

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM412656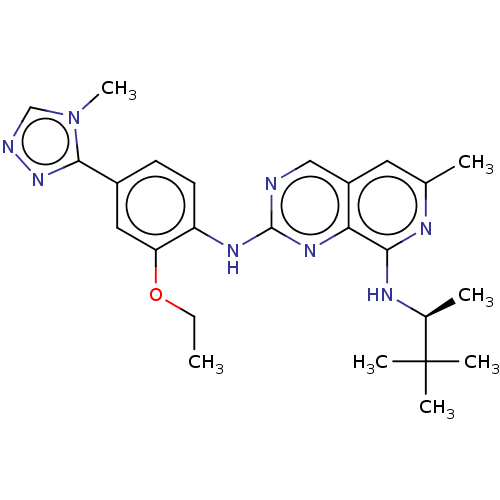 (US10399974, Example 54) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241208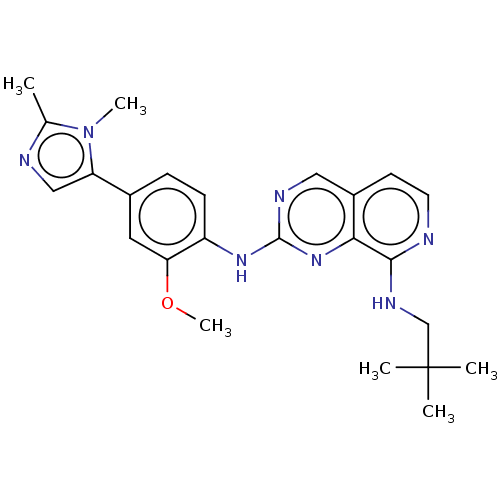 (US11046688, Example 50 | US9409907, 50) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50464039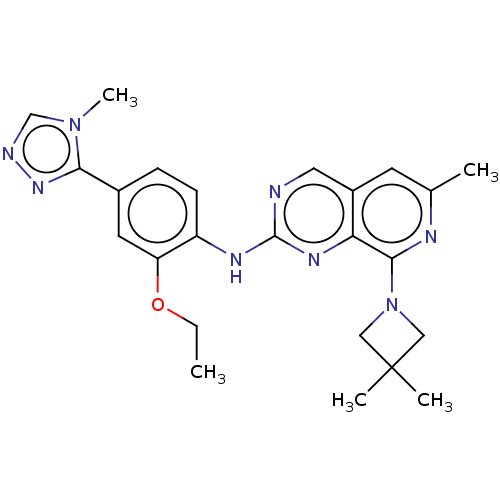 (CHEMBL4245639) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241333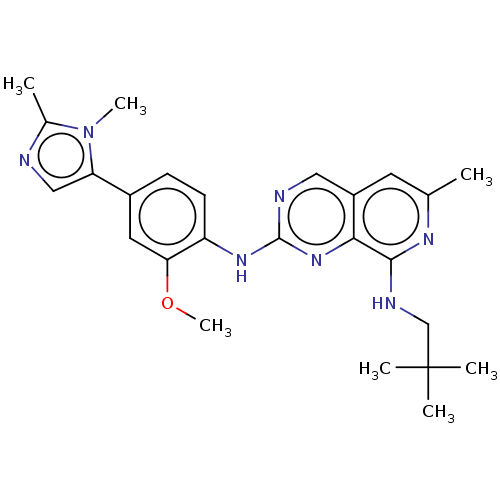 (US10479788, Example 177 | US11046688, Example 177 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241338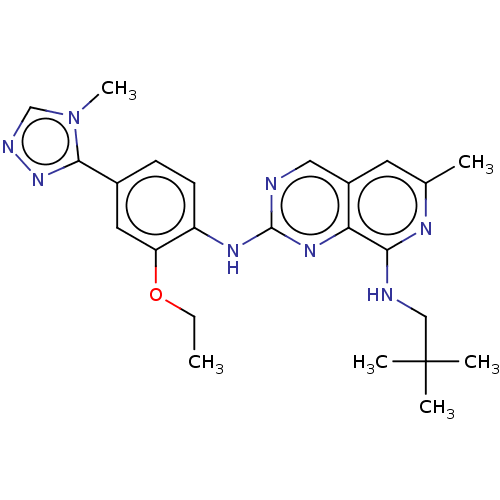 (US10479788, Example 182 | US11046688, Example 182 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM412611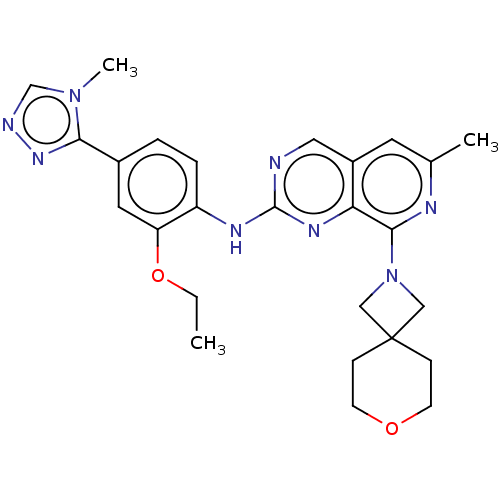 (N-(2-ethoxy-4-(4-methyl-4H-1,2,4-triazol-3-yl)phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM412614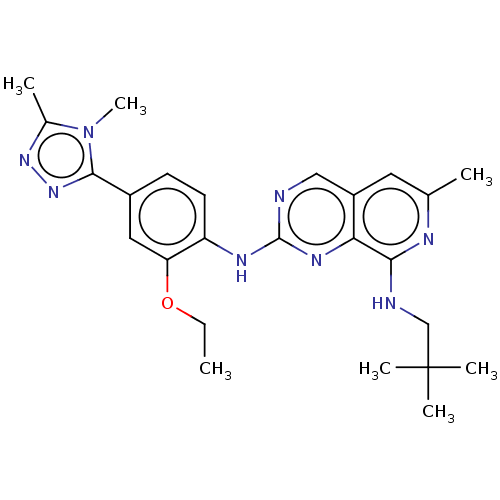 (N2-(4-(4,5-dimethyl-4H-1,2,4-triazol-3-yl)-2-ethox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50464037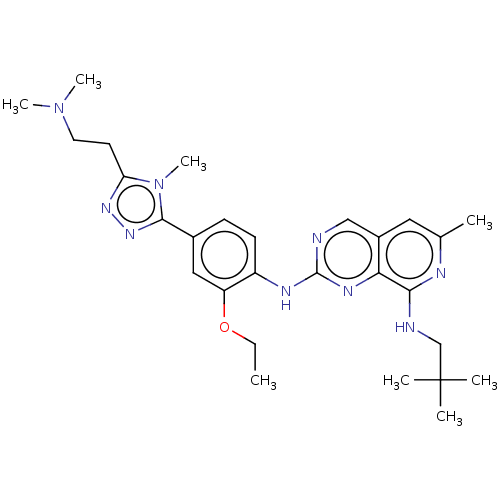 (CHEMBL4240502) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241335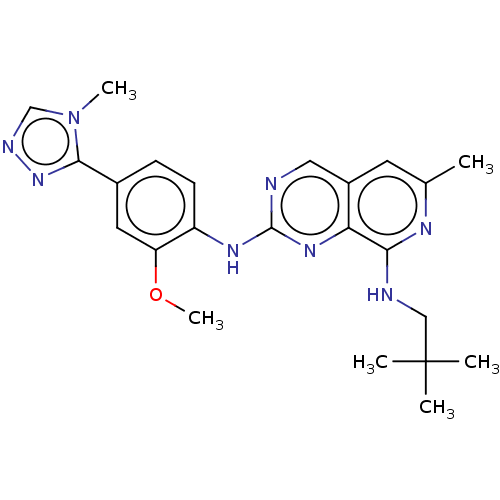 (US9409907, 179) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50464040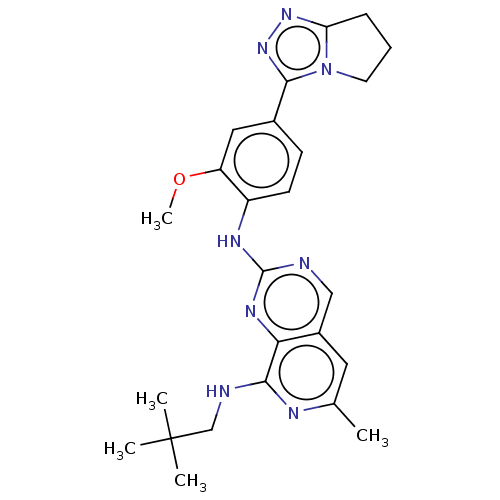 (CHEMBL4251352) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241235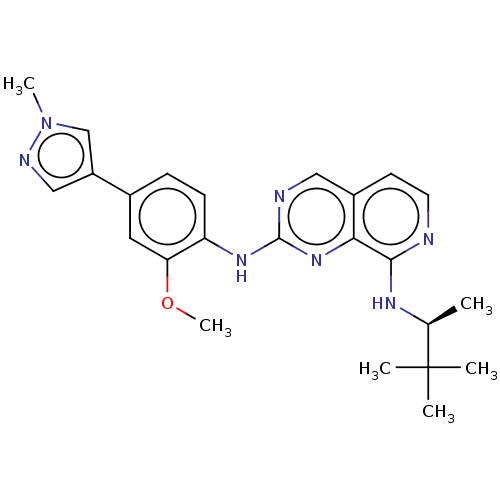 (US10479788, Example 77 | US11046688, Example 77 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human full length MPS1 expressed in recombinant baculovirus infected Sf9 insect cells using 5FAM-DHTGFLTEYVATRCONH2 as substrate after ... | J Med Chem 59: 3671-88 (2016) Article DOI: 10.1021/acs.jmedchem.5b01811 BindingDB Entry DOI: 10.7270/Q2DZ0B6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM412610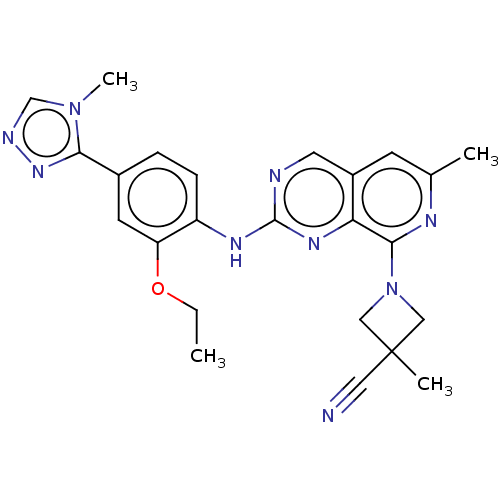 (1-(2-((2-ethoxy-4-(4-methyl-4H-1,2,4-triazol-3-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50514549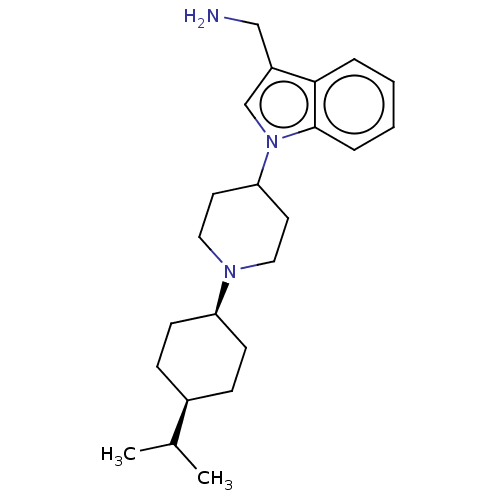 (CHEMBL4548515) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astraea Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]N/OFQ from human NOP expressed in CHO cell membrane incubated for 60 mins by liquid scintillation counting method | J Med Chem 63: 2688-2704 (2020) Article DOI: 10.1021/acs.jmedchem.9b02134 BindingDB Entry DOI: 10.7270/Q2CJ8HV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM50464038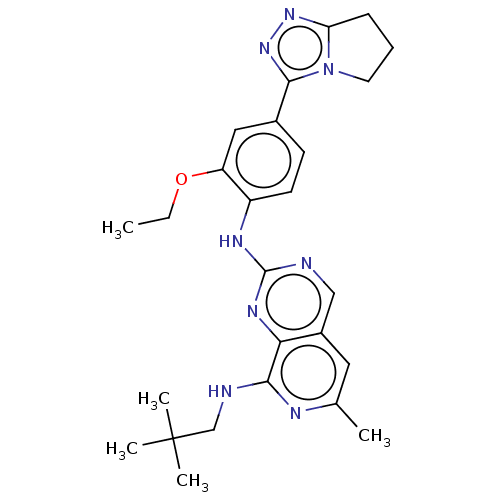 (CHEMBL4250961) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241226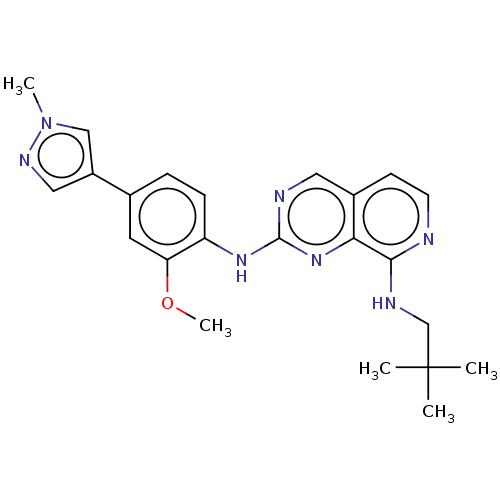 (US10479788, Example 68 | US11046688, Example 68 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241226 (US10479788, Example 68 | US11046688, Example 68 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human full length MPS1 expressed in recombinant baculovirus infected Sf9 insect cells using 5FAM-DHTGFLTEYVATRCONH2 as substrate after ... | J Med Chem 59: 3671-88 (2016) Article DOI: 10.1021/acs.jmedchem.5b01811 BindingDB Entry DOI: 10.7270/Q2DZ0B6T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241207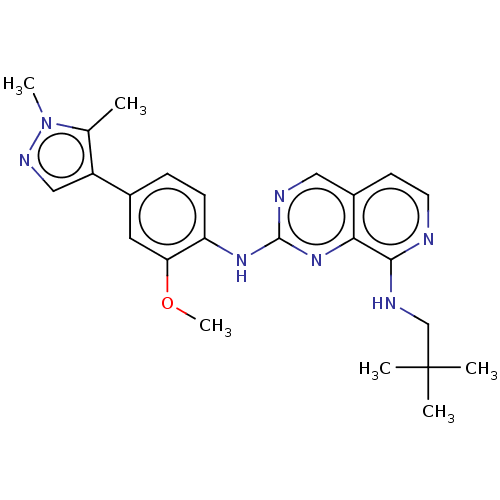 (US11046688, Example 49 | US9409907, 49) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM412658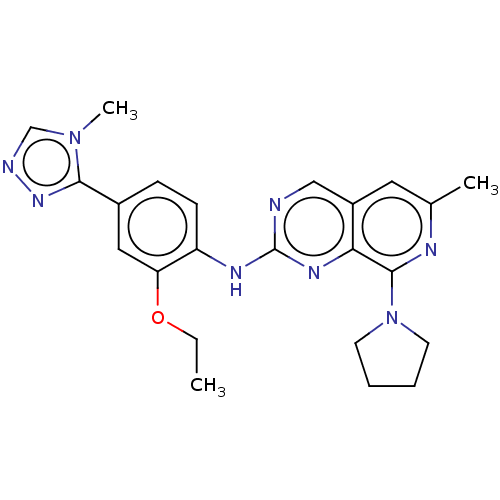 (US10399974, Example 56) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50091825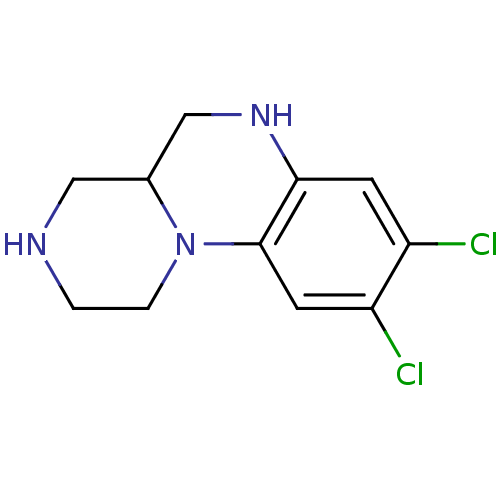 (8,9-Dichloro-2,3,4,4a,5,6-hexahydro-1H-pyrazino[1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Binding affinity using [125I]-DOI as radioligand with membranes isolated from a CHO-k cell line expressing the human 5-hydroxytryptamine 2C receptor | Bioorg Med Chem Lett 10: 1991-4 (2001) BindingDB Entry DOI: 10.7270/Q2Z89BMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241213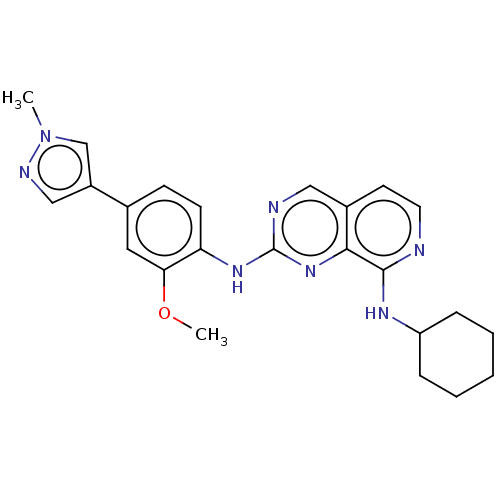 (US10479788, Example 55 | US11046688, Example 55 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human full length MPS1 expressed in recombinant baculovirus infected Sf9 insect cells using 5FAM-DHTGFLTEYVATRCONH2 as substrate after ... | J Med Chem 59: 3671-88 (2016) Article DOI: 10.1021/acs.jmedchem.5b01811 BindingDB Entry DOI: 10.7270/Q2DZ0B6T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50514557 (CHEMBL4541337) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astraea Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]N/OFQ from human NOP expressed in CHO cell membrane incubated for 60 mins by liquid scintillation counting method | J Med Chem 63: 2688-2704 (2020) Article DOI: 10.1021/acs.jmedchem.9b02134 BindingDB Entry DOI: 10.7270/Q2CJ8HV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50514553 (CHEMBL4439811) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astraea Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]N/OFQ from human NOP expressed in CHO cell membrane incubated for 60 mins by liquid scintillation counting method | J Med Chem 63: 2688-2704 (2020) Article DOI: 10.1021/acs.jmedchem.9b02134 BindingDB Entry DOI: 10.7270/Q2CJ8HV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50514550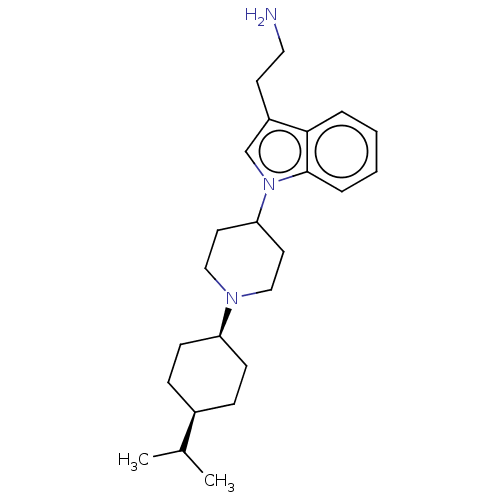 (CHEMBL4465668) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astraea Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]N/OFQ from human NOP expressed in CHO cell membrane incubated for 60 mins by liquid scintillation counting method | J Med Chem 63: 2688-2704 (2020) Article DOI: 10.1021/acs.jmedchem.9b02134 BindingDB Entry DOI: 10.7270/Q2CJ8HV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50514549 (CHEMBL4548515) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astraea Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human MOP expressed in CHO cell membrane incubated for 60 mins by liquid scintillation counting method | J Med Chem 63: 2688-2704 (2020) Article DOI: 10.1021/acs.jmedchem.9b02134 BindingDB Entry DOI: 10.7270/Q2CJ8HV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241216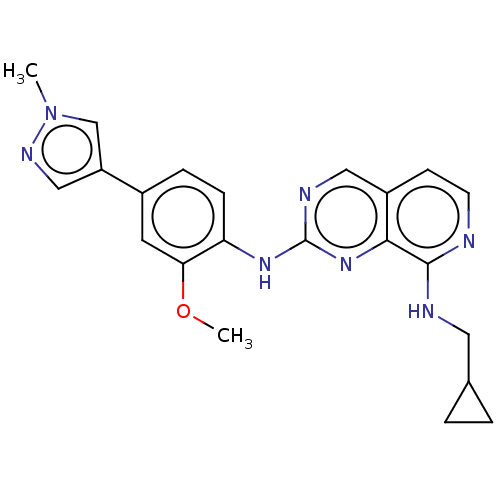 (US10479788, Example 58 | US11046688, Example 58 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human full length MPS1 expressed in