 Found 16 hits with Last Name = 'macdonald' and Initial = 'ir'
Found 16 hits with Last Name = 'macdonald' and Initial = 'ir' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholinesterase
(Homo sapiens (Human)) | BDBM50219206
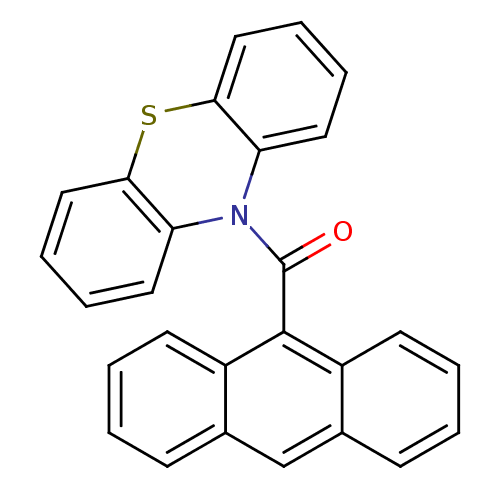
(Anthracen-9-yl (10H-phenothiazine-10yl) methanone,...)Show SMILES O=C(N1c2ccccc2Sc2ccccc12)c1c2ccccc2cc2ccccc12 Show InChI InChI=1S/C27H17NOS/c29-27(26-20-11-3-1-9-18(20)17-19-10-2-4-12-21(19)26)28-22-13-5-7-15-24(22)30-25-16-8-6-14-23(25)28/h1-17H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
| Assay Description
Inhibition constant using AChE or BuChE. |
Biochemistry 51: 7046-53 (2012)
Article DOI: 10.1021/bi300955k
BindingDB Entry DOI: 10.7270/Q2J101R6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50308418

(CHEMBL605824 | N-[2-(N',N'-diisopropylamin...)Show InChI InChI=1S/C21H27N3OS/c1-15(2)23(16(3)4)14-13-22-21(25)24-17-9-5-7-11-19(17)26-20-12-8-6-10-18(20)24/h5-12,15-16H,13-14H2,1-4H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
| Assay Description
Inhibition constant using AChE or BuChE. |
Biochemistry 51: 7046-53 (2012)
Article DOI: 10.1021/bi300955k
BindingDB Entry DOI: 10.7270/Q2J101R6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50292603
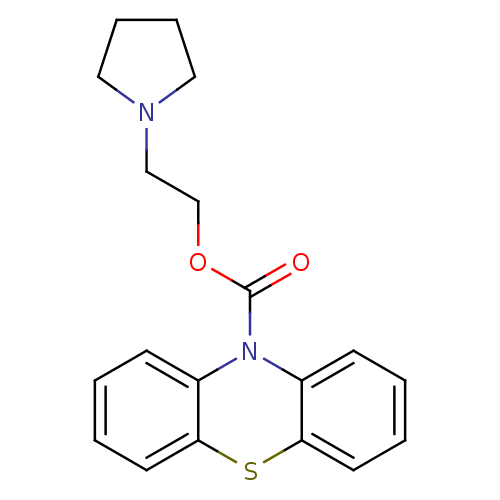
(2-(pyrrolidin-1-yl)ethyl 10H-phenothiazine-10-carb...)Show InChI InChI=1S/C19H20N2O2S/c22-19(23-14-13-20-11-5-6-12-20)21-15-7-1-3-9-17(15)24-18-10-4-2-8-16(18)21/h1-4,7-10H,5-6,11-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor (unknown origin) by PDSP assay |
Bioorg Med Chem Lett 23: 3822-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.082
BindingDB Entry DOI: 10.7270/Q2H133DC |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50292604
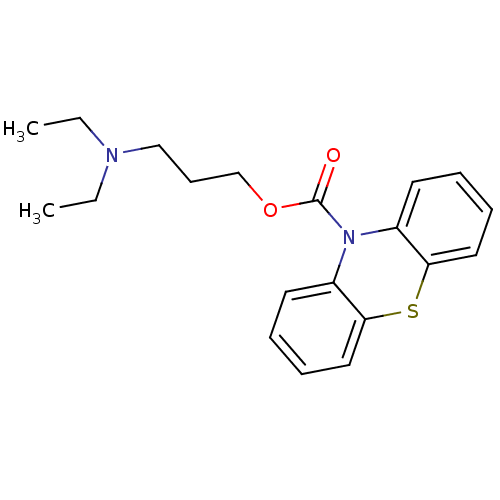
(3-(diethylamino)propyl 10H-phenothiazine-10-carbox...)Show InChI InChI=1S/C20H24N2O2S/c1-3-21(4-2)14-9-15-24-20(23)22-16-10-5-7-12-18(16)25-19-13-8-6-11-17(19)22/h5-8,10-13H,3-4,9,14-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor (unknown origin) by PDSP assay |
Bioorg Med Chem Lett 23: 3822-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.082
BindingDB Entry DOI: 10.7270/Q2H133DC |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50292603

