

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50179206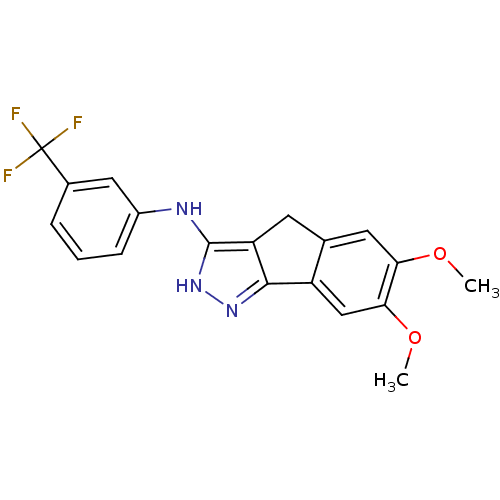 (3-trifluoromethyl-N-(6,7-dimethoxy-2,4-dihydroinde...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antiproliferative activity against PDGF-BB stimulated HCASMC | J Med Chem 48: 8163-73 (2005) Article DOI: 10.1021/jm050680m BindingDB Entry DOI: 10.7270/Q2W095HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM339271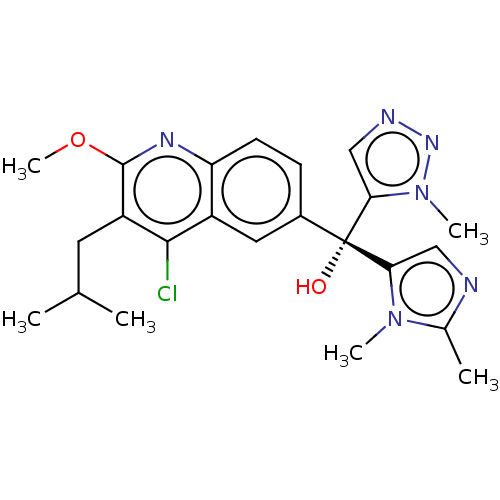 (US10201546, Example 134b) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description For the RORγt construct used in the ThermoFluorŪ assay, numbering for the nucleotide sequences was based on the reference sequence for human ROR... | US Patent US10201546 (2019) BindingDB Entry DOI: 10.7270/Q2F47R8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50179207 (3-fluoro-N-(6,7-dimethoxy-2,4-dihydroindeno[1,2-c]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antiproliferative activity against PDGF-BB stimulated HCASMC | J Med Chem 48: 8163-73 (2005) Article DOI: 10.1021/jm050680m BindingDB Entry DOI: 10.7270/Q2W095HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50179212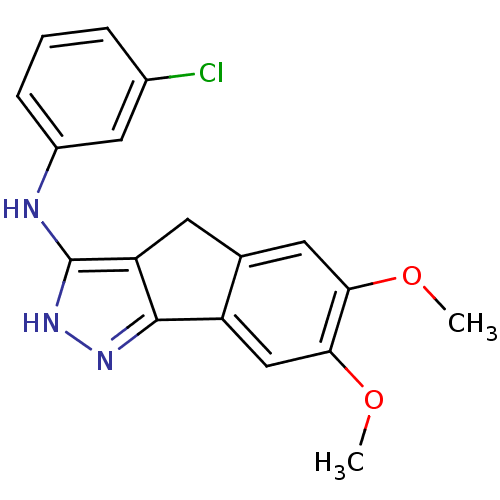 (3-chloro-N-(6,7-dimethoxy-2,4-dihydroindeno[1,2-c]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against PDGFRbeta kinase | J Med Chem 48: 8163-73 (2005) Article DOI: 10.1021/jm050680m BindingDB Entry DOI: 10.7270/Q2W095HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM339272 (US10201546, Example 134c) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description For the RORγt construct used in the ThermoFluorŪ assay, numbering for the nucleotide sequences was based on the reference sequence for human ROR... | US Patent US10201546 (2019) BindingDB Entry DOI: 10.7270/Q2F47R8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50179207 (3-fluoro-N-(6,7-dimethoxy-2,4-dihydroindeno[1,2-c]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against PDGFRbeta kinase | J Med Chem 48: 8163-73 (2005) Article DOI: 10.1021/jm050680m BindingDB Entry DOI: 10.7270/Q2W095HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM339171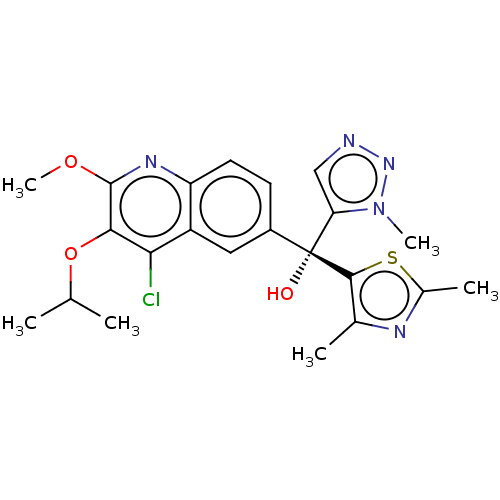 (US10201546, Example 85c) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description For the RORγt construct used in the ThermoFluorŪ assay, numbering for the nucleotide sequences was based on the reference sequence for