recombinant baculovirus infected Sf9 insect cells using 5FAM-DHTGFLTEYVATRCONH2 as substrate after ... | J Med Chem 59: 3671-88 (2016) Article DOI: 10.1021/acs.jmedchem.5b01811 BindingDB Entry DOI: 10.7270/Q2DZ0B6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50091831 ((R)-8,9-Dichloro-2,3,4,4a-tetrahydro-1H,6H-pyrazin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Binding affinity using [3H]-mesulergine as radioligand with receptor membranes isolated from a CHO-k cell line expressing the human 5-hydroxytryptami... | Bioorg Med Chem Lett 10: 1991-4 (2001) BindingDB Entry DOI: 10.7270/Q2Z89BMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241195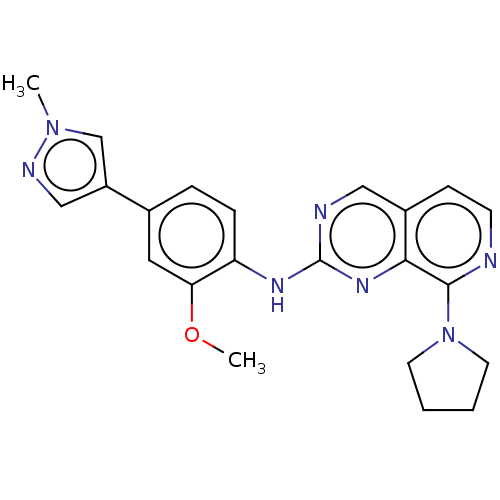 (US10479788, Example 37 | US11046688, Example 37 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human full length MPS1 expressed in recombinant baculovirus infected Sf9 insect cells using 5FAM-DHTGFLTEYVATRCONH2 as substrate after ... | J Med Chem 59: 3671-88 (2016) Article DOI: 10.1021/acs.jmedchem.5b01811 BindingDB Entry DOI: 10.7270/Q2DZ0B6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241223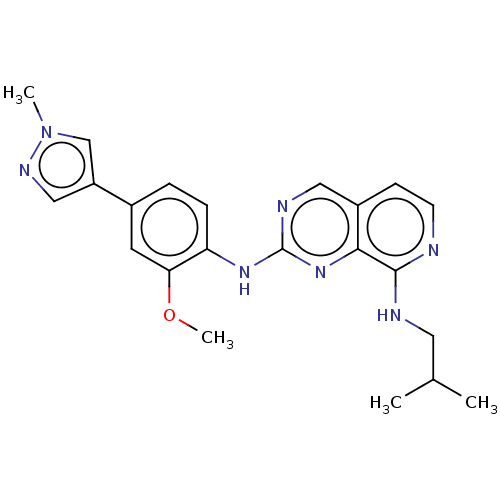 (US10479788, Example 65 | US11046688, Example 65 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human full length MPS1 expressed in recombinant baculovirus infected Sf9 insect cells using 5FAM-DHTGFLTEYVATRCONH2 as substrate after ... | J Med Chem 59: 3671-88 (2016) Article DOI: 10.1021/acs.jmedchem.5b01811 BindingDB Entry DOI: 10.7270/Q2DZ0B6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50091822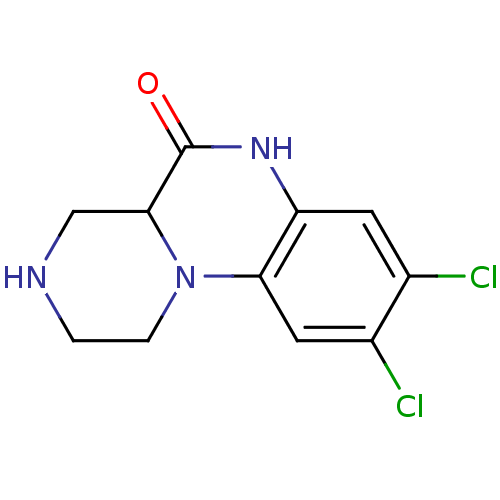 (8,9-Dichloro-2,3,4,4a-tetrahydro-1H,6H-pyrazino[1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Binding affinity using [125I]-DOI as radioligand with membranes isolated from a CHO-k cell line expressing the human 5-hydroxytryptamine 2C receptor | Bioorg Med Chem Lett 10: 1991-4 (2001) BindingDB Entry DOI: 10.7270/Q2Z89BMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM412644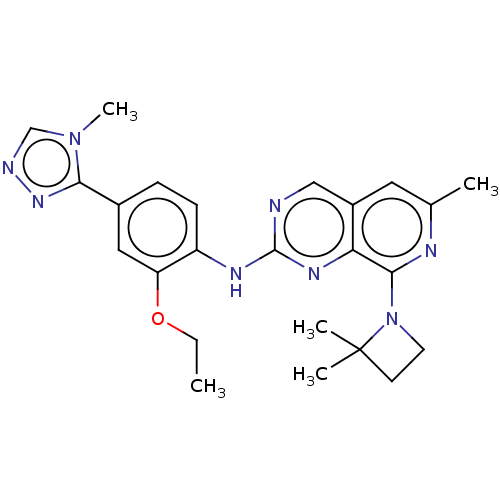 (US10399974, Example 42) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase TTK (Homo sapiens (Human)) | BDBM241234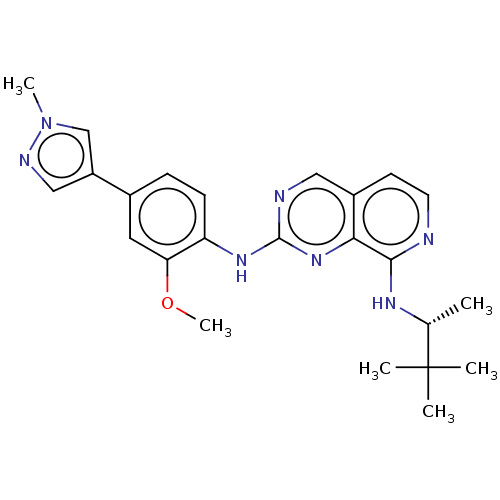 (US10479788, Example 76 | US11046688, Example 76 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human full length MPS1 expressed in recombinant baculovirus infected Sf9 insect cells using 5FAM-DHTGFLTEYVATRCONH2 as substrate after ... | J Med Chem 59: 3671-88 (2016) Article DOI: 10.1021/acs.jmedchem.5b01811 BindingDB Entry DOI: 10.7270/Q2DZ0B6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50091829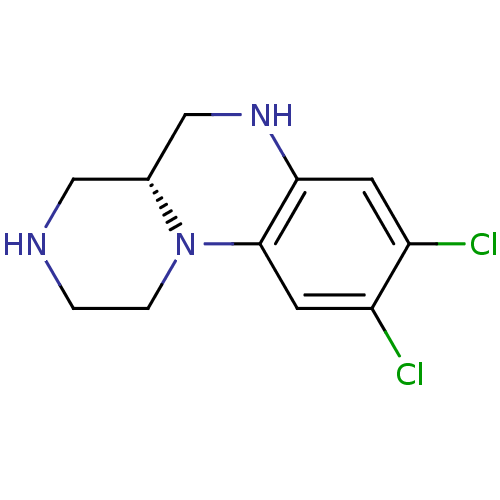 ((R)-8,9-Dichloro-2,3,4,4a,5,6-hexahydro-1H-pyrazin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Binding affinity using [125I]-DOI as radioligand with membranes isolated from a CHO-k cell line expressing the human 5-hydroxytryptamine 2C receptor | Bioorg Med Chem Lett 10: 1991-4 (2001) BindingDB Entry DOI: 10.7270/Q2Z89BMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM241331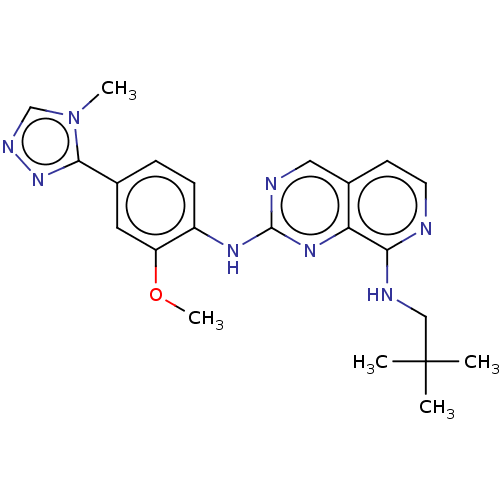 (US10479788, Example 175 | US11046688, Example 175 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal His-tagged CDK2/cyclinA expressed in baculovirus expression system using 5FAM- peptide18 as substrate afte... | J Med Chem 61: 8226-8240 (2018) Article DOI: 10.1021/acs.jmedchem.8b00690 BindingDB Entry DOI: 10.7270/Q2J105S9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50091826 ((S)-8,9-Dichloro-2,3,4,4a,5,6-hexahydro-1H-pyrazin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Binding affinity using [125I]-DOI as radioligand with membranes isolated from a CHO-k cell line expressing the human 5-hydroxytryptamine 2C receptor | Bioorg Med Chem Lett 10: 1991-4 (2001) BindingDB Entry DOI: 10.7270/Q2Z89BMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50091830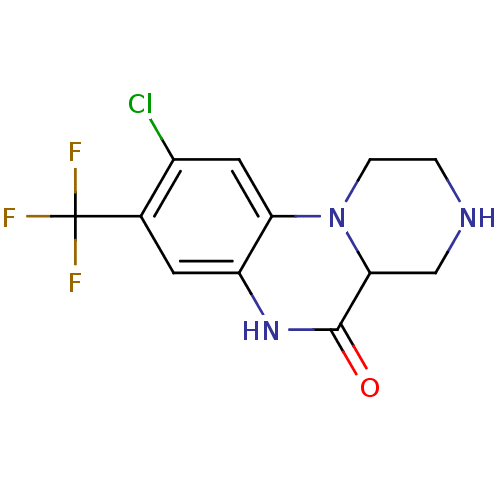 (9-Chloro-8-trifluoromethyl-2,3,4,4a-tetrahydro-1H,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Binding affinity using [125I]-DOI as radioligand with membranes isolated from a CHO-k cell line