(2-(pyrrolidin-1-yl)ethyl 10H-phenothiazine-10-carb...)Show InChI InChI=1S/C19H20N2O2S/c22-19(23-14-13-20-11-5-6-12-20)21-15-7-1-3-9-17(15)24-18-10-4-2-8-16(18)21/h1-4,7-10H,5-6,11-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H2 receptor (unknown origin) by PDSP assay |
Bioorg Med Chem Lett 23: 3822-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.082
BindingDB Entry DOI: 10.7270/Q2H133DC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM120262
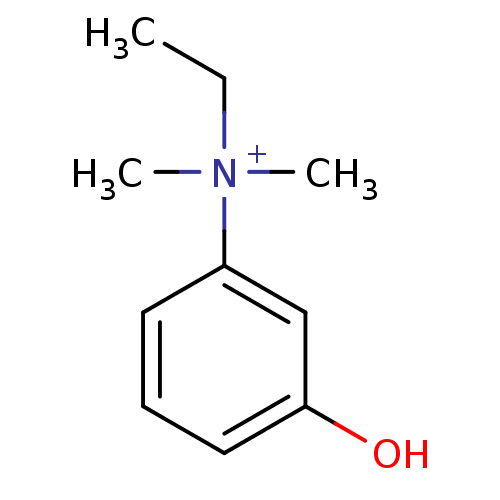
(EDROPHONIUM BROMIDE | EDROPHONIUM CHLORIDE | Edrop...)Show InChI InChI=1S/C10H15NO/c1-4-11(2,3)9-6-5-7-10(12)8-9/h5-8H,4H2,1-3H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
| Assay Description
Inhibition constant using AChE or BuChE. |
Biochemistry 51: 7046-53 (2012)
Article DOI: 10.1021/bi300955k
BindingDB Entry DOI: 10.7270/Q2J101R6 |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50292604

(3-(diethylamino)propyl 10H-phenothiazine-10-carbox...)Show InChI InChI=1S/C20H24N2O2S/c1-3-21(4-2)14-9-15-24-20(23)22-16-10-5-7-12-18(16)25-19-13-8-6-11-17(19)22/h5-8,10-13H,3-4,9,14-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H2 receptor (unknown origin) by PDSP assay |
Bioorg Med Chem Lett 23: 3822-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.082
BindingDB Entry DOI: 10.7270/Q2H133DC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50308418

(CHEMBL605824 | N-[2-(N',N'-diisopropylamin...)Show InChI InChI=1S/C21H27N3OS/c1-15(2)23(16(3)4)14-13-22-21(25)24-17-9-5-7-11-19(17)26-20-12-8-6-10-18(20)24/h5-12,15-16H,13-14H2,1-4H3,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
| Assay Description
Inhibition constant using AChE or BuChE. |
Biochemistry 51: 7046-53 (2012)
Article DOI: 10.1021/bi300955k
BindingDB Entry DOI: 10.7270/Q2J101R6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50292603

(2-(pyrrolidin-1-yl)ethyl 10H-phenothiazine-10-carb...)Show InChI InChI=1S/C19H20N2O2S/c22-19(23-14-13-20-11-5-6-12-20)21-15-7-1-3-9-17(15)24-18-10-4-2-8-16(18)21/h1-4,7-10H,5-6,11-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Antagonist activity at serotonin 5HT3 receptor (unknown origin) by PDSP assay |
Bioorg Med Chem Lett 23: 3822-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.082
BindingDB Entry DOI: 10.7270/Q2H133DC |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM31904
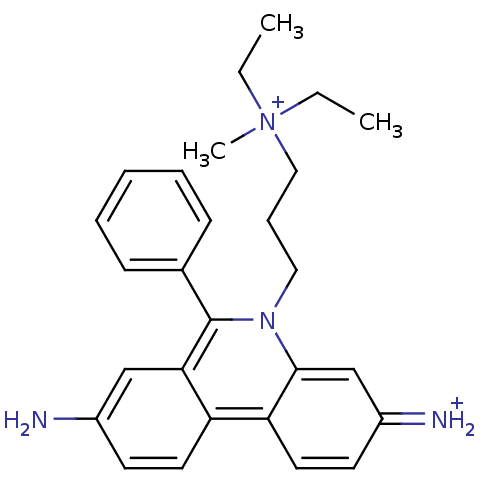
(CHEMBL345124 | Propidium | Propidium Iodide, 2 | p...)Show SMILES CC[N+](C)(CC)CCCn1c(-c2ccccc2)c2cc(N)ccc2c2ccc(=[NH2+])cc12 Show InChI InChI=1S/C27H33N4/c1-4-31(3,5-2)17-9-16-30-26-19-22(29)13-15-24(26)23-14-12-21(28)18-25(23)27(30)20-10-7-6-8-11-20/h6-8,10-15,18-19,29H,4-5,9,16-17,28H2,1-3H3/q+1/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
| Assay Description
Inhibition constant using AChE or BuChE. |
Biochemistry 51: 7046-53 (2012)
Article DOI: 10.1021/bi300955k
BindingDB Entry DOI: 10.7270/Q2J101R6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50100134

