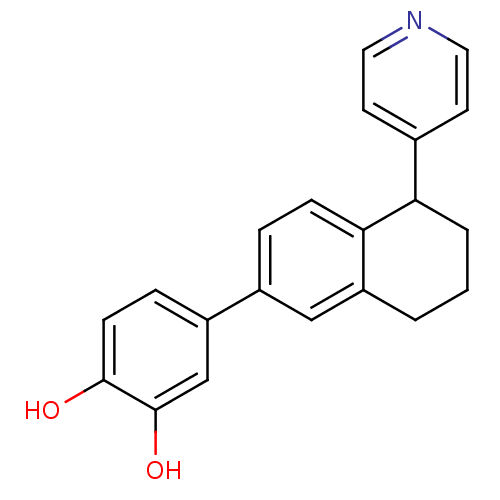 (4-[5-(pyridin-4-yl)-5,6,7,8-tetrahydronaphthalen-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 144 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM25446 (4-[6-(4-fluorophenyl)-1H-inden-3-yl]pyridine | Abi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 159 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description To measure CYP3A4 activity, the product of testosterone 6-hydroxylation, 6beta-hydroxytestosterone was determined. After incubation, the reaction was... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25454 (4-[6-(4-fluorophenyl)-1,2,3,4-tetrahydronaphthalen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 163 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25451 (2-fluoro-4-[5-(pyridin-4-yl)-7,8-dihydronaphthalen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 188 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25453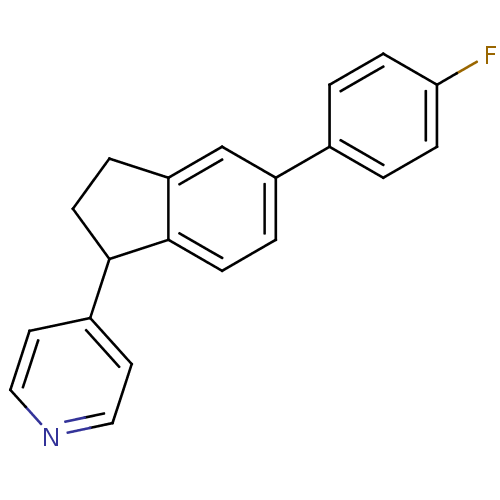 (4-[5-(4-fluorophenyl)-2,3-dihydro-1H-inden-1-yl]py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 233 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM25441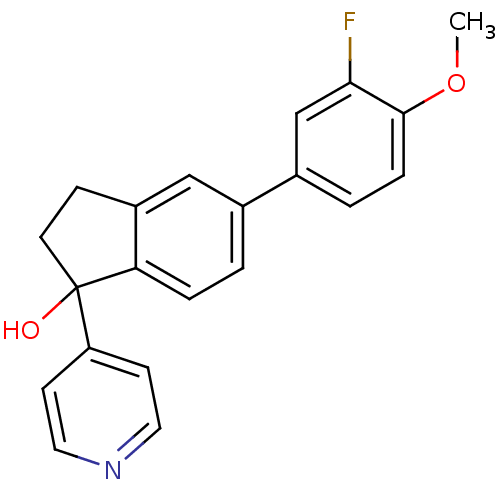 (5-(3-fluoro-4-methoxyphenyl)-1-(pyridin-4-yl)-2,3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 291 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25450 (4-[5-(pyridin-4-yl)-7,8-dihydronaphthalen-2-yl]ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 307 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM25449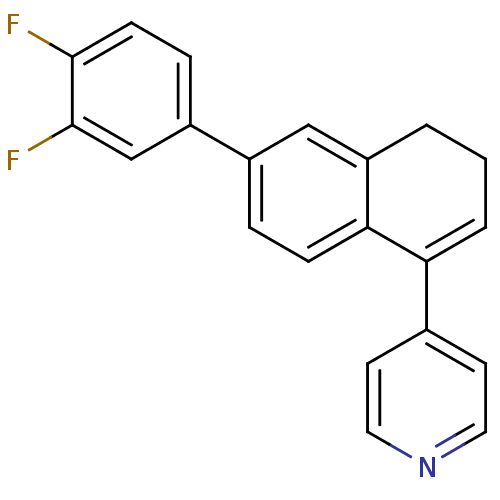 (4-[6-(3,4-difluorophenyl)-3,4-dihydronaphthalen-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 311 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25439 (5-(4-fluorophenyl)-1-(pyridin-4-yl)-2,3-dihydro-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 333 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM25450 (4-[5-(pyridin-4-yl)-7,8-dihydronaphthalen-2-yl]ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 357 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description To measure CYP3A4 activity, the product of testosterone 6-hydroxylation, 6beta-hydroxytestosterone was determined. After incubation, the reaction was... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25443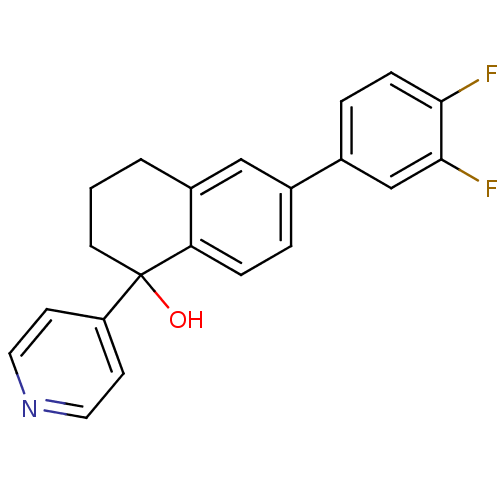 (6-(3,4-difluorophenyl)-1-(pyridin-4-yl)-1,2,3,4-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 423 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM25441 (5-(3-fluoro-4-methoxyphenyl)-1-(pyridin-4-yl)-2,3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 436 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM25454 (4-[6-(4-fluorophenyl)-1,2,3,4-tetrahydronaphthalen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 518 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM25453 (4-[5-(4-fluorophenyl)-2,3-dihydro-1H-inden-1-yl]py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 543 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM25455 (4-[6-(3,4-difluorophenyl)-1,2,3,4-tetrahydronaphth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 567 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM25457 (2-fluoro-4-[5-(pyridin-4-yl)-5,6,7,8-tetrahydronap...