

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118462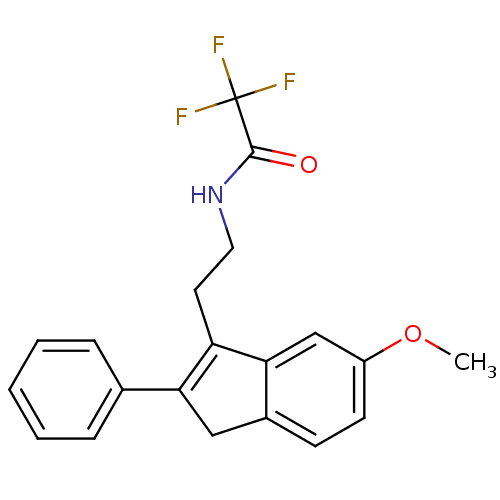 (2,2,2-Trifluoro-N-[2-(6-methoxy-2-phenyl-3H-inden-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00602 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118435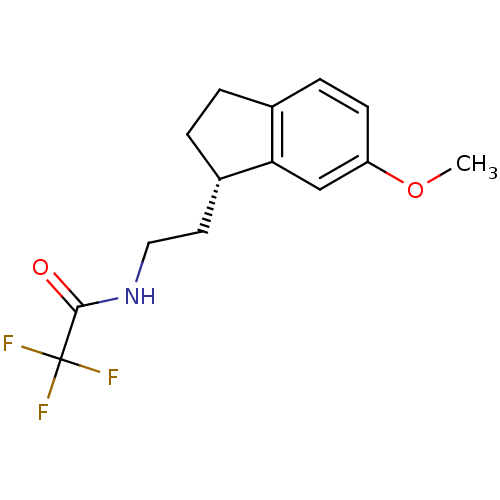 (2,2,2-Trifluoro-N-[2-(6-methoxy-indan-1-yl)-ethyl]...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118446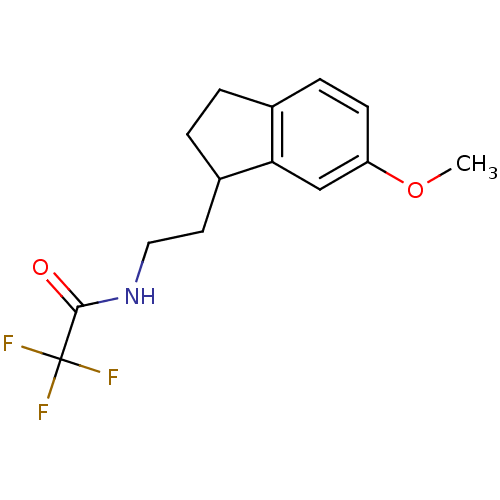 (2,2,2-Trifluoro-N-[2-(6-methoxy-indan-1-yl)-ethyl]...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0225 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118456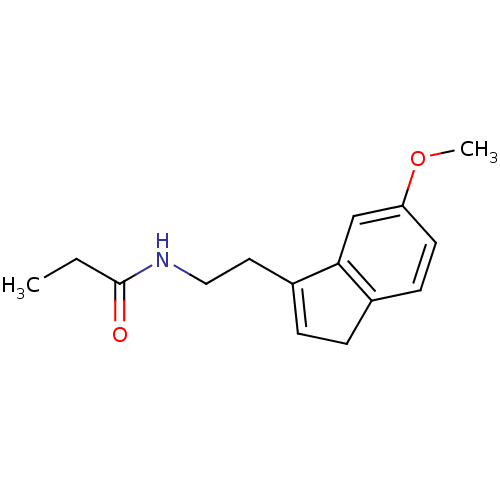 (CHEMBL334645 | N-[2-(6-Methoxy-3H-inden-1-yl)-ethy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0231 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50521464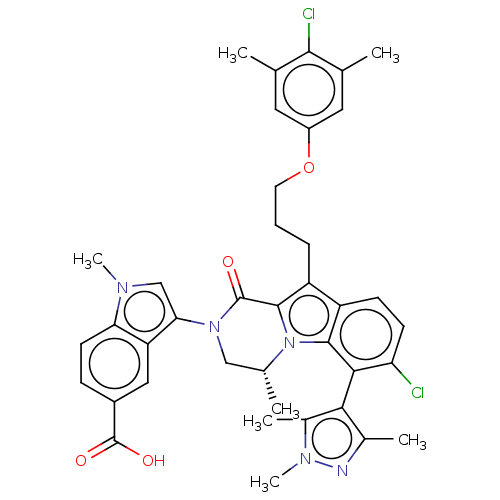 (CHEMBL4469869 | US11596639, Example 78) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of FITC-labeled Bak peptide binding to MBP-fused Mcl-1 (unknown origin) measured after 3 hrs by TR-FRET assay | J Med Chem 62: 3971-3988 (2019) Article DOI: 10.1021/acs.jmedchem.8b01991 BindingDB Entry DOI: 10.7270/Q2V69P04 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50521464 (CHEMBL4469869 | US11596639, Example 78) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of FITC-labeled Bak peptide binding to MBP-fused Mcl-1 (unknown origin) measured after 3 hrs by TR-FRET assay | J Med Chem 62: 3971-3988 (2019) Article DOI: 10.1021/acs.jmedchem.8b01991 BindingDB Entry DOI: 10.7270/Q2V69P04 