

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM85134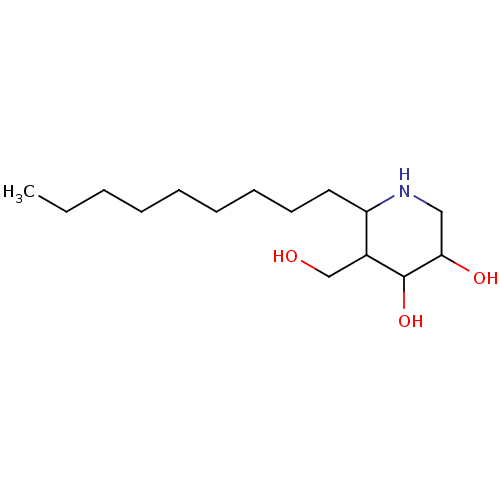 (Isofagomine derivative, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
University of British Columbia | Assay Description Fluorescene-based assay using beta-glucocerebrosidase. | Chembiochem 12: 2151-4 (2011) Article DOI: 10.1002/cbic.201100332 BindingDB Entry DOI: 10.7270/Q2WD3Z3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM85134 (Isofagomine derivative, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
University of British Columbia | Assay Description Fluorescene-based assay using beta-glucocerebrosidase. | Chembiochem 12: 2151-4 (2011) Article DOI: 10.1002/cbic.201100332 BindingDB Entry DOI: 10.7270/Q2WD3Z3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383315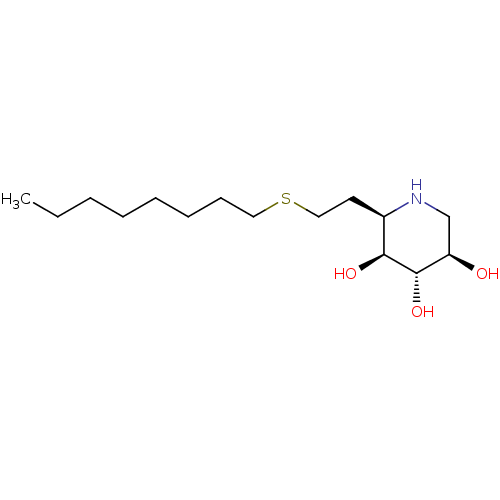 (CHEMBL2029773) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 4-methylumbelliferyl beta-D-glucopyranoside as substrate after 15 mins by Dixon an... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383327 (CHEMBL2029772) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 4-methylumbelliferyl beta-D-glucopyranoside as substrate after 15 mins by Dixon an... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383321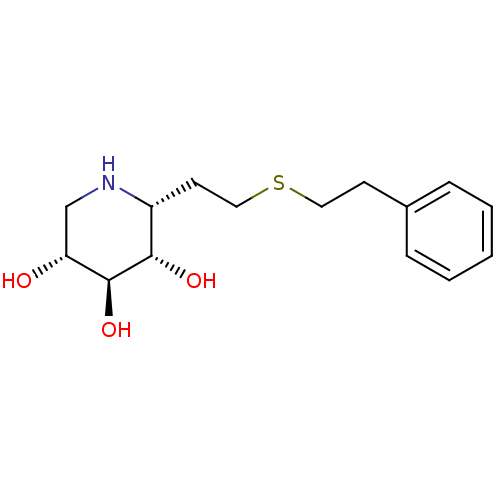 (CHEMBL2029780) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 4-methylumbelliferyl beta-D-glucopyranoside as substrate after 15 mins by Dixon an... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383323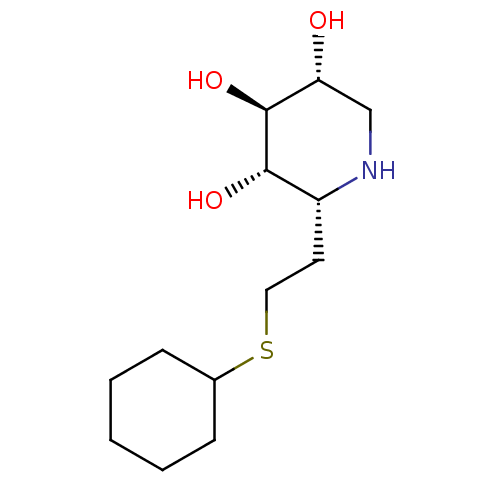 (CHEMBL2029778) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 4-methylumbelliferyl beta-D-glucopyranoside as substrate after 15 mins by Dixon an... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383325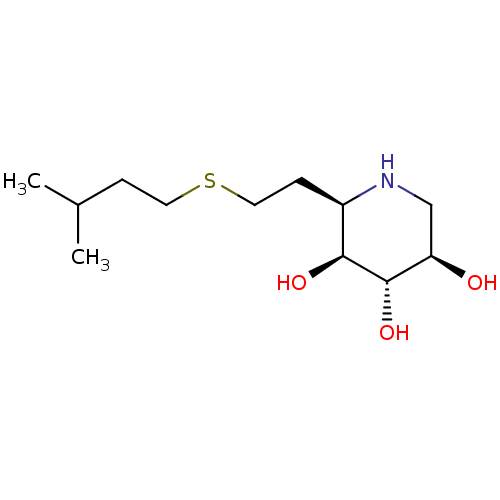 (CHEMBL2029776) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 4-methylumbelliferyl beta-D-glucopyranoside as substrate after 15 mins by Dixon an... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50075408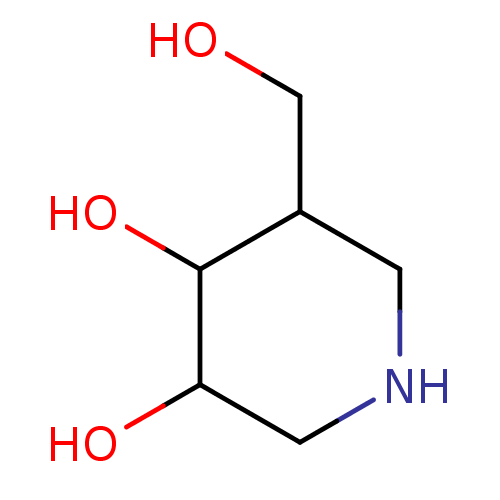 (5-(hydroxymethyl)piperidine-3,4-diol | CHEMBL34512...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
University of British Columbia | Assay Description Fluorescene-based assay using beta-glucocerebrosidase. | Chembiochem 12: 2151-4 (2011) Article DOI: 10.1002/cbic.201100332 BindingDB Entry DOI: 10.7270/Q2WD3Z3W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383324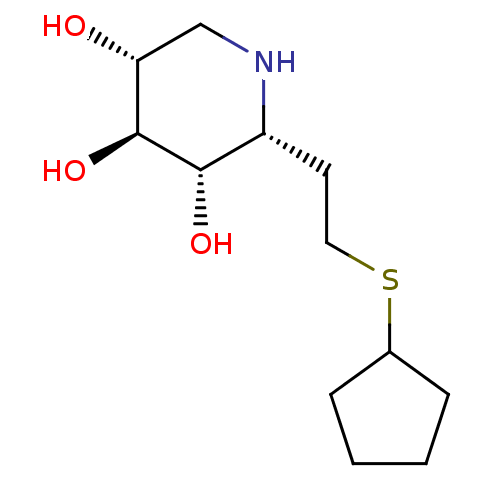 (CHEMBL2029777) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 4-methylumbelliferyl beta-D-glucopyranoside as substrate after 15 mins by Dixon an... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383319 (CHEMBL2029782) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 4-methylumbelliferyl beta-D-glucopyranoside as substrate after 15 mins by Dixon an... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383328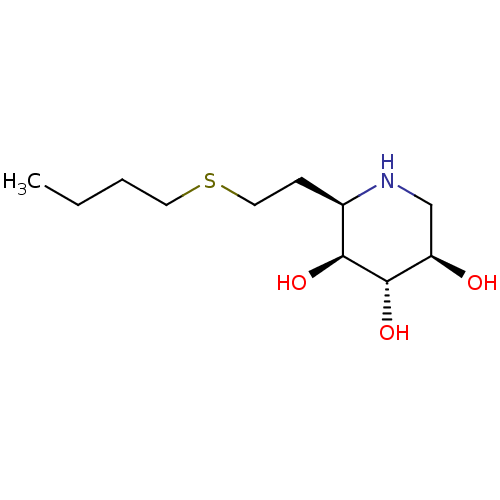 (CHEMBL2029771) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 4-methylumbelliferyl beta-D-glucopyranoside as substrate after 15 mins by Dixon an... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383322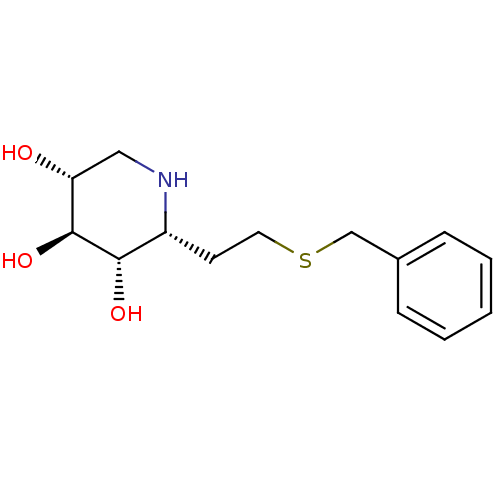 (CHEMBL2029779) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 4-methylumbelliferyl beta-D-glucopyranoside as substrate after 15 mins by Dixon an... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50075408 (5-(hydroxymethyl)piperidine-3,4-diol | CHEMBL34512...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
University of British Columbia | Assay Description Fluorescene-based assay using beta-glucocerebrosidase. | Chembiochem 12: 2151-4 (2011) Article DOI: 10.1002/cbic.201100332 BindingDB Entry DOI: 10.7270/Q2WD3Z3W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383326 (CHEMBL2029775) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 2,4-dinitrophenyl beta-D-glucopyranoside as substrate after 3 mins by Dixon and Li... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383318 (CHEMBL2029783) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 2,4-dinitrophenyl beta-D-glucopyranoside as substrate after 3 mins by Dixon and Li... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383314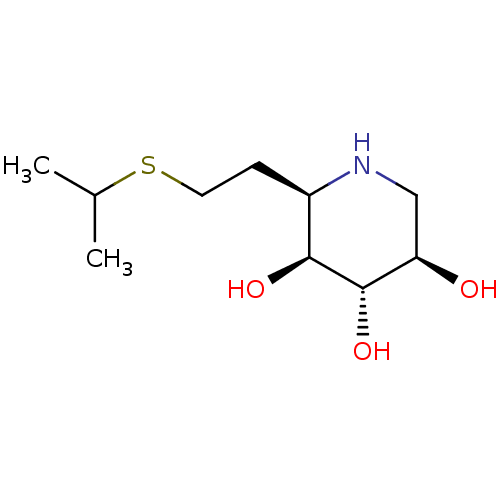 (CHEMBL2029774) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 2,4-dinitrophenyl beta-D-glucopyranoside as substrate after 3 mins by Dixon and Li... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM85133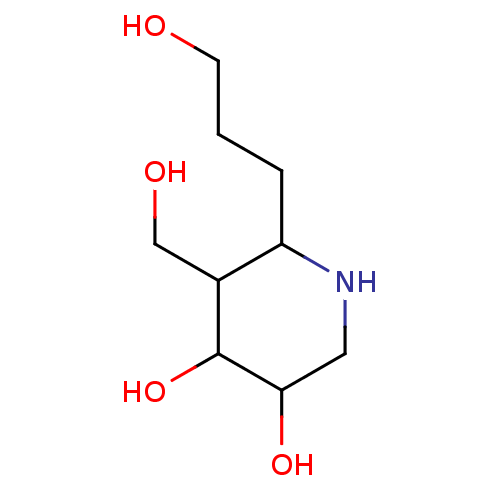 (Isofagomine derivative, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