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 587 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25442 (6-(4-fluorophenyl)-1-(pyridin-4-yl)-1,2,3,4-tetrah...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 587 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM25456 (4-[5-(pyridin-4-yl)-5,6,7,8-tetrahydronaphthalen-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 632 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description To measure CYP3A4 activity, the product of testosterone 6-hydroxylation, 6beta-hydroxytestosterone was determined. After incubation, the reaction was... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM25444 (6-(3-fluoro-4-methoxyphenyl)-1-(pyridin-4-yl)-1,2,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 686 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM25439 (5-(4-fluorophenyl)-1-(pyridin-4-yl)-2,3-dihydro-1H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 816 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM25442 (6-(4-fluorophenyl)-1-(pyridin-4-yl)-1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM25444 (6-(3-fluoro-4-methoxyphenyl)-1-(pyridin-4-yl)-1,2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 945 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM25451 (2-fluoro-4-[5-(pyridin-4-yl)-7,8-dihydronaphthalen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 991 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM25453 (4-[5-(4-fluorophenyl)-2,3-dihydro-1H-inden-1-yl]py...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM25453 (4-[5-(4-fluorophenyl)-2,3-dihydro-1H-inden-1-yl]py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description To measure CYP3A4 activity, the product of testosterone 6-hydroxylation, 6beta-hydroxytestosterone was determined. After incubation, the reaction was... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM25442 (6-(4-fluorophenyl)-1-(pyridin-4-yl)-1,2,3,4-tetrah...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25455 (4-[6-(3,4-difluorophenyl)-1,2,3,4-tetrahydronaphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM25457 (2-fluoro-4-[5-(pyridin-4-yl)-5,6,7,8-tetrahydronap...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM25450 (4-[5-(pyridin-4-yl)-7,8-dihydronaphthalen-2-yl]ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM25458 ((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM25443 (6-(3,4-difluorophenyl)-1-(pyridin-4-yl)-1,2,3,4-te...