human ROR... | US Patent US10201546 (2019) BindingDB Entry DOI: 10.7270/Q2F47R8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50179211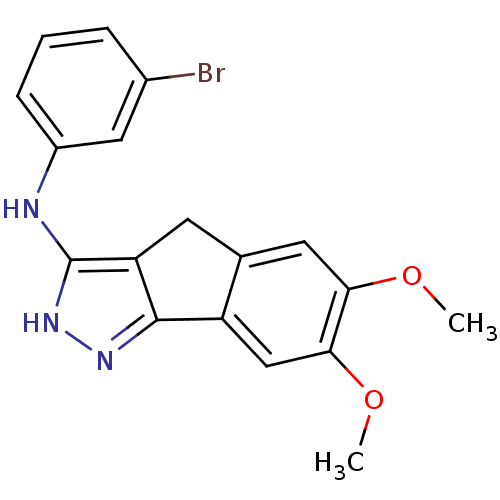 (3-bromo-N-(6,7-dimethoxy-2,4-dihydroindeno[1,2-c]p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against PDGFRbeta kinase | J Med Chem 48: 8163-73 (2005) Article DOI: 10.1021/jm050680m BindingDB Entry DOI: 10.7270/Q2W095HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50451743 (CHEMBL4205065) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, LLC Curated by ChEMBL | Assay Description Inverse agonist activity at GAL4 DBD-fused wild type human RORgammat LBD (850 to 1635 residues) expressed in HEK293T cells assessed as inhibition of ... | Bioorg Med Chem Lett 27: 5277-5283 (2017) Article DOI: 10.1016/j.bmcl.2017.10.027 BindingDB Entry DOI: 10.7270/Q2V98BN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM339269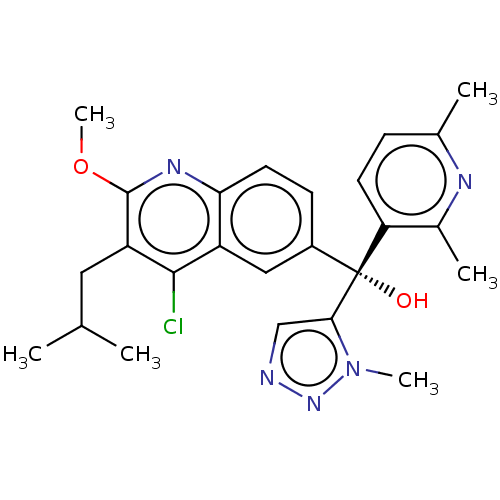 (US10201546, Example 133c) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description For the RORγt construct used in the ThermoFluorŪ assay, numbering for the nucleotide sequences was based on the reference sequence for human ROR... | US Patent US10201546 (2019) BindingDB Entry DOI: 10.7270/Q2F47R8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM220218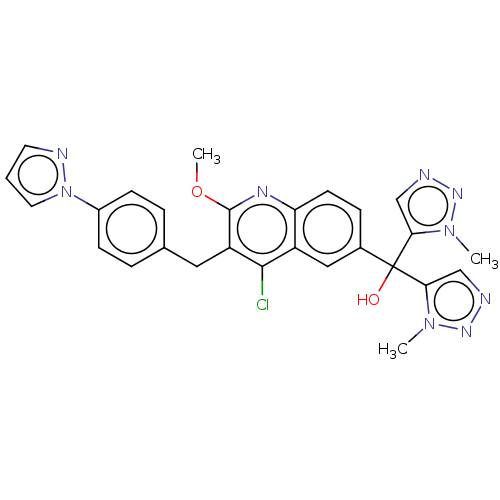 (US9290476, 61) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, LLC Curated by ChEMBL | Assay Description Inverse agonist activity at GAL4 DBD-fused wild type human RORgammat LBD (850 to 1635 residues) expressed in HEK293T cells assessed as inhibition of ... | Bioorg Med Chem Lett 27: 5277-5283 (2017) Article DOI: 10.1016/j.bmcl.2017.10.027 BindingDB Entry DOI: 10.7270/Q2V98BN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50239059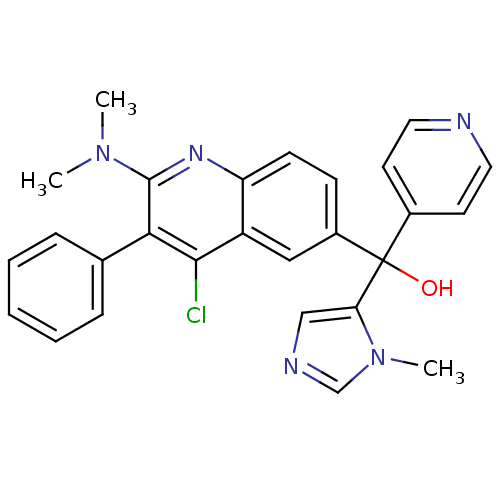 (CHEMBL4059503) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inverse agonist activity at GAL4 DBD-fused human RORgammat expressed in HEK293T cells assessed as inhibition of transcriptional activity after 24 hrs... | Bioorg Med Chem Lett 27: 2047-2057 (2017) Article DOI: 10.1016/j.bmcl.2017.02.044 BindingDB Entry DOI: 10.7270/Q25H7JCJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50239059 (CHEMBL4059503) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inverse agonist activity at GAL4 DBD-fused human RORgammat expressed in HEK293T cells assessed