University of British Columbia | Assay Description Fluorescene-based assay using beta-glucocerebrosidase. | Chembiochem 12: 2151-4 (2011) Article DOI: 10.1002/cbic.201100332 BindingDB Entry DOI: 10.7270/Q2WD3Z3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM85133 (Isofagomine derivative, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
University of British Columbia | Assay Description Fluorescene-based assay using beta-glucocerebrosidase. | Chembiochem 12: 2151-4 (2011) Article DOI: 10.1002/cbic.201100332 BindingDB Entry DOI: 10.7270/Q2WD3Z3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383329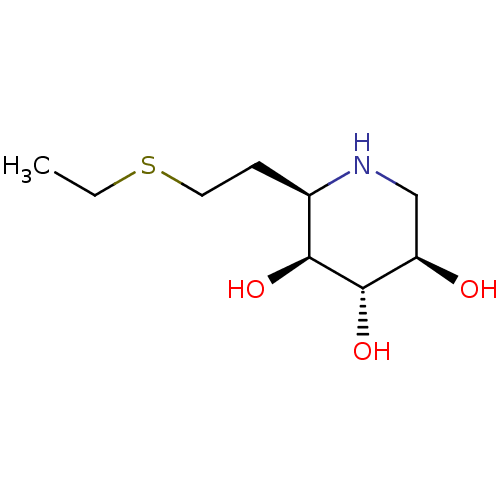 (CHEMBL2029770) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 2,4-dinitrophenyl beta-D-glucopyranoside as substrate after 3 mins by Dixon and Li... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383320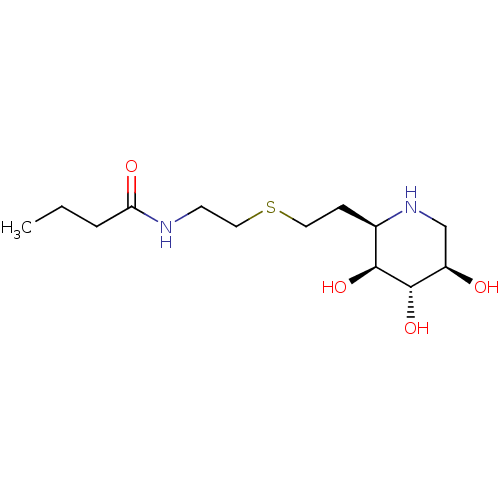 (CHEMBL2029781) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 2,4-dinitrophenyl beta-D-glucopyranoside as substrate after 3 mins by Dixon and Li... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM85131 (Isofagomine derivative, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
University of British Columbia | Assay Description Fluorescene-based assay using beta-glucocerebrosidase. | Chembiochem 12: 2151-4 (2011) Article DOI: 10.1002/cbic.201100332 BindingDB Entry DOI: 10.7270/Q2WD3Z3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383317 (CHEMBL2029784) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 2,4-dinitrophenyl beta-D-glucopyranoside as substrate after 3 mins by Dixon and Li... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383316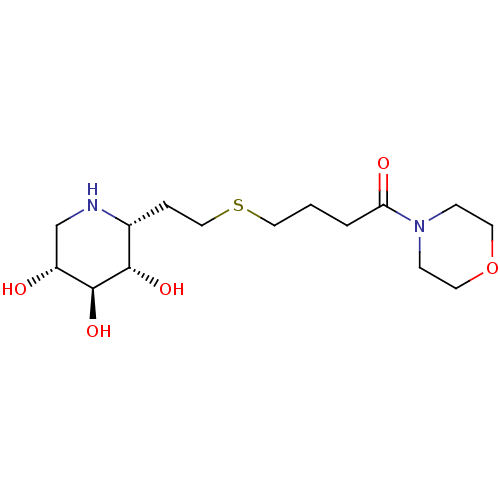 (CHEMBL2029785) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 2,4-dinitrophenyl beta-D-glucopyranoside as substrate after 3 mins by Dixon and Li... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM85132 (Isofagomine derivative, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
University of British Columbia | Assay Description Fluorescene-based assay using beta-glucocerebrosidase. | Chembiochem 12: 2151-4 (2011) Article DOI: 10.1002/cbic.201100332 BindingDB Entry DOI: 10.7270/Q2WD3Z3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM85131 (Isofagomine derivative, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 610 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
