 Found 473 hits with Last Name = 'mao' and Initial = 't'
Found 473 hits with Last Name = 'mao' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytidine deaminase
(Mus musculus) | BDBM50367349
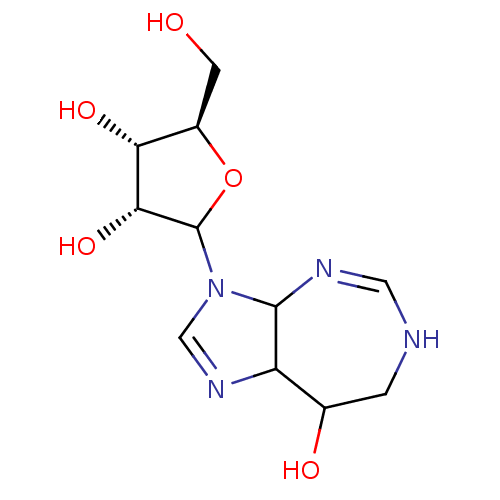
(CHEMBL604436)Show SMILES OC[C@H]1OC([C@H](O)[C@@H]1O)N1C=NC2C1N=CNCC2O |r,c:11,16| Show InChI InChI=1S/C11H18N4O5/c16-2-6-8(18)9(19)11(20-6)15-4-14-7-5(17)1-12-3-13-10(7)15/h3-11,16-19H,1-2H2,(H,12,13)/t5?,6-,7?,8-,9-,10?,11?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibitory constant for mouse kidney cytidine deaminase |
J Med Chem 29: 1374-80 (1986)
BindingDB Entry DOI: 10.7270/Q26T0N69 |
More data for this
Ligand-Target Pair | |
Cytidine deaminase
(Mus musculus) | BDBM50391219

(CHEMBL2093931)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)N1CC[C@@H](O)CNC1=O |r| Show InChI InChI=1S/C10H18N2O6/c13-4-6-7(15)8(16)9(18-6)12-2-1-5(14)3-11-10(12)17/h5-9,13-16H,1-4H2,(H,11,17)/t5-,6-,7-,8-,9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibitory constant for mouse kidney cytidine deaminase |
J Med Chem 29: 1374-80 (1986)
BindingDB Entry DOI: 10.7270/Q26T0N69 |
More data for this
Ligand-Target Pair | |
Cytidine deaminase
(Mus musculus) | BDBM50367346

(CHEMBL605877)Show SMILES OC[C@H]1OC([C@H](O)[C@@H]1O)N1C=CC(CO)NC1=O |r,c:11| Show InChI InChI=1S/C10H16N2O6/c13-3-5-1-2-12(10(17)11-5)9-8(16)7(15)6(4-14)18-9/h1-2,5-9,13-16H,3-4H2,(H,11,17)/t5?,6-,7-,8-,9?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibitory constant for mouse kidney cytidine deaminase |
J Med Chem 29: 1374-80 (1986)
BindingDB Entry DOI: 10.7270/Q26T0N69 |
More data for this
Ligand-Target Pair | |
Cytidine deaminase
(Mus musculus) | BDBM50421666
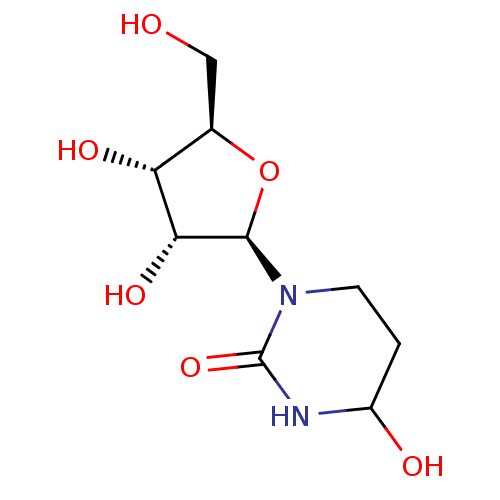
(CHEMBL2311128 | US9040501, 876404)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)N1CCC(O)NC1=O Show InChI InChI=1S/C9H16N2O6/c12-3-4-6(14)7(15)8(17-4)11-2-1-5(13)10-9(11)16/h4-8,12-15H,1-3H2,(H,10,16)/t4-,5?,6-,7-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibitory constant for mouse kidney cytidine deaminase |
J Med Chem 29: 1374-80 (1986)
BindingDB Entry DOI: 10.7270/Q26T0N69 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Mus musculus) | BDBM50226311
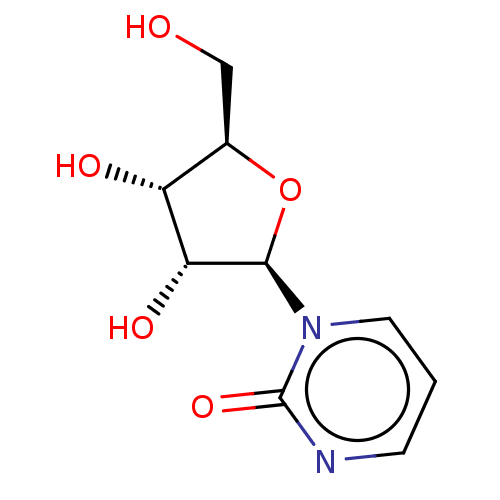
(CHEBI:46938 | Zebularine)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cccnc1=O |r| Show InChI InChI=1S/C9H12N2O5/c12-4-5-6(13)7(14)8(16-5)11-3-1-2-10-9(11)15/h1-3,5-8,12-14H,4H2/t5-,6-,7-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibitory constant for adenosine deaminase (ADA) |
J Med Chem 29: 1374-80 (1986)
BindingDB Entry DOI: 10.7270/Q26T0N69 |
More data for this
Ligand-Target Pair | |
Cytidine deaminase
(Mus musculus) | BDBM50226311