as inhibition of transcriptional activity after 24 hrs... | Bioorg Med Chem Lett 27: 2047-2057 (2017) Article DOI: 10.1016/j.bmcl.2017.02.044 BindingDB Entry DOI: 10.7270/Q25H7JCJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM339385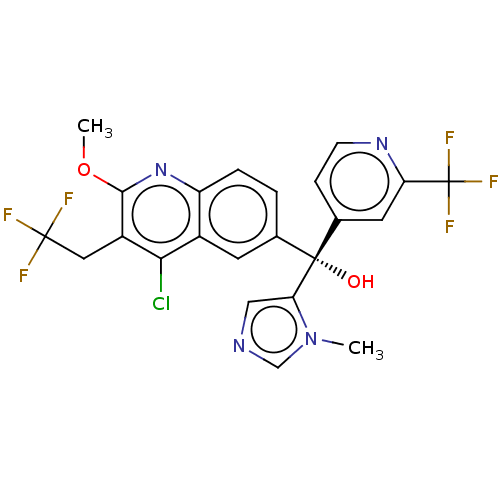 (US10201546, Example 225c) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description A reporter assay was used to test functional activity of RORγt modulatory compounds on transcriptional activation driven by the RORγt LBD. ... | US Patent US10201546 (2019) BindingDB Entry DOI: 10.7270/Q2F47R8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM339273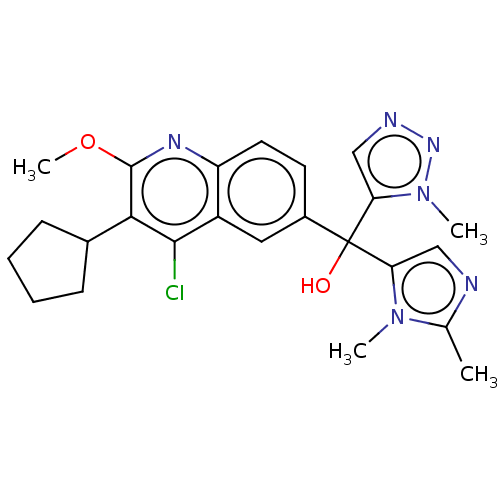 (US10201546, Example 135a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description For the RORγt construct used in the ThermoFluorŪ assay, numbering for the nucleotide sequences was based on the reference sequence for human ROR... | US Patent US10201546 (2019) BindingDB Entry DOI: 10.7270/Q2F47R8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50179210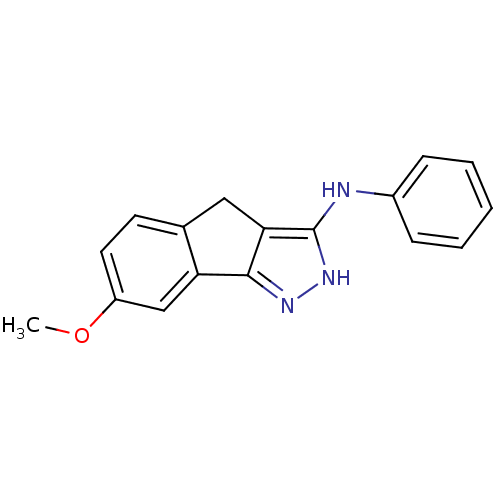 ((7-methoxy-2,4-dihydroindeno[1,2-c]pyrazol-3-yl)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antiproliferative activity against PDGF-BB stimulated HCASMC | J Med Chem 48: 8163-73 (2005) Article DOI: 10.1021/jm050680m BindingDB Entry DOI: 10.7270/Q2W095HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50179209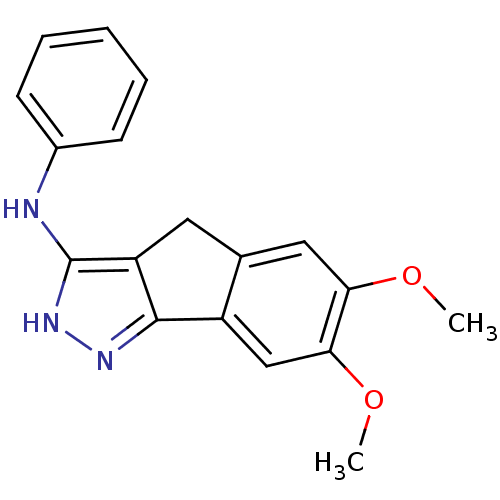 ((6,7-dimethoxy-2,4-dihydroindeno[1,2-c]pyrazol-3-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against PDGFRbeta kinase | J Med Chem 48: 8163-73 (2005) Article DOI: 10.1021/jm050680m BindingDB Entry DOI: 10.7270/Q2W095HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50179209 ((6,7-dimethoxy-2,4-dihydroindeno[1,2-c]pyrazol-3-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against PDGFRbeta kinase | J Med Chem 48: 8163-73 (2005) Article DOI: 10.1021/jm050680m BindingDB Entry DOI: 10.7270/Q2W095HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM339207 (US10201546, Example 103a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description For the RORγt construct used in the ThermoFluorŪ assay, numbering for the nucleotide sequences was based on the reference sequence for human ROR... | US Patent US10201546 (2019) BindingDB Entry DOI: 10.7270/Q2F47R8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50179204 (3-methoxy-N-(6,7-dimethoxy-2,4-dihydroindeno[1,2-c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against PDGFRbeta kinase | J Med Chem 48: 8163-73 (2005) Article DOI: 10.1021/jm050680m BindingDB Entry DOI: 10.7270/Q2W095HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50179204 (3-methoxy-N-(6,7-dimethoxy-2,4-dihydroindeno[1,2-c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against PDGFRbeta kinase | J Med Chem 48: 8163-73 (2005) Article DOI: 10.1021/jm050680m BindingDB Entry DOI: 10.7270/Q2W095HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma [258-518,Y502F] (Homo sapiens (Human)) | BDBM221253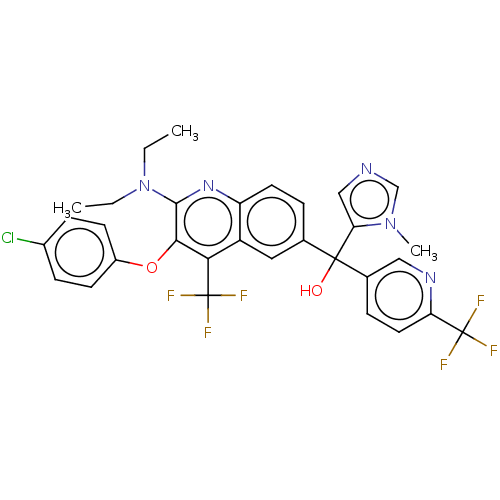 (US9303015, 27a | US9303015, 27b | US9303015, 27c) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The reporter assay was performed by transiently transfecting HEK293T cells with 5 μg of pBIND-RORγt LBD or pBIND-RORγt LBD-AF2 and 5 &... | US Patent US9303015 (2016) BindingDB Entry DOI: 10.7270/Q29P30H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50239056 (CHEMBL4062264) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inverse agonist activity at GAL4 DBD-fused human RORgammat expressed in HEK293T cells assessed as inhibition of transcriptional activity after 24 hrs... | Bioorg Med Chem Lett 27: 2047-2057 (2017) Article DOI: 10.1016/j.bmcl.2017.02.044 BindingDB Entry DOI: 10.7270/Q25H7JCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50239059 (CHEMBL4059503) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inverse agonist activity at GAL4 DBD-fused human RORgammat expressed in HEK293T cells assessed as inhibition of transcriptional activity after 24 hrs... | Bioorg Med Chem Lett 27: 2047-2057 (2017) Article DOI: 10.1016/j.bmcl.2017.02.044 BindingDB Entry DOI: 10.7270/Q25H7JCJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM339267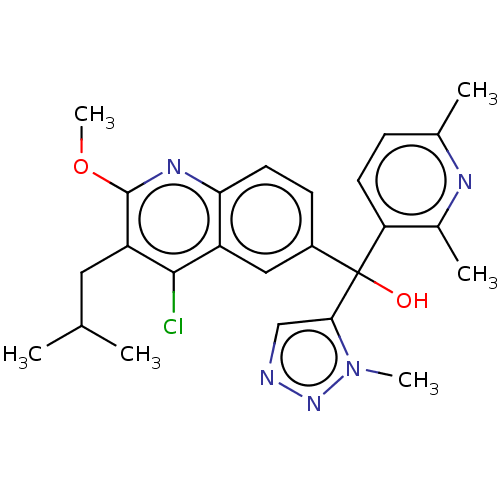 (US10201546, Example 133a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description For the RORγt construct used in the ThermoFluorŪ assay, numbering for the nucleotide sequences was based on the reference sequence for human ROR... | US Patent US10201546 (2019) BindingDB Entry DOI: 10.7270/Q2F47R8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM339278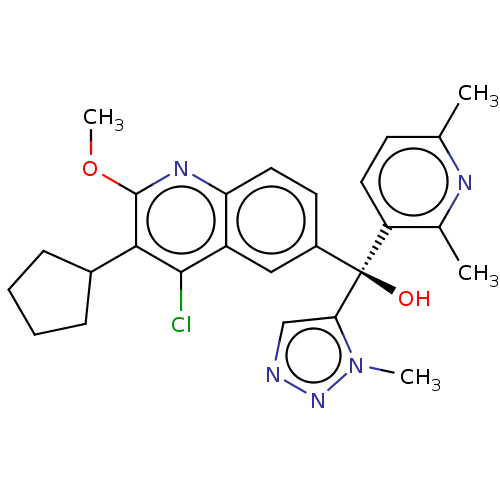 (US10201546, Example 136c) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description A reporter assay was used to test functional activity of