expressing the human 5-hydroxytryptamine 2C receptor | Bioorg Med Chem Lett 10: 1991-4 (2001) BindingDB Entry DOI: 10.7270/Q2Z89BMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50514541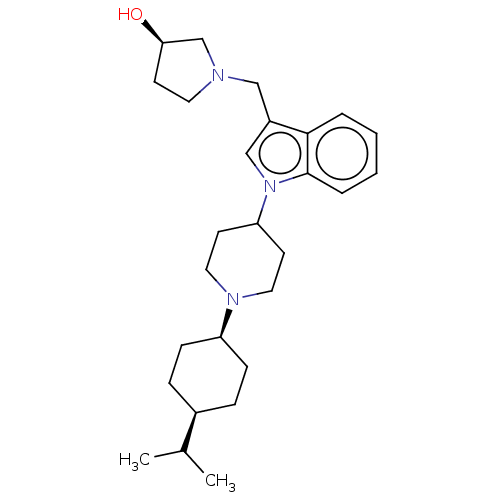 (CHEMBL4473045) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astraea Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]N/OFQ from human NOP expressed in CHO cell membrane incubated for 60 mins by liquid scintillation counting method | J Med Chem 63: 2688-2704 (2020) Article DOI: 10.1021/acs.jmedchem.9b02134 BindingDB Entry DOI: 10.7270/Q2CJ8HV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50514545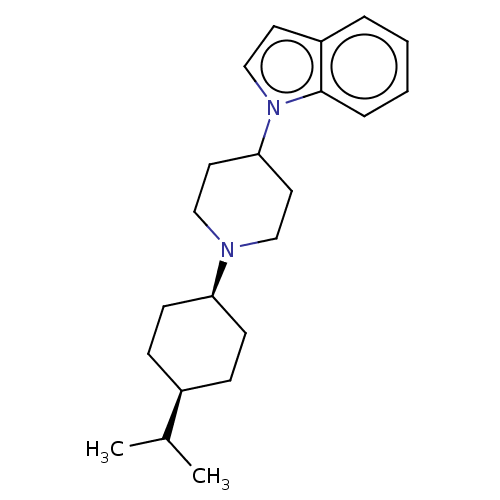 (CHEMBL4435280) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astraea Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]N/OFQ from human NOP expressed in CHO cell membrane incubated for 60 mins by liquid scintillation counting method | J Med Chem 63: 2688-2704 (2020) Article DOI: 10.1021/acs.jmedchem.9b02134 BindingDB Entry DOI: 10.7270/Q2CJ8HV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50514548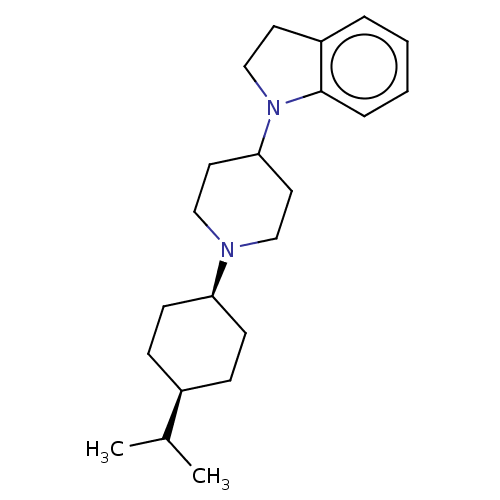 (CHEMBL4570328) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astraea Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]N/OFQ from human NOP expressed in CHO cell membrane incubated for 60 mins by liquid scintillation counting method | J Med Chem 63: 2688-2704 (2020) Article DOI: 10.1021/acs.jmedchem.9b02134 BindingDB Entry DOI: 10.7270/Q2CJ8HV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50246834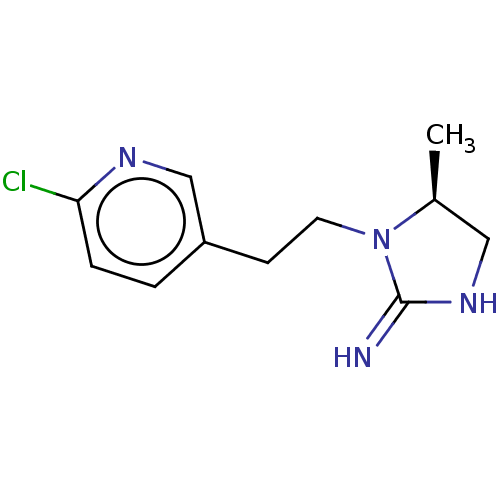 (CHEMBL4067640) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha4beta2 nAChR expressed in HEK cell membranes after 2 hrs | J Med Chem 60: 10092-10104 (2017) Article DOI: 10.1021/acs.jmedchem.7b01250 BindingDB Entry DOI: 10.7270/Q2PG1V5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50514552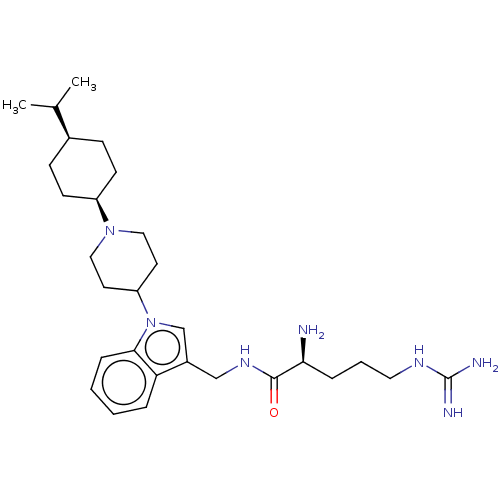 (CHEMBL4594096) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astraea Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]N/OFQ from human NOP expressed in CHO cell membrane incubated for 60 mins by liquid scintillation counting method | J Med Chem 63: 2688-2704 (2020) Article DOI: 10.1021/acs.jmedchem.9b02134 BindingDB Entry DOI: 10.7270/Q2CJ8HV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50514539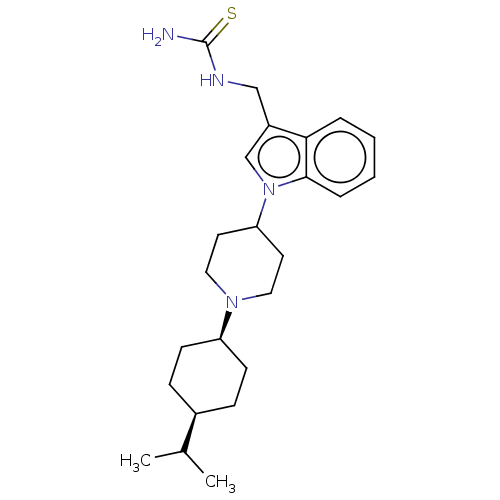 (CHEMBL4460695) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astraea Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]N/OFQ from human NOP expressed in CHO cell membrane incubated for 60 mins by liquid scintillation counting method | J Med Chem 63: 2688-2704 (2020) Article DOI: 10.1021/acs.jmedchem.9b02134 BindingDB Entry DOI: 10.7270/Q2CJ8HV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50145565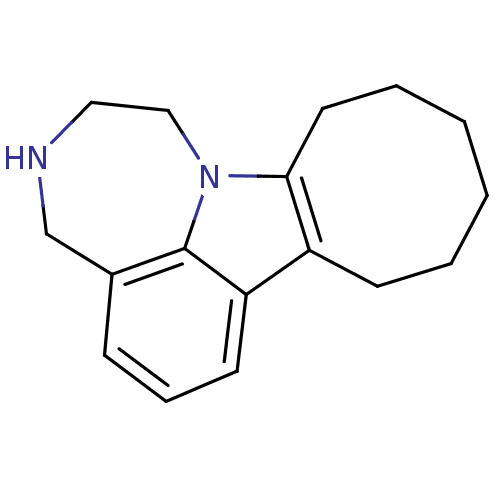 (1,2,3,4,8,9,10,11,12,13-decahydrocycloocta[b][1,4]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Agonistic binding affinity against human 5-hydroxytryptamine 2C receptor in CHO cells using [125I]- DOI | Bioorg Med Chem Lett 14: 2603-7 (2004) Article DOI: 10.1016/j.bmcl.2004.02.100 BindingDB Entry DOI: 10.7270/Q2QZ29F3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50246823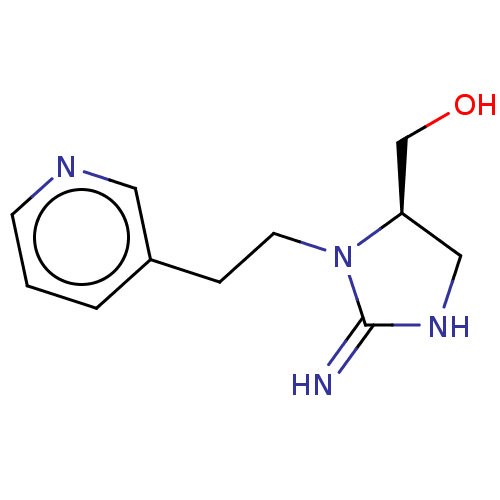 (CHEMBL4071277) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha4beta2 nAChR expressed in HEK cell membranes after 2 hrs | J Med Chem 60: 10092-10104 (2017) Article DOI: 10.1021/acs.jmedchem.7b01250 BindingDB Entry DOI: 10.7270/Q2PG1V5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50570559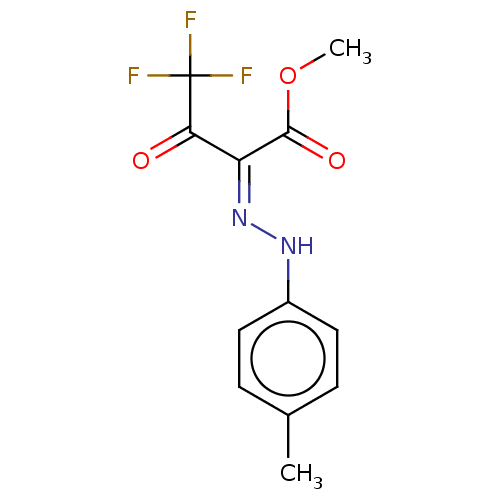 (CHEMBL4868687) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of porcine liver carboxylesterase by double reciprocal Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50514558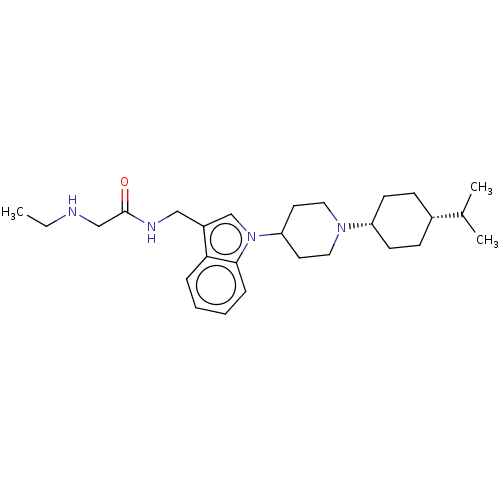 (CHEMBL4519453) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astraea Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]N/OFQ from human NOP expressed in CHO cell membrane incubated for 60 mins by liquid scintillation counting method | J Med