(CHEBI:46938 | Zebularine)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cccnc1=O |r| Show InChI InChI=1S/C9H12N2O5/c12-4-5-6(13)7(14)8(16-5)11-3-1-2-10-9(11)15/h1-3,5-8,12-14H,4H2/t5-,6-,7-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibitory constant for mouse kidney cytidine deaminase |
J Med Chem 29: 1374-80 (1986)
BindingDB Entry DOI: 10.7270/Q26T0N69 |
More data for this
Ligand-Target Pair | |
Cytidine deaminase
(Mus musculus) | BDBM50367344
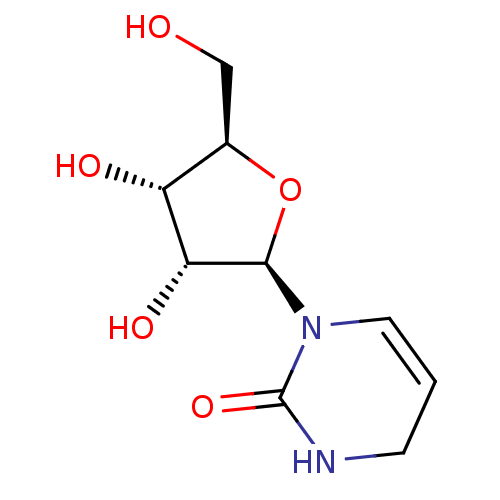
(CHEMBL1232227 | CHEMBL604639)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)N1C=CCNC1=O |r,c:11| Show InChI InChI=1S/C9H14N2O5/c12-4-5-6(13)7(14)8(16-5)11-3-1-2-10-9(11)15/h1,3,5-8,12-14H,2,4H2,(H,10,15)/t5-,6-,7-,8?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibitory constant for mouse kidney cytidine deaminase |
J Med Chem 29: 1374-80 (1986)
BindingDB Entry DOI: 10.7270/Q26T0N69 |
More data for this
Ligand-Target Pair | |
Cytidine deaminase
(Mus musculus) | BDBM50367345
(CHEMBL604220)Show SMILES OC[C@H]1OC([C@H](O)[C@@H]1O)N1CCC=NC1=O |r,c:13| Show InChI InChI=1S/C9H14N2O5/c12-4-5-6(13)7(14)8(16-5)11-3-1-2-10-9(11)15/h2,5-8,12-14H,1,3-4H2/t5-,6-,7-,8?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibitory constant for mouse kidney cytidine deaminase |
J Med Chem 29: 1374-80 (1986)
BindingDB Entry DOI: 10.7270/Q26T0N69 |
More data for this
Ligand-Target Pair | |
Cytidine deaminase
(Mus musculus) | BDBM50335291