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50521464 (CHEMBL4469869 | US11596639, Example 78) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of FITC-labeled Bak peptide binding to MBP-fused Mcl-1 (unknown origin) measured after 3 hrs by TR-FRET assay | J Med Chem 62: 3971-3988 (2019) Article DOI: 10.1021/acs.jmedchem.8b01991 BindingDB Entry DOI: 10.7270/Q2V69P04 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118453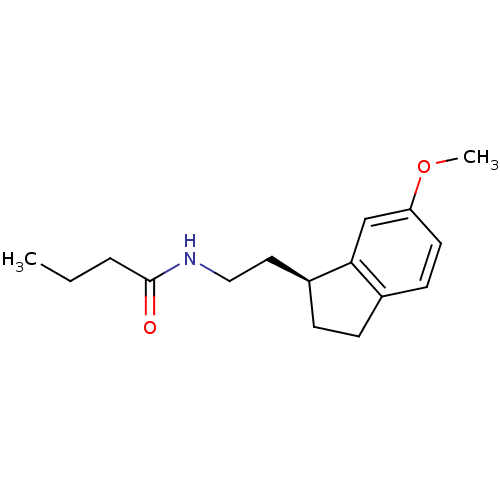 ((S) N-[2-(6-Methoxy-indan-1-yl)-ethyl]-butyramide ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0321 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118440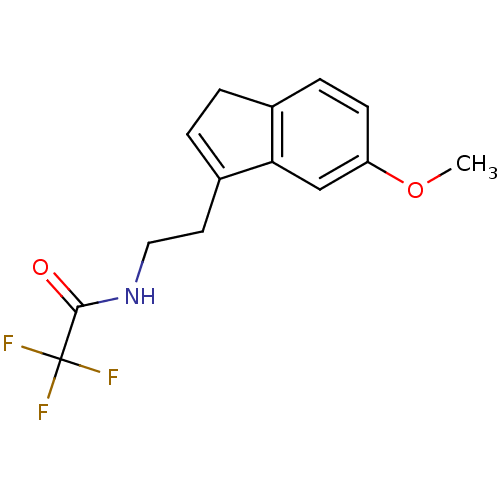 (2,2,2-Trifluoro-N-[2-(6-methoxy-3H-inden-1-yl)-eth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0408 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50518241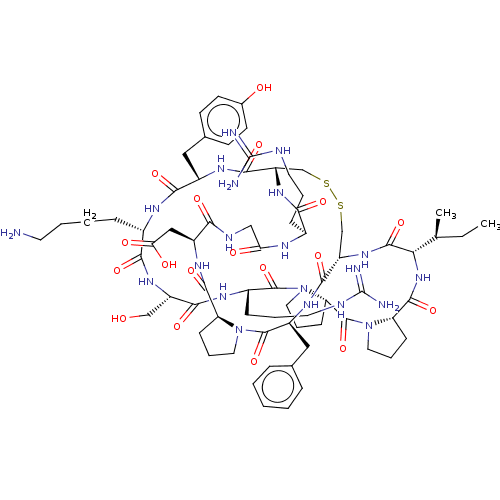 (CHEMBL4569923) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins | J Med Chem 62: 552-560 (2019) Article DOI: 10.1021/acs.jmedchem.8b01139 BindingDB Entry DOI: 10.7270/Q2MS3X3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118430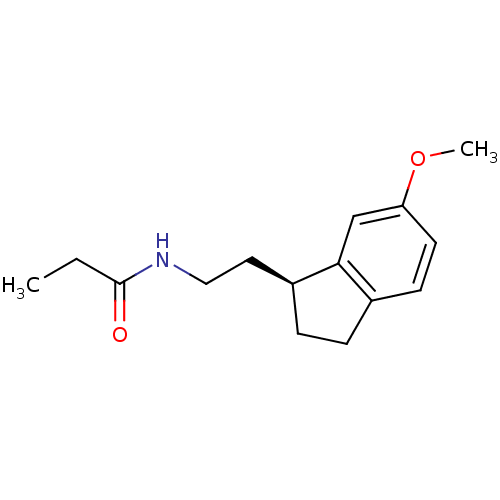 ((S) N-[2-(6-Methoxy-indan-1-yl)-ethyl]-propionamid...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118463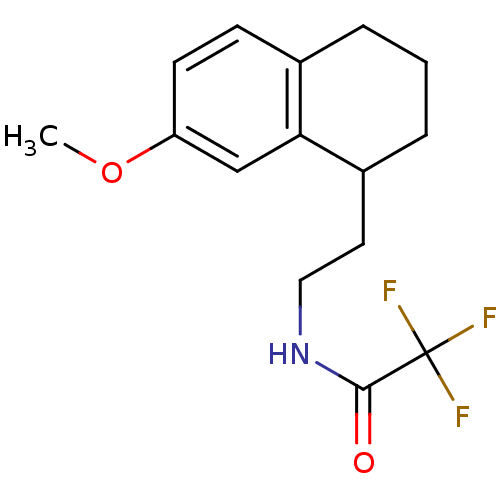 (2,2,2-Trifluoro-N-[2-(7-methoxy-1,2,3,4-tetrahydro...