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM25452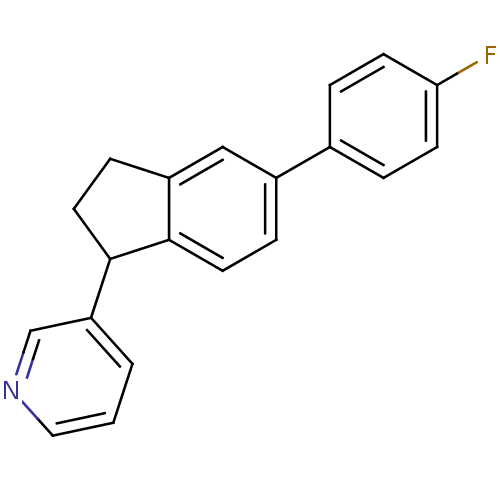 (3-[5-(4-fluorophenyl)-2,3-dihydro-1H-inden-1-yl]py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description To measure CYP3A4 activity, the product of testosterone 6-hydroxylation, 6beta-hydroxytestosterone was determined. After incubation, the reaction was... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM25458 ((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM25451 (2-fluoro-4-[5-(pyridin-4-yl)-7,8-dihydronaphthalen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description To measure CYP3A4 activity, the product of testosterone 6-hydroxylation, 6beta-hydroxytestosterone was determined. After incubation, the reaction was... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM25456 (4-[5-(pyridin-4-yl)-5,6,7,8-tetrahydronaphthalen-2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM25454 (4-[6-(4-fluorophenyl)-1,2,3,4-tetrahydronaphthalen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description To measure CYP3A4 activity, the product of testosterone 6-hydroxylation, 6beta-hydroxytestosterone was determined. After incubation, the reaction was... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM25455 (4-[6-(3,4-difluorophenyl)-1,2,3,4-tetrahydronaphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description To measure CYP3A4 activity, the product of testosterone 6-hydroxylation, 6beta-hydroxytestosterone was determined. After incubation, the reaction was... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM25456 (4-[5-(pyridin-4-yl)-5,6,7,8-tetrahydronaphthalen-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25445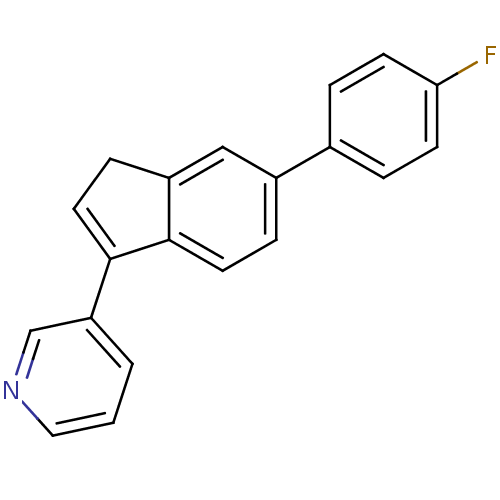 (3-[6-(4-fluorophenyl)-1H-inden-3-yl]pyridine | Abi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.35E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM25447 (4-[6-(4-methoxyphenyl)-1H-inden-3-yl]pyridine | Ab...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description To measure CYP3A4 activity, the product of testosterone 6-hydroxylation, 6beta-hydroxytestosterone was determined. After incubation, the reaction was... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM25458 ((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description To measure CYP3A4 activity, the product of testosterone 6-hydroxylation, 6beta-hydroxytestosterone was determined. After incubation, the reaction was... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM25451 (2-fluoro-4-[5-(pyridin-4-yl)-7,8-dihydronaphthalen...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.78E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM25443 (6-(3,4-difluorophenyl)-1-(pyridin-4-yl)-1,2,3,4-te...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 76 total ) | Next | Last >> |