(9-(beta-D-ribofuranosyl)purine | CHEMBL1399702 | n...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2cncnc12 Show InChI InChI=1S/C10H12N4O4/c15-2-6-7(16)8(17)10(18-6)14-4-13-5-1-11-3-12-9(5)14/h1,3-4,6-8,10,15-17H,2H2/t6-,7-,8-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibitory constant for mouse kidney cytidine deaminase |
J Med Chem 29: 1374-80 (1986)
BindingDB Entry DOI: 10.7270/Q26T0N69 |
More data for this
Ligand-Target Pair | |
Cytidine deaminase
(Mus musculus) | BDBM50367348
(CHEMBL3218780 | CHEMBL604226)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2C(CO)NC=Nc12 |r,c:18| Show InChI InChI=1S/C11H16N4O5/c16-1-5-7-10(13-3-12-5)15(4-14-7)11-9(19)8(18)6(2-17)20-11/h3-6,8-9,11,16-19H,1-2H2,(H,12,13)/t5?,6-,8-,9-,11?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibitory constant for mouse kidney cytidine deaminase |
J Med Chem 29: 1374-80 (1986)
BindingDB Entry DOI: 10.7270/Q26T0N69 |
More data for this
Ligand-Target Pair | |
Cytidine deaminase
(Mus musculus) | BDBM50367351
(CHEMBL604221)Show SMILES OC[C@H]1OC([C@H](O)[C@@H]1O)N1CCC(NC1=O)C(O)O |r| Show InChI InChI=1S/C10H18N2O7/c13-3-5-6(14)7(15)8(19-5)12-2-1-4(9(16)17)11-10(12)18/h4-9,13-17H,1-3H2,(H,11,18)/t4?,5-,6-,7-,8?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibitory constant for mouse kidney cytidine deaminase |
J Med Chem 29: 1374-80 (1986)
BindingDB Entry DOI: 10.7270/Q26T0N69 |
More data for this
Ligand-Target Pair | |
Cytidine deaminase
(Mus musculus) | BDBM50367347

(CHEMBL605678)Show SMILES OC[C@H]1OC([C@H](O)[C@@H]1O)n1ccc(CO)nc1=O |r| Show InChI InChI=1S/C10H14N2O6/c13-3-5-1-2-12(10(17)11-5)9-8(16)7(15)6(4-14)18-9/h1-2,6-9,13-16H,3-4H2/t6-,7-,8-,9?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibitory constant for mouse kidney cytidine deaminase |
J Med Chem 29: 1374-80 (1986)
BindingDB Entry DOI: 10.7270/Q26T0N69 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fer
(Homo sapiens (Human)) | BDBM50501949
(CHEMBL4461851)Show SMILES Cc1cccc(Nc2nc(N[C@@H]3CCCC[C@@H]3N)c(C#N)c3cn[nH]c(=O)c23)c1 |r| Show InChI InChI=1S/C21H23N7O/c1-12-5-4-6-13(9-12)25-20-18-15(11-24-28-21(18)29)14(10-22)19(27-20)26-17-8-3-2-7-16(17)23/h4-6,9,11,16-17H,2-3,7-8,23H2,1H3,(H,28,29)(H2,25,26,27)/t16-,17+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His-tagged human FER (SH2 domain to C-terminal) expressed in Escherichia coli assessed as decrease in FL-Peptide... |
ACS Med Chem Lett 10: 737-742 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00631
BindingDB Entry DOI: 10.7270/Q2WS8XG3 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50264689
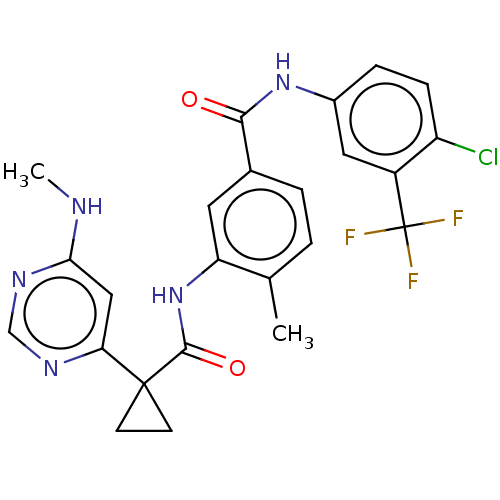
(CHEMBL4060057)Show SMILES CNc1cc(ncn1)C1(CC1)C(=O)Nc1cc(ccc1C)C(=O)Nc1ccc(Cl)c(c1)C(F)(F)F Show InChI InChI=1S/C24H21ClF3N5O2/c1-13-3-4-14(21(34)32-15-5-6-17(25)16(10-15)24(26,27)28)9-18(13)33-22(35)23(7-8-23)19-11-20(29-2)31-12-30-19/h3-6,9-12H,7-8H2,1-2H3,(H,32,34)(H,33,35)(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of CRAF (unknown origin) using human His6-tagged MEK1 K97R mutant as substrate pretreated for 20 mins followed by [33P]-ATP addition measu... |
Eur J Med Chem 130: 86-106 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.041
BindingDB Entry DOI: 10.7270/Q2DN47JG |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50394470