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0469 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50518240 (CHEMBL4439523) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins | J Med Chem 62: 552-560 (2019) Article DOI: 10.1021/acs.jmedchem.8b01139 BindingDB Entry DOI: 10.7270/Q2MS3X3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118450 (CHEMBL335437 | N-[2-(6-Methoxy-indan-1-yl)-ethyl]-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0553 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118458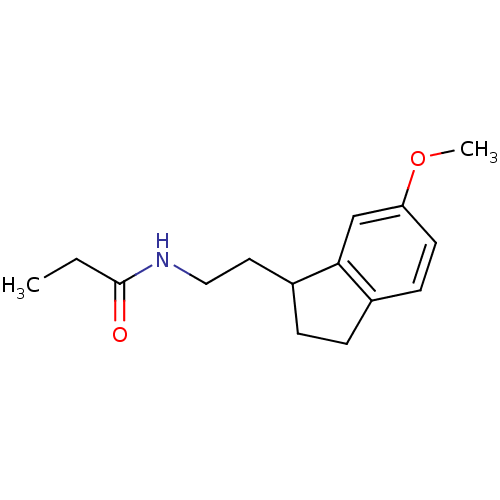 (CHEMBL337837 | N-[2-(6-Methoxy-indan-1-yl)-ethyl]-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0728 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118460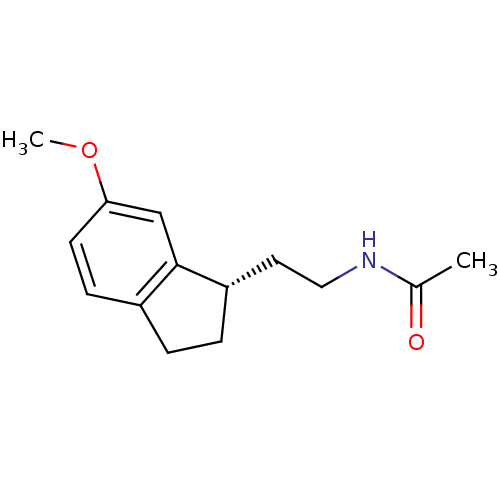 ((S) N-[2-(6-Methoxy-indan-1-yl)-ethyl]-acetamide |...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0733 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50521465 (CHEMBL4449014 | US11596639, Example 88) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of FITC-labeled Bak peptide binding to MBP-fused Mcl-1 (unknown origin) measured after 3 hrs by TR-FRET assay | J Med Chem 62: 3971-3988 (2019) Article DOI: 10.1021/acs.jmedchem.8b01991 BindingDB Entry DOI: 10.7270/Q2V69P04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 0.0823 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50212159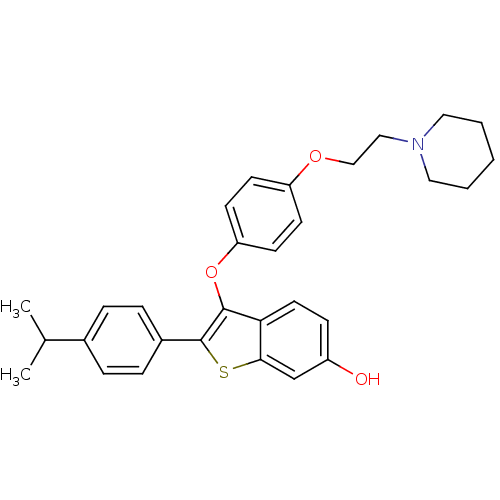 (2-(4-isopropylphenyl)-3-(4-(2-(piperidin-1-yl)etho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERalpha | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118436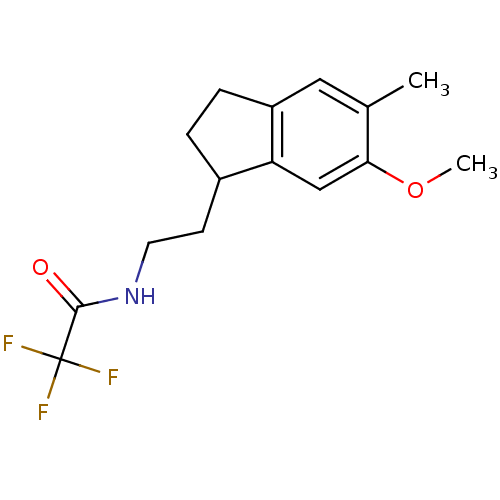 (2,2,2-Trifluoro-N-[2-(6-methoxy-5-methyl-indan-1-y...