RORγt modulatory compounds on transcriptional activation driven by the RORγt LBD. ... | US Patent US10201546 (2019) BindingDB Entry DOI: 10.7270/Q2F47R8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM339279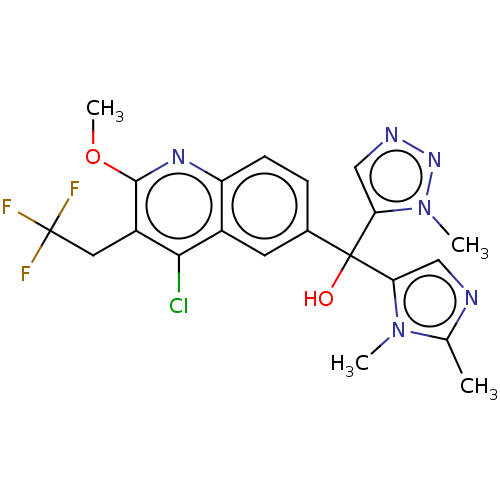 (US10201546, Example 137) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description A reporter assay was used to test functional activity of RORγt modulatory compounds on transcriptional activation driven by the RORγt LBD. ... | US Patent US10201546 (2019) BindingDB Entry DOI: 10.7270/Q2F47R8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50179216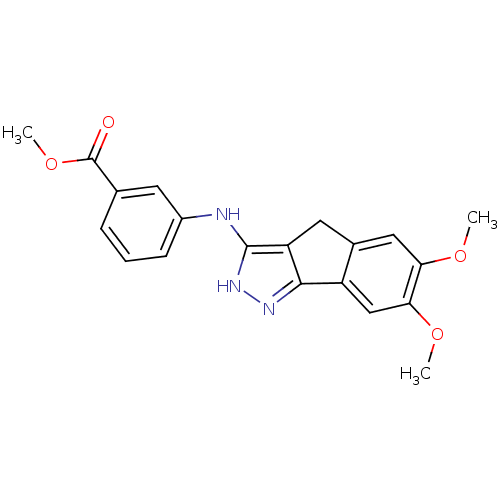 (3-carbomethoxy-N-(6,7-dimethoxy-2,4-dihydroindeno[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antiproliferative activity against PDGF-BB stimulated HCASMC | J Med Chem 48: 8163-73 (2005) Article DOI: 10.1021/jm050680m BindingDB Entry DOI: 10.7270/Q2W095HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50179216 (3-carbomethoxy-N-(6,7-dimethoxy-2,4-dihydroindeno[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antiproliferative activity against PDGF-BB stimulated HCASMC | J Med Chem 48: 8163-73 (2005) Article DOI: 10.1021/jm050680m BindingDB Entry DOI: 10.7270/Q2W095HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM339259 (US10201546, Example 129c) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description A reporter assay was used to test functional activity of RORγt modulatory compounds on transcriptional activation driven by the RORγt LBD. ... | US Patent US10201546 (2019) BindingDB Entry DOI: 10.7270/Q2F47R8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM339200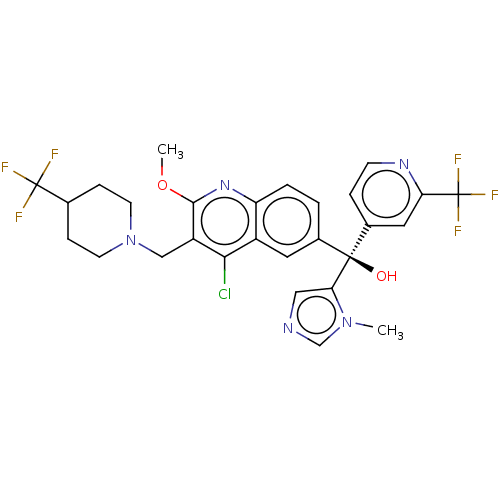 (US10201546, Example 100c) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description For the RORγt construct used in the ThermoFluorŪ assay, numbering for the nucleotide sequences was based on the reference sequence for human ROR... | US Patent US10201546 (2019) BindingDB Entry DOI: 10.7270/Q2F47R8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM339405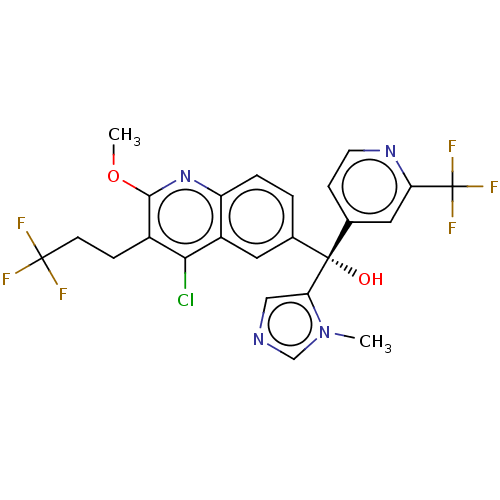 (US10201546, Example 244c) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description A reporter assay was used to test functional activity of RORγt modulatory compounds on transcriptional activation driven by the RORγt LBD. ... | US Patent US10201546 (2019) BindingDB Entry DOI: 