Chem 63: 2688-2704 (2020) Article DOI: 10.1021/acs.jmedchem.9b02134 BindingDB Entry DOI: 10.7270/Q2CJ8HV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50091834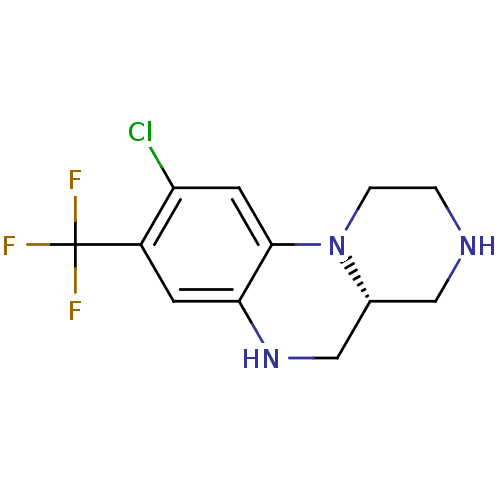 ((R)-9-Chloro-8-trifluoromethyl-2,3,4,4a,5,6-hexahy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Binding affinity using [125I]-DOI as radioligand with membranes isolated from a CHO-k cell line expressing the human 5-hydroxytryptamine 2C receptor | Bioorg Med Chem Lett 10: 1991-4 (2001) BindingDB Entry DOI: 10.7270/Q2Z89BMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50091825 (8,9-Dichloro-2,3,4,4a,5,6-hexahydro-1H-pyrazino[1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Binding affinity using [125I]-DOI as radioligand with membranes isolated from a CHO-k cell line expressing the human 5-hydroxytryptamine 2C receptor | Bioorg Med Chem Lett 10: 1991-4 (2001) BindingDB Entry DOI: 10.7270/Q2Z89BMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50246826 (CHEMBL4084577) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha4beta2 nAChR expressed in HEK cell membranes after 2 hrs | J Med Chem 60: 10092-10104 (2017) Article DOI: 10.1021/acs.jmedchem.7b01250 BindingDB Entry DOI: 10.7270/Q2PG1V5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50246835 (CHEMBL4077474) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha4beta2 nAChR expressed in HEK cell membranes after 2 hrs | J Med Chem 60: 10092-10104 (2017) Article DOI: 10.1021/acs.jmedchem.7b01250 BindingDB Entry DOI: 10.7270/Q2PG1V5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50091832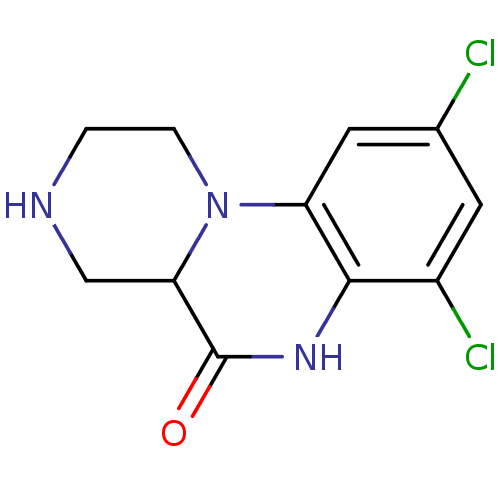 (7,9-Dichloro-2,3,4,4a-tetrahydro-1H,6H-pyrazino[1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Agonistic activity for 5-HT2c (5-HT2C) by measuring [3H]-inositol monophosphate fromation in CHO cells in which the human 5-HT2C receptor subtype was... | Bioorg Med Chem Lett 10: 1991-4 (2001) BindingDB Entry DOI: 10.7270/Q2Z89BMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2000 total ) | Next | Last >> |