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0984 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118447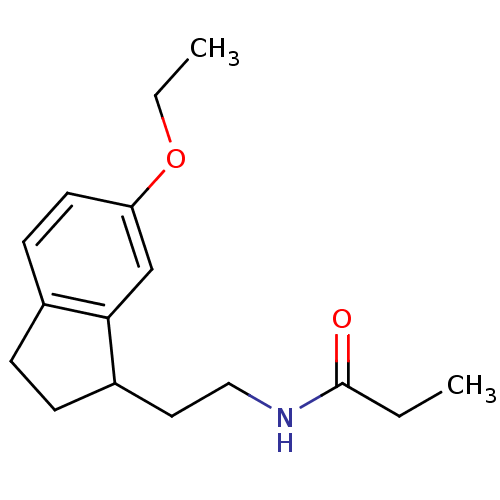 (CHEMBL134878 | N-[2-(6-Ethoxy-indan-1-yl)-ethyl]-p...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50100983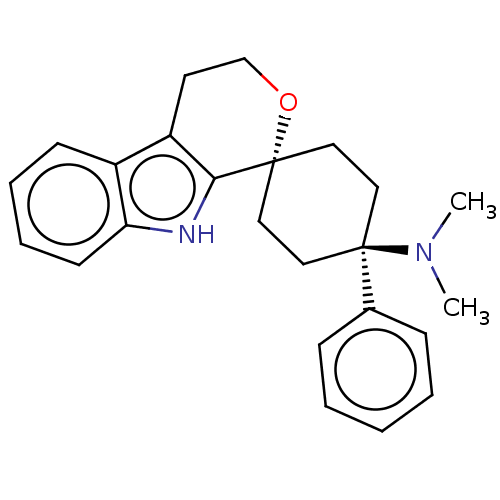 (CHEMBL3326224) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM86446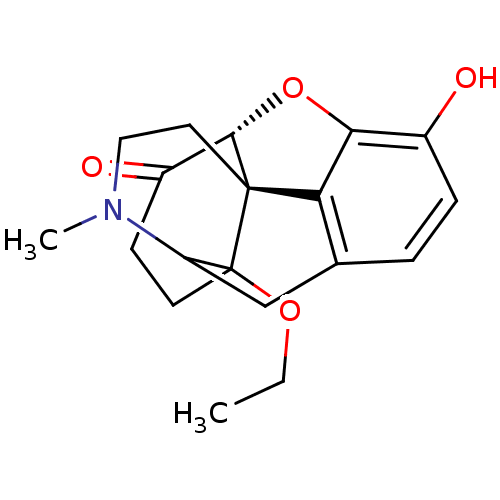 (14-O-methyloxymorphone) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by PDSP Ki Database | Eur J Pharmacol 483: 301-8 (2004) Article DOI: 10.1016/j.ejphar.2003.10.049 BindingDB Entry DOI: 10.7270/Q2TQ603X | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50036833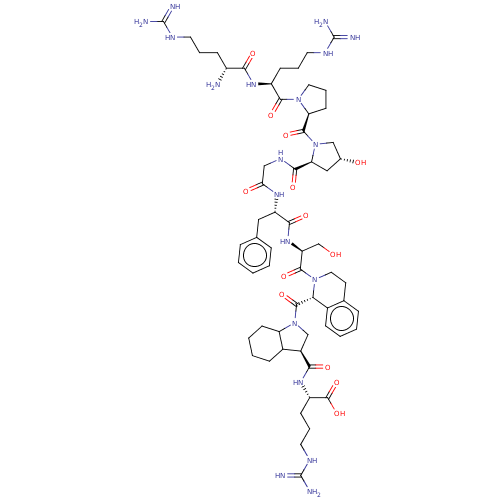 (CHEMBL2371961 | D-Arg-Arg-Pro-Hyp-Gly-Phe-Ser-D-Ti...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc. Curated by ChEMBL | Assay Description Binding affinity towards bradykinin receptor B2 using [3H]-bradykinin | J Med Chem 37: 1347-54 (1994) BindingDB Entry DOI: 10.7270/Q200014T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50046660 (2-[4-(2-Amino-6-methyl-4-oxo-3,4-dihydro-quinazoli...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Compound was tested for TS activity against human Thymidylate synthase by tight binding kinetics | J Med Chem 36: 733-46 (1993) BindingDB Entry DOI: 10.7270/Q2HM57JF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50494549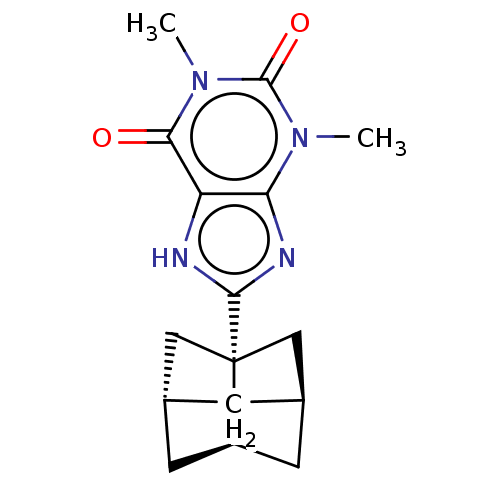 (CHEMBL3093327) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Binding affinity to rat adenosine A1 receptor | Bioorg Med Chem 21: 7435-52 (2013) Article DOI: 10.1016/j.bmc.2013.09.044 BindingDB Entry DOI: 