University of British Columbia | Assay Description Fluorescene-based assay using beta-glucocerebrosidase. | Chembiochem 12: 2151-4 (2011) Article DOI: 10.1002/cbic.201100332 BindingDB Entry DOI: 10.7270/Q2WD3Z3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383330 (CHEMBL2029769) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 2,4-dinitrophenyl beta-D-glucopyranoside as substrate after 3 mins by Dixon and Li... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM85132 (Isofagomine derivative, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
University of British Columbia | Assay Description Fluorescene-based assay using beta-glucocerebrosidase. | Chembiochem 12: 2151-4 (2011) Article DOI: 10.1002/cbic.201100332 BindingDB Entry DOI: 10.7270/Q2WD3Z3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50383331 (CHEMBL2029768) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 2,4-dinitrophenyl beta-D-glucopyranoside as substrate after 3 mins by Dixon and Li... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50182798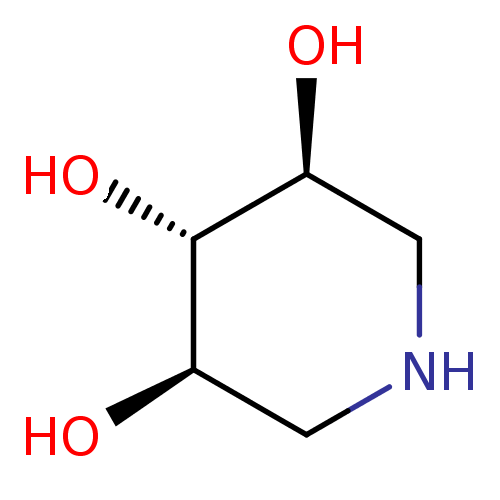 ((3R,4r,5S)-piperidine-3,4,5-triol | 1,5-Dideoxy-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Competitive inhibition of wild type human glucocerebrosidase using 2,4-dinitrophenyl beta-D-glucopyranoside as substrate after 3 mins by Dixon and Li... | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit alpha (Homo sapiens (Human)) | BDBM68271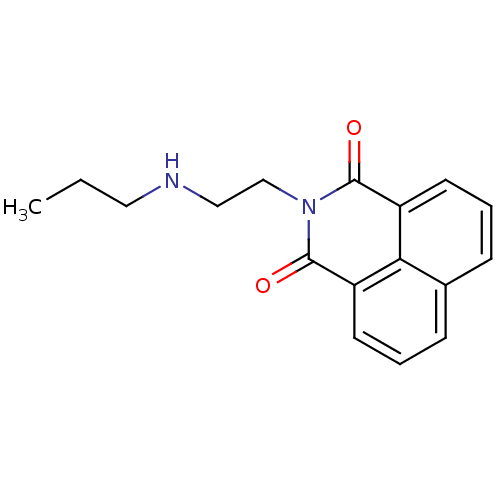 (M-31850) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | 25 |
Research Institute, Hospital for Sick Children | Assay Description Enzyme assay based on 4-MU substrates with HTS to identify inhibitory compounds for Hex. | Chem Biol 14: 153-64 (2007) Article DOI: 10.1016/j.chembiol.2006.12.006 BindingDB Entry DOI: 10.7270/Q25B00W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic beta-glucosidase (Homo sapiens (Human)) | BDBM50383315 (CHEMBL2029773) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Inhibition of human cytosolic beta-glucosidase using 4-methylumbelliferyl beta-D-glucopyranoside as substrate after 15 mins by fluorimetric analysis | J Med Chem 55: 2737-45 (2012) Article DOI: 10.1021/jm201633y BindingDB Entry DOI: 10.7270/Q2639QSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit alpha (Homo sapiens (Human)) | BDBM18512 (5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Genetics and Genome Biology, SickKids, PGCRL 686 Bay Street, Toronto, Ontario M5G 0A4, Canada. Curated by ChEMBL | Assay Description Inhibition of human placental HexA using MUGS substrate incubated for 1 to 2 hrs at pH 7.0 by spectrofluorometry | J Med Chem 58: 4483-93 (2015) Article DOI: 10.1021/jm5017895 BindingDB Entry DOI: 10.7270/Q28054CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM18512 (5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Genetics and Genome Biology, SickKids, PGCRL 686 Bay Street, Toronto, Ontario M5G 0A4, Canada. Curated by ChEMBL | Assay Description Inhibition of human placental HexB using MUGS substrate incubated for 1 to 2 hrs at pH 7.0 by spectrofluorometry | J Med Chem 58: 4483-93 (2015) Article DOI: 10.1021/jm5017895 BindingDB Entry DOI: 10.7270/Q28054CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit alpha (Homo sapiens (Human)) | BDBM68272 (LU79953) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
Research Institute, Hospital for Sick Children | Assay Description Enzyme assay based on 4-MU substrates with HTS to identify inhibitory compounds for Hex. | Chem Biol 14: 153-64 (2007) Article DOI: 10.1016/j.chembiol.2006.12.006 BindingDB Entry DOI: 10.7270/Q25B00W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM84610 (5-(3,5-dichlorophenoxy)-N-(4-pyrdinyl)-2- furamide...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Research Institute, Hospital for Sick Children | Assay Description Beta-glucocerebrosidase (GCase) activity was measured by release of 4-methylumbelliferyl fluorophore from MUClc. | Chembiochem 9: 2650-62 (2008) Article DOI: 10.1002/cbic.200800304 BindingDB Entry DOI: 10.7270/Q2JQ0ZJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18046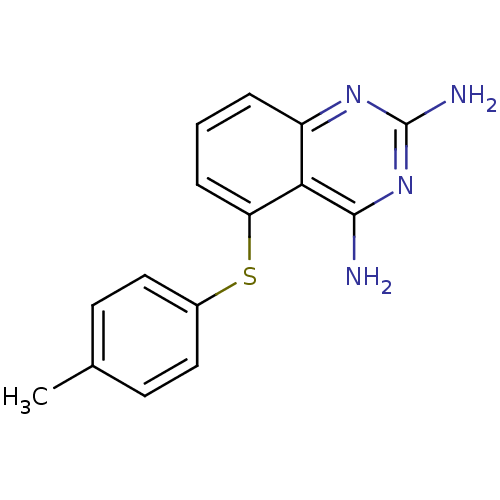 (5-((4-methylphenyl)thio)-quinazoline 2,4-diamine, ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Research Institute, Hospital for Sick Children | Assay Description Beta-glucocerebrosidase (GCase) activity was measured by release of 4-methylumbelliferyl fluorophore from MUClc. | Chembiochem 9: 2650-62 (2008) Article DOI: 10.1002/cbic.200800304 BindingDB Entry DOI: 10.7270/Q2JQ0ZJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50246569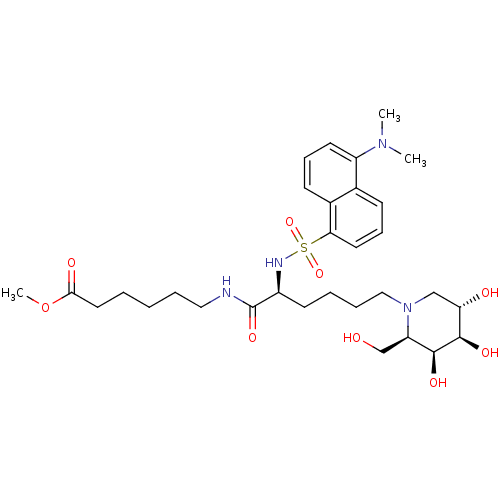 (CHEMBL505422 | Methyl 6-[N2-dansyl-N6-(1,5-dideoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz Curated by ChEMBL | Assay Description Inhibition of human lysosomal beta galactosidase | Bioorg Med Chem 16: 10216-20 (2008) Article DOI: 10.1016/j.bmc.2008.10.054 BindingDB Entry DOI: 10.7270/Q2DZ085J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit alpha (Homo sapiens (Human)) | BDBM18512 (5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | 4.5 | n/a |
Genetics and Genome Biology, SickKids, PGCRL 686 Bay Street, Toronto, Ontario M5G 0A4, Canada. Curated by ChEMBL | Assay Description Inhibition of HexA alpha G269S mutant in ATSD patient fibroblasts using MUGS substrate incubated for 1 to 2 hrs at pH 4.5 by spectrofluorometry | J Med Chem 58: 4483-93 (2015) Article DOI: 10.1021/jm5017895 BindingDB Entry DOI: 10.7270/Q28054CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit alpha (Homo sapiens (Human)) | BDBM18512 (5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | 4.5 | n/a |
Genetics and Genome Biology, SickKids, PGCRL 686 Bay Street, Toronto, Ontario M5G 0A4, Canada. Curated by ChEMBL | Assay Description Inhibition of human placental HexA using MUGS substrate incubated for 1 to 2 hrs at pH 4.5 by spectrofluorometry | J Med Chem 58: 4483-93 (2015) Article DOI: 10.1021/jm5017895 BindingDB Entry DOI: 10.7270/Q28054CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM18512 (5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | 4.5 | n/a |
Genetics and Genome Biology, SickKids, PGCRL 686 Bay Street, Toronto, Ontario M5G 0A4, Canada. Curated by ChEMBL | Assay Description Inhibition of human placental HexB using MUGS substrate incubated for 1 to 2 hrs at pH 4.5 by spectrofluorometry | J Med Chem 58: 4483-93 (2015) Article DOI: 10.1021/jm5017895 BindingDB Entry DOI: 10.7270/Q28054CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit alpha (Homo sapiens (Human)) | BDBM18788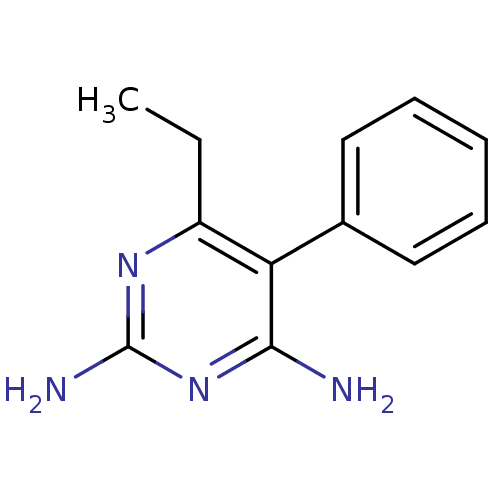 (6-ethyl-5-phenylpyrimidine-2,4-diamine | CHEMBL221...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Genetics and Genome Biology, SickKids, PGCRL 686 Bay Street, Toronto, Ontario M5G 0A4, Canada. Curated by ChEMBL | Assay Description Inhibition of human placental HexA using MUGS substrate incubated for 1 to 2 hrs at pH 7.0 by spectrofluorometry | J Med Chem 58: 4483-93 (2015) Article DOI: 10.1021/jm5017895 BindingDB Entry DOI: 10.7270/Q28054CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit alpha (Homo sapiens (Human)) | BDBM18512 (5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | 4.5 | n/a |