10.7270/Q2F47R8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50179217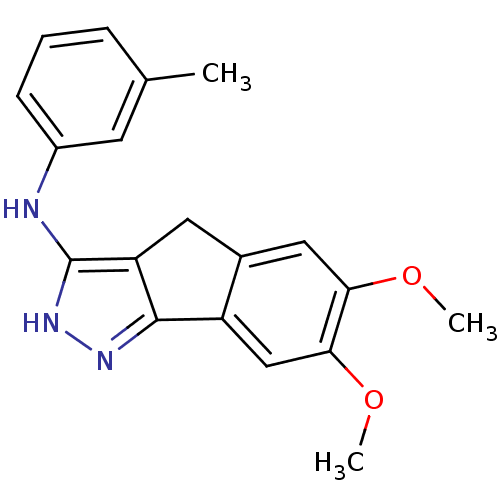 (3-methyl-N-(6,7-dimethoxy-2,4-dihydroindeno[1,2-c]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against PDGFRbeta kinase | J Med Chem 48: 8163-73 (2005) Article DOI: 10.1021/jm050680m BindingDB Entry DOI: 10.7270/Q2W095HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50451754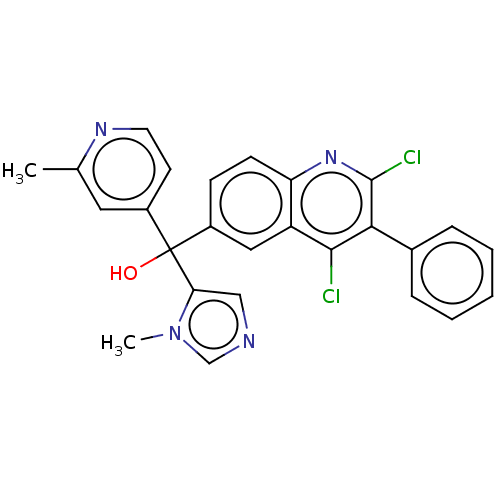 (CHEMBL4217859) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, LLC Curated by ChEMBL | Assay Description Inverse agonist activity at GAL4 DBD-fused wild type human RORgammat LBD (850 to 1635 residues) expressed in HEK293T cells assessed as inhibition of ... | Bioorg Med Chem Lett 27: 5277-5283 (2017) Article DOI: 10.1016/j.bmcl.2017.10.027 BindingDB Entry DOI: 10.7270/Q2V98BN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50451750 (CHEMBL4204175) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, LLC Curated by ChEMBL | Assay Description Inverse agonist activity at GAL4 DBD-fused wild type human RORgammat LBD (850 to 1635 residues) expressed in HEK293T cells assessed as inhibition of ... | Bioorg Med Chem Lett 27: 5277-5283 (2017) Article DOI: 10.1016/j.bmcl.2017.10.027 BindingDB Entry DOI: 10.7270/Q2V98BN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50451750 (CHEMBL4204175) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, LLC Curated by ChEMBL | Assay Description Inverse agonist activity at GAL4 DBD-fused wild type human RORgammat LBD (850 to 1635 residues) expressed in HEK293T cells assessed as inhibition of ... | Bioorg Med Chem Lett 27: 5277-5283 (2017) Article DOI: 10.1016/j.bmcl.2017.10.027 BindingDB Entry DOI: 10.7270/Q2V98BN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM339363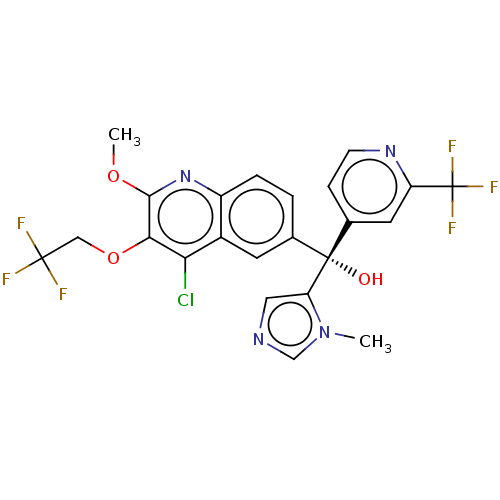 (US10201546, Example 213c) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description A reporter assay was used to test functional activity of RORγt modulatory compounds on transcriptional activation driven by the RORγt LBD. ... | US Patent US10201546 (2019) BindingDB Entry DOI: 10.7270/Q2F47R8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM339377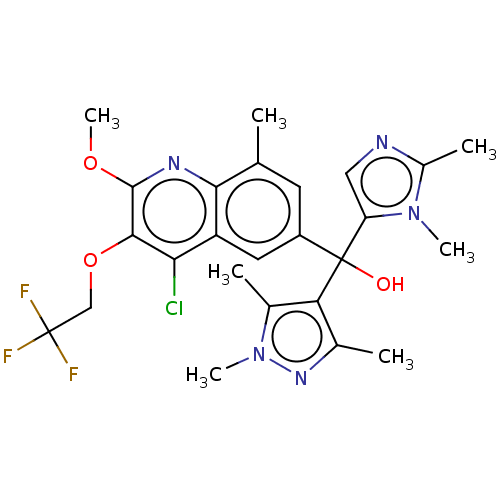 (US10201546, Example 220) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description A reporter assay was used to test functional activity of RORγt modulatory compounds on transcriptional activation