10.7270/Q2TT4TWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50048908 (CHEMBL415788) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H] UR-MK300 from human neurotensin receptor 1 expressed in HT-29 cells incubated in dark after 30 mins by liquid scintillation coun... | ACS Med Chem Lett 11: 16-22 (2020) Article DOI: 10.1021/acsmedchemlett.9b00462 BindingDB Entry DOI: 10.7270/Q2SF30GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118441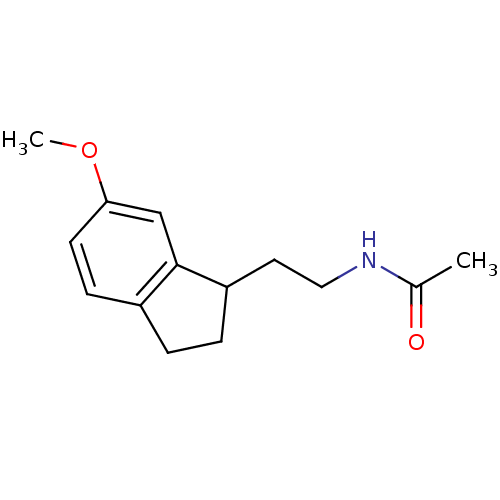 (CHEMBL134171 | N-[2-(6-Methoxy-indan-1-yl)-ethyl]-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50153628 (4-[3-(3-Naphthalen-2-yl-8-aza-bicyclo[3.2.1]oct-2-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro binding affinity for serotonin transporter | Bioorg Med Chem Lett 14: 5281-4 (2004) Article DOI: 10.1016/j.bmcl.2004.08.030 BindingDB Entry DOI: 10.7270/Q2F76C1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50518249 (CHEMBL4588827) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins | J Med Chem 62: 552-560 (2019) Article DOI: 10.1021/acs.jmedchem.8b01139 BindingDB Entry DOI: 10.7270/Q2MS3X3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50448167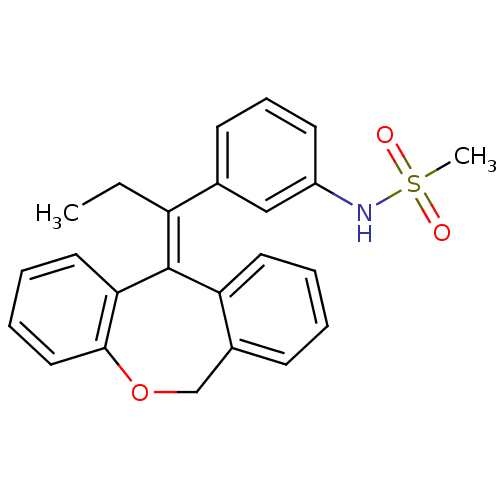 (CHEMBL3120318) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells | J Med Chem 57: 849-60 (2014) Article DOI: 10.1021/jm401616g BindingDB Entry DOI: 10.7270/Q23X885F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM86261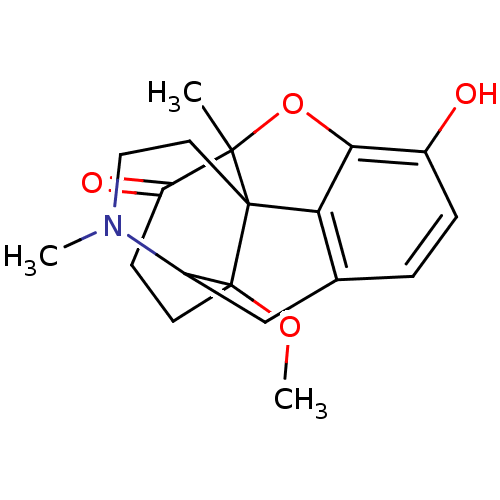 (14-methoxymetopon | CAS_131575-03-6 | NSC_125489) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by PDSP