Genetics and Genome Biology, SickKids, PGCRL 686 Bay Street, Toronto, Ontario M5G 0A4, Canada. Curated by ChEMBL | Assay Description Inhibition of HexA in normal human fibroblasts using MUGS substrate incubated for 1 to 2 hrs at pH 4.5 by spectrofluorometry | J Med Chem 58: 4483-93 (2015) Article DOI: 10.1021/jm5017895 BindingDB Entry DOI: 10.7270/Q28054CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM18788 (6-ethyl-5-phenylpyrimidine-2,4-diamine | CHEMBL221...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Genetics and Genome Biology, SickKids, PGCRL 686 Bay Street, Toronto, Ontario M5G 0A4, Canada. Curated by ChEMBL | Assay Description Inhibition of human placental HexB using MUGS substrate incubated for 1 to 2 hrs at pH 7.0 by spectrofluorometry | J Med Chem 58: 4483-93 (2015) Article DOI: 10.1021/jm5017895 BindingDB Entry DOI: 10.7270/Q28054CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit alpha (Homo sapiens (Human)) | BDBM50089680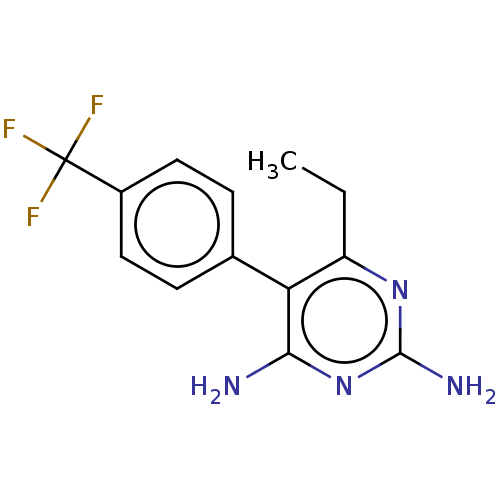 (CHEMBL3577321) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genetics and Genome Biology, SickKids, PGCRL 686 Bay Street, Toronto, Ontario M5G 0A4, Canada. Curated by ChEMBL | Assay Description Inhibition of human placental HexA using pNPGlcNAc substrate | J Med Chem 58: 4483-93 (2015) Article DOI: 10.1021/jm5017895 BindingDB Entry DOI: 10.7270/Q28054CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit alpha/beta (Homo sapiens (Human)) | BDBM18512 (5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genetics and Genome Biology, SickKids, PGCRL 686 Bay Street, Toronto, Ontario M5G 0A4, Canada. Curated by ChEMBL | Assay Description Inhibition of beta-N-acetylhexosaminidase (unknown origin) | J Med Chem 58: 4483-93 (2015) Article DOI: 10.1021/jm5017895 BindingDB Entry DOI: 10.7270/Q28054CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit alpha (Homo sapiens (Human)) | BDBM68270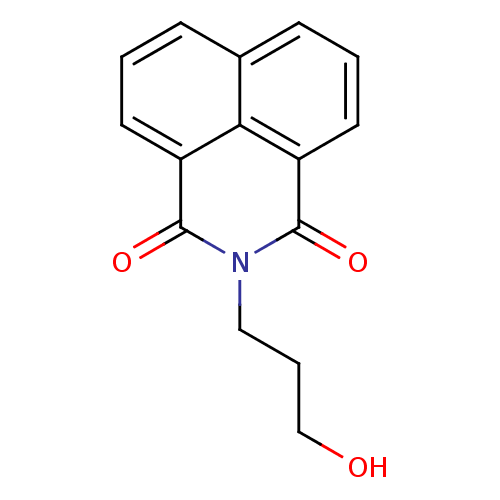 (5141402) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