driven by the RORγt LBD. ... | US Patent US10201546 (2019) BindingDB Entry DOI: 10.7270/Q2F47R8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM339252 (US10201546, Example 126b) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description A reporter assay was used to test functional activity of RORγt modulatory compounds on transcriptional activation driven by the RORγt LBD. ... | US Patent US10201546 (2019) BindingDB Entry DOI: 10.7270/Q2F47R8C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM339278 (US10201546, Example 136c) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description For the RORγt construct used in the ThermoFluorŪ assay, numbering for the nucleotide sequences was based on the reference sequence for human ROR... | US Patent US10201546 (2019) BindingDB Entry DOI: 10.7270/Q2F47R8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM339165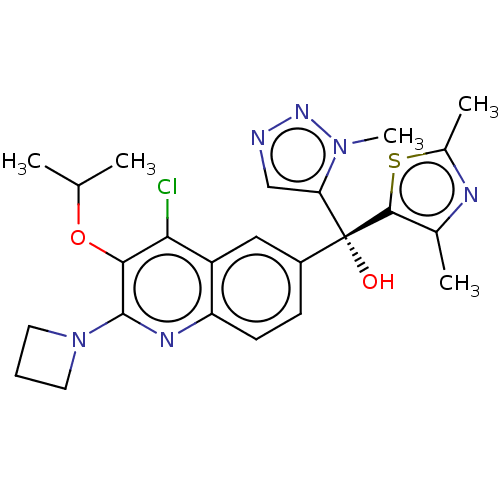 (US10201546, Example 82c) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description For the RORγt construct used in the ThermoFluorŪ assay, numbering for the nucleotide sequences was based on the reference sequence for human ROR... | US Patent US10201546 (2019) BindingDB Entry DOI: 10.7270/Q2F47R8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM339389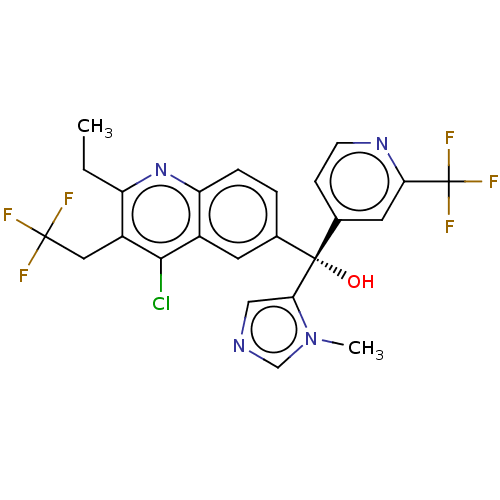 (US10201546, Example 227c) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description A reporter assay was used to test functional activity of RORγt modulatory compounds on transcriptional activation driven by the RORγt LBD. ... | US Patent US10201546 (2019) BindingDB Entry DOI: 10.7270/Q2F47R8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM339270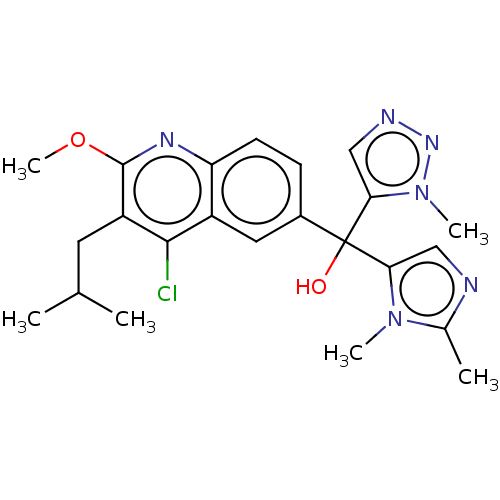 (US10201546, Example 134a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description For the RORγt construct used in the ThermoFluorŪ assay, numbering for the nucleotide sequences was based on the reference sequence for human ROR... | US Patent US10201546 (2019) BindingDB Entry DOI: 10.7270/Q2F47R8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma [258-518,Y502F] (Artificial Sequence) | BDBM231231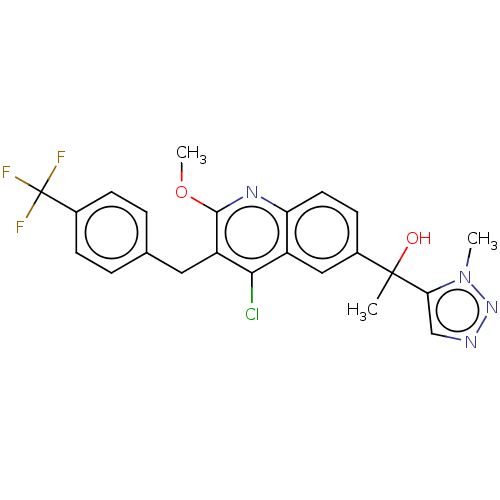 (US9346782, 11a | US9346782, 11b | US9346782, 11c) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description The reporter assay was performed by transiently transfecting HEK293T cells with 5 μg of pBIND-RORγt LBD or pBIND-RORγt