Ki Database | Eur J Pharmacol 483: 301-8 (2004) Article DOI: 10.1016/j.ejphar.2003.10.049 BindingDB Entry DOI: 10.7270/Q2TQ603X | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50268151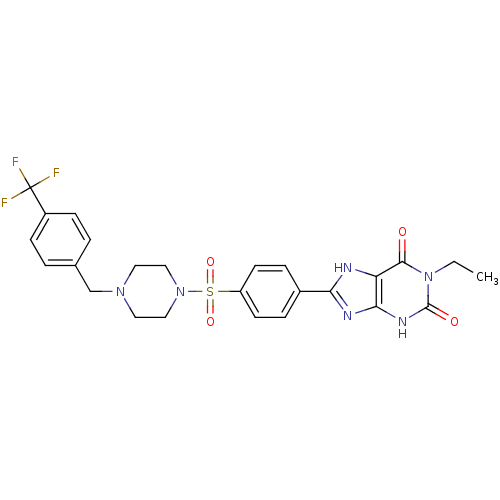 (1-Ethyl-8-(4-(4-(4-trifluoromethylbenzyl)piperazin...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Displacement of [3H]PSB-603 from human recombinant adenosine A2B receptor expressed in CHO cells | J Med Chem 52: 3994-4006 (2009) Article DOI: 10.1021/jm900413e BindingDB Entry DOI: 10.7270/Q24J0G1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin (Homo sapiens (Human)) | BDBM50533536 (CHEMBL4483694) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of trypsin (unknown origin) using Bz-FVRpNA as substrate incubated for 30 mins measured for 7 mins by morrison plot analysis | J Med Chem 59: 7287-92 (2016) Article DOI: 10.1021/acs.jmedchem.6b00557 BindingDB Entry DOI: 10.7270/Q29G5R98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50212148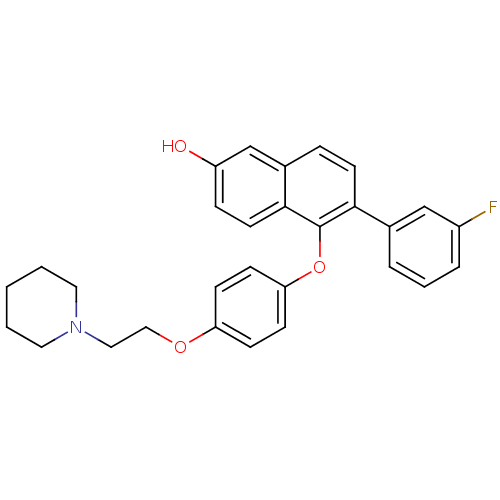 (6-(3-fluorophenyl)-5-(4-(2-(piperidin-1-yl)ethoxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERbeta | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50212148 (6-(3-fluorophenyl)-5-(4-(2-(piperidin-1-yl)ethoxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERalpha | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50101099 (CHEMBL3326228) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19423 (HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | <0.200 | <-55.4 | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50153629 (4-[2-(3-Naphthalen-2-yl-8-aza-bicyclo[3.2.1]oct-2-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description In vitro binding affinity for serotonin transporter | Bioorg Med Chem Lett 14: 5281-4 (2004) Article DOI: 10.1016/j.bmcl.2004.08.030 BindingDB Entry DOI: 10.7270/Q2F76C1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50212157 (6-(3,4-difluorophenyl)-5-(4-(2-(piperidin-1-yl)eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERalpha | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118438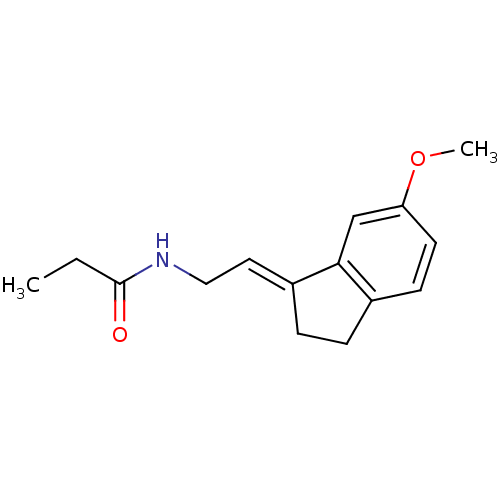 ((E) N-[2-(6-Methoxy-indan-1-ylidene)-ethyl]-propio...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.208 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50212149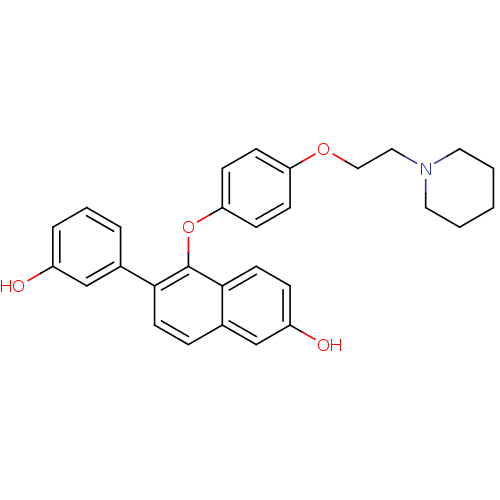 (6-(3-hydroxyphenyl)-5-(4-(2-(piperidin-1-yl)ethoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERalpha | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50518247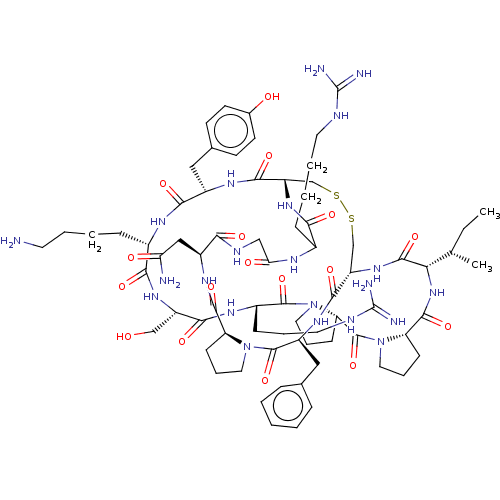 (CHEMBL4454304) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins | J Med Chem 62: 552-560 (2019) Article DOI: 10.1021/acs.jmedchem.8b01139 BindingDB Entry DOI: 10.7270/Q2MS3X3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM429076 (US10533010, Example I-174 | US11208415, Example I-...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of FITC-labeled Bak peptide binding to MBP-fused Mcl-1 (unknown origin) measured after 3 hrs by TR-FRET assay | J Med Chem 62: 3971-3988 (2019) Article DOI: 10.1021/acs.jmedchem.8b01991 BindingDB Entry DOI: 10.7270/Q2V69P04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50268109 (8-(4-(4-(4-chlorobenzyl)piperazin-1-ylsulfonyl)phe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.215 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Displacement of [3H]PSB-603 from human recombinant adenosine A2B receptor expressed in CHO cells | J Med Chem 52: 3994-4006 (2009) Article DOI: 10.1021/jm900413e BindingDB Entry DOI: 10.7270/Q24J0G1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50448165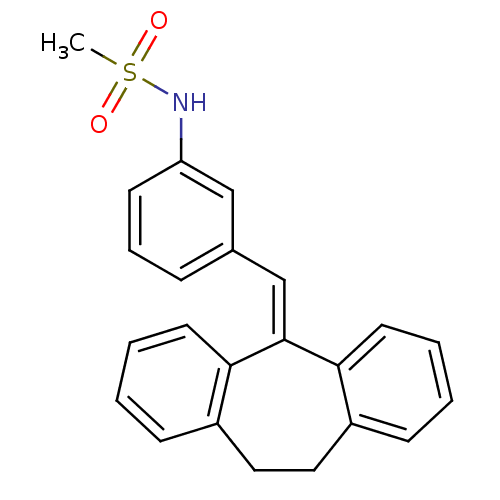 (CHEMBL3120320) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.226 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells | J Med Chem 57: 849-60 (2014) Article DOI: 10.1021/jm401616g BindingDB Entry DOI: 10.7270/Q23X885F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19967 (6-(4-fluorophenyl)-5-{4-[2-(piperidin-1-yl)ethoxy]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERalpha | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50048908 (CHEMBL415788) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Binding affinity to human neurotensin receptor 1 | ACS Med Chem Lett 11: 16-22 (2020) Article DOI: 10.1021/acsmedchemlett.9b00462 BindingDB Entry DOI: 10.7270/Q2SF30GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50101306 (CHEMBL3326229) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from human MOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50212160 (3-(6-hydroxy-1-(4-(2-(piperidin-1-yl)ethoxy)phenox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERalpha | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 45286 total ) | Next | Last >> |