(2-(4-Dimethylamino-phenyl)-3,6-dimethyl-benzothiaz...)Show InChI InChI=1S/C17H19N2S/c1-12-5-10-15-16(11-12)20-17(19(15)4)13-6-8-14(9-7-13)18(2)3/h5-11H,1-4H3/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 3.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
| Assay Description
Inhibition constant using AChE or BuChE. |
Biochemistry 51: 7046-53 (2012)
Article DOI: 10.1021/bi300955k
BindingDB Entry DOI: 10.7270/Q2J101R6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50292604

(3-(diethylamino)propyl 10H-phenothiazine-10-carbox...)Show InChI InChI=1S/C20H24N2O2S/c1-3-21(4-2)14-9-15-24-20(23)22-16-10-5-7-12-18(16)25-19-13-8-6-11-17(19)22/h5-8,10-13H,3-4,9,14-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Antagonist activity at serotonin 5HT3 receptor (unknown origin) by PDSP assay |
Bioorg Med Chem Lett 23: 3822-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.082
BindingDB Entry DOI: 10.7270/Q2H133DC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50100134

(2-(4-Dimethylamino-phenyl)-3,6-dimethyl-benzothiaz...)Show InChI InChI=1S/C17H19N2S/c1-12-5-10-15-16(11-12)20-17(19(15)4)13-6-8-14(9-7-13)18(2)3/h5-11H,1-4H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
| Assay Description
Inhibition constant using AChE or BuChE. |
Biochemistry 51: 7046-53 (2012)
Article DOI: 10.1021/bi300955k
BindingDB Entry DOI: 10.7270/Q2J101R6 |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50219223
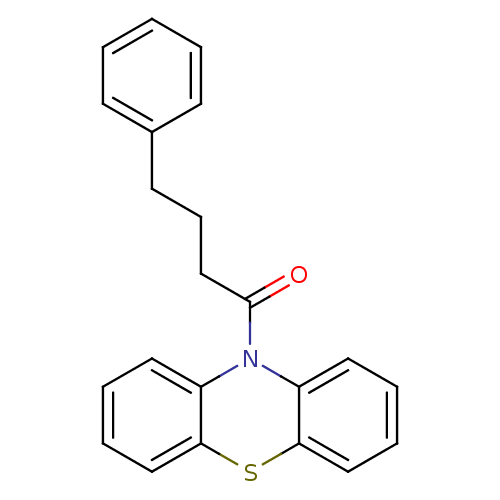
(1-(10H-phenothiazin-10-yl)-4-phenylbutan-1-one | C...)Show InChI InChI=1S/C22H19NOS/c24-22(16-8-11-17-9-2-1-3-10-17)23-18-12-4-6-14-20(18)25-21-15-7-5-13-19(21)23/h1-7,9-10,12-15H,8,11,16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H2 receptor (unknown origin) by PDSP assay |
Bioorg Med Chem Lett 23: 3822-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.082
BindingDB Entry DOI: 10.7270/Q2H133DC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM31904

(CHEMBL345124 | Propidium | Propidium Iodide, 2 | p...)Show SMILES CC[N+](C)(CC)CCCn1c(-c2ccccc2)c2cc(N)ccc2c2ccc(=[NH2+])cc12 Show InChI InChI=1S/C27H33N4/c1-4-31(3,5-2)17-9-16-30-26-19-22(29)13-15-24(26)23-14-12-21(28)18-25(23)27(30)20-10-7-6-8-11-20/h6-8,10-15,18-19,29H,4-5,9,16-17,28H2,1-3H3/q+1/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 9.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
| Assay Description
Inhibition constant using AChE or BuChE. |
Biochemistry 51: 7046-53 (2012)
Article DOI: 10.1021/bi300955k
BindingDB Entry DOI: 10.7270/Q2J101R6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM120262

(EDROPHONIUM BROMIDE | EDROPHONIUM CHLORIDE | Edrop...)Show InChI InChI=1S/C10H15NO/c1-4-11(2,3)9-6-5-7-10(12)8-9/h5-8H,4H2,1-3H3/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 1.98E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
| Assay Description
Inhibition constant using AChE or BuChE. |
Biochemistry 51: 7046-53 (2012)
Article DOI: 10.1021/bi300955k
BindingDB Entry DOI: 10.7270/Q2J101R6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data