LBD-AF2 and 5 &... | US Patent US9346782 (2016) BindingDB Entry DOI: 10.7270/Q2DB80PS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma [258-518,Y502F] (Homo sapiens (Human)) | BDBM221234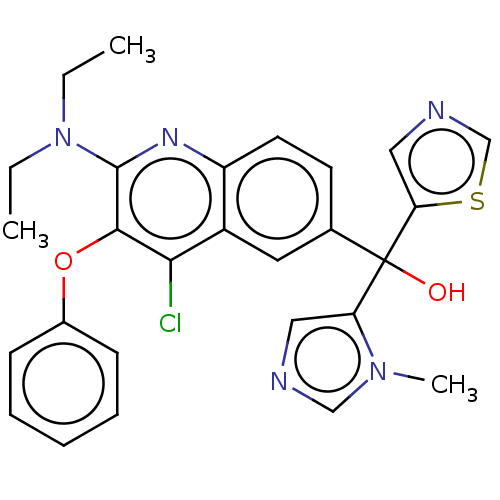 (US9303015, 8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The reporter assay was performed by transiently transfecting HEK293T cells with 5 μg of pBIND-RORγt LBD or pBIND-RORγt LBD-AF2 and 5 &... | US Patent US9303015 (2016) BindingDB Entry DOI: 10.7270/Q29P30H5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM339214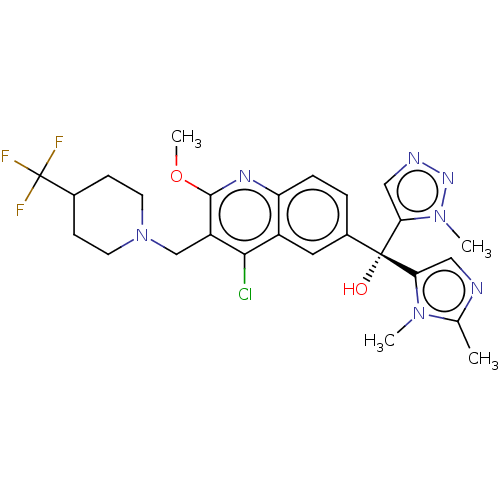 (US10201546, Example 106b) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description For the RORγt construct used in the ThermoFluorŪ assay, numbering for the nucleotide sequences was based on the reference sequence for human ROR... | US Patent US10201546 (2019) BindingDB Entry DOI: 10.7270/Q2F47R8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM339215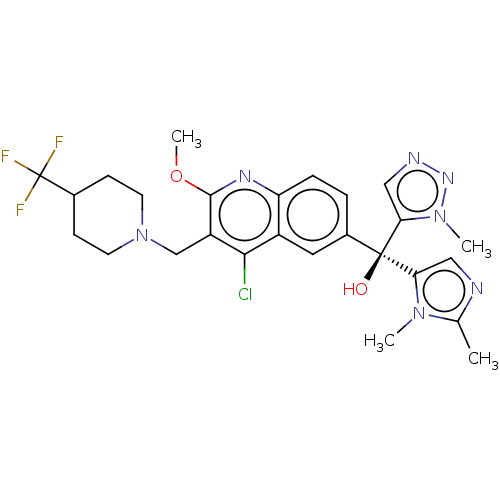 (US10201546, Example 106c) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description For the RORγt construct used in the ThermoFluorŪ assay, numbering for the nucleotide sequences was based on the reference sequence for human ROR... | US Patent US10201546 (2019) BindingDB Entry DOI: 10.7270/Q2F47R8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50179208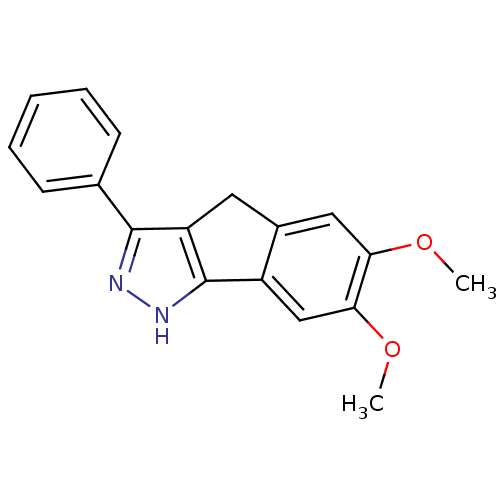 (6,7-dimethoxy-3-phenyl-1,4-dihydroindeno[1,2-c]pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against PDGFRbeta kinase | J Med Chem 48: 8163-73 (2005) Article DOI: 10.1021/jm050680m BindingDB Entry DOI: 10.7270/Q2W095HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50179208 (6,7-dimethoxy-3-phenyl-1,4-dihydroindeno[1,2-c]pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against PDGFRbeta kinase | J Med Chem 48: 8163-73 (2005) Article DOI: 10.1021/jm050680m BindingDB Entry DOI: 10.7270/Q2W095HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM339374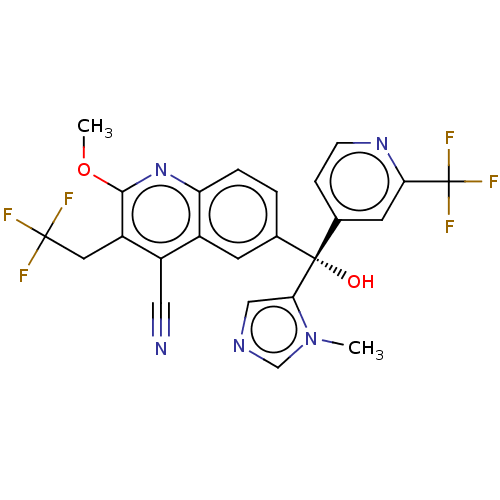 (US10201546, Example 218c) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description A reporter assay was used to test functional activity of RORγt modulatory compounds on transcriptional activation driven by the RORγt LBD. ... | US Patent US10201546 (2019) BindingDB Entry DOI: 10.7270/Q2F47R8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1325 total ) | Next | Last >> |