Research Institute, Hospital for Sick Children | Assay Description Enzyme assay based on 4-MU substrates with HTS to identify inhibitory compounds for Hex. | Chem Biol 14: 153-64 (2007) Article DOI: 10.1016/j.chembiol.2006.12.006 BindingDB Entry DOI: 10.7270/Q25B00W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit alpha (Homo sapiens (Human)) | BDBM18512 (5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genetics and Genome Biology, SickKids, PGCRL 686 Bay Street, Toronto, Ontario M5G 0A4, Canada. Curated by ChEMBL | Assay Description Inhibition of human placental HexA using pNPGlcNAc substrate | J Med Chem 58: 4483-93 (2015) Article DOI: 10.1021/jm5017895 BindingDB Entry DOI: 10.7270/Q28054CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit alpha (Homo sapiens (Human)) | BDBM18780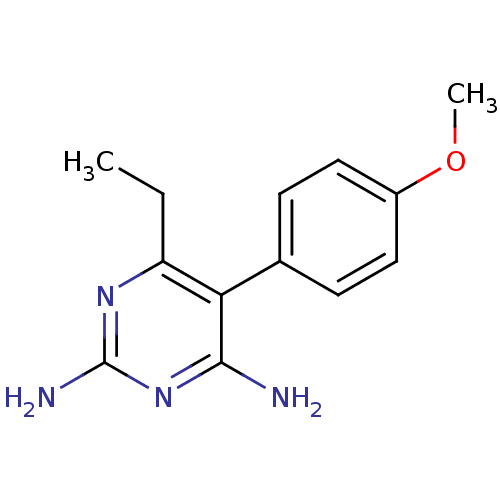 (6-ethyl-5-(4-methoxyphenyl)pyrimidine-2,4-diamine ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genetics and Genome Biology, SickKids, PGCRL 686 Bay Street, Toronto, Ontario M5G 0A4, Canada. Curated by ChEMBL | Assay Description Inhibition of human placental HexA using pNPGlcNAc substrate | J Med Chem 58: 4483-93 (2015) Article DOI: 10.1021/jm5017895 BindingDB Entry DOI: 10.7270/Q28054CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit alpha (Homo sapiens (Human)) | BDBM50089676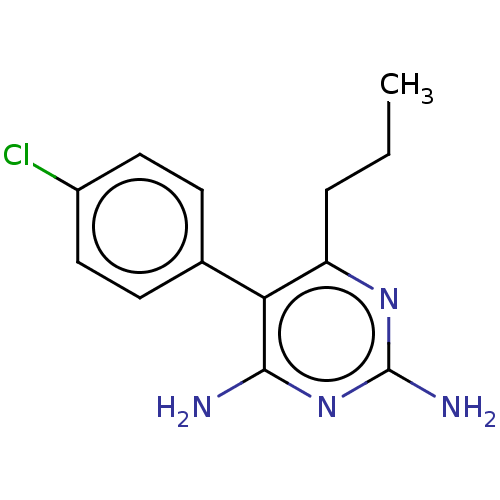 (CHEMBL3577317) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genetics and Genome Biology, SickKids, PGCRL 686 Bay Street, Toronto, Ontario M5G 0A4, Canada. Curated by ChEMBL | Assay Description Inhibition of human placental HexA using pNPGlcNAc substrate | J Med Chem 58: 4483-93 (2015) Article DOI: 10.1021/jm5017895 BindingDB Entry DOI: 10.7270/Q28054CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit alpha (Homo sapiens (Human)) | BDBM50106194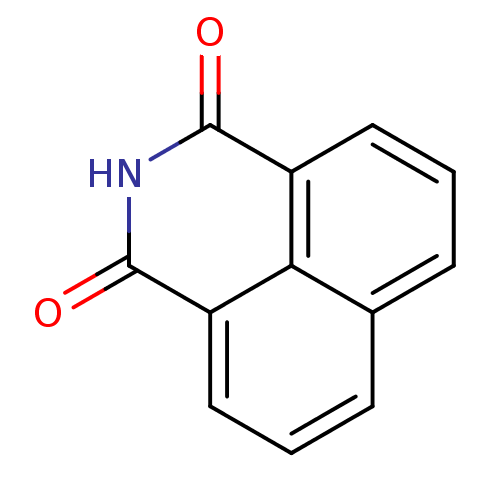 (1,3-dioxo-1H-benz[de]isoquinoline | 1,8-Naphthalim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
Research Institute, Hospital for Sick Children | Assay Description Enzyme assay based on 4-MU substrates with HTS to identify inhibitory compounds for Hex. | Chem Biol 14: 153-64 (2007) Article DOI: 10.1016/j.chembiol.2006.12.006 BindingDB Entry DOI: 10.7270/Q25B00W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 74 total ) | Next | Last >> |