(CHEMBL2160099)Show SMILES OCCOc1ccccc1NC(=O)N[C@@H]1CCN(Cc2ccc3cc(F)ccc3c2)C1 |r| Show InChI InChI=1S/C24H26FN3O3/c25-20-8-7-18-13-17(5-6-19(18)14-20)15-28-10-9-21(16-28)26-24(30)27-22-3-1-2-4-23(22)31-12-11-29/h1-8,13-14,21,29H,9-12,15-16H2,(H2,26,27,30)/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR3 receptor in human eosinophils assessed as inhibition of CCL11-induced degranulation after 4 hrs by ELISA |
Bioorg Med Chem Lett 22: 6876-81 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.035
BindingDB Entry DOI: 10.7270/Q2QC04MN |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297172
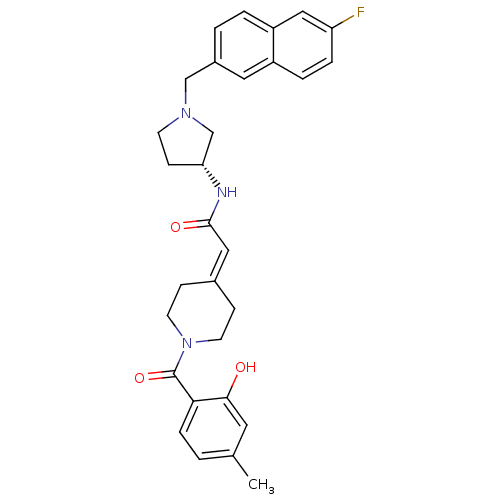
(CHEMBL560275 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...)Show SMILES [#6]-c1ccc(-[#6](=O)-[#7]-2-[#6]-[#6]\[#6](-[#6]-[#6]-2)=[#6]/[#6](=O)-[#7]-[#6@@H]-2-[#6]-[#6]-[#7](-[#6]-c3ccc4cc(F)ccc4c3)-[#6]-2)c(-[#8])c1 |r| Show InChI InChI=1S/C30H32FN3O3/c1-20-2-7-27(28(35)14-20)30(37)34-12-8-21(9-13-34)16-29(36)32-26-10-11-33(19-26)18-22-3-4-24-17-25(31)6-5-23(24)15-22/h2-7,14-17,26,35H,8-13,18-19H2,1H3,(H,32,36)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297171
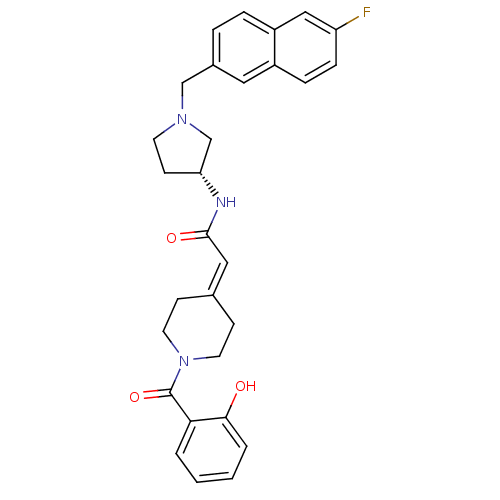
(CHEMBL551735 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...)Show SMILES [#8]-c1ccccc1-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]/[#6](=O)-[#7]-[#6@@H]-1-[#6]-[#6]-[#7](-[#6]-c2ccc3cc(F)ccc3c2)-[#6]-1 |r| Show InChI InChI=1S/C29H30FN3O3/c30-24-8-7-22-15-21(5-6-23(22)17-24)18-32-12-11-25(19-32)31-28(35)16-20-9-13-33(14-10-20)29(36)26-3-1-2-4-27(26)34/h1-8,15-17,25,34H,9-14,18-19H2,(H,31,35)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells by functional inhibition curve analysis |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297181

(2-[1-(1,3-Benzodioxol-5-ylcarbonyl)piperidin-4-yli...)Show SMILES Fc1ccc2cc(-[#6]-[#7]-3-[#6]-[#6]-[#6@H](-[#6]-3)-[#7]-[#6](=O)\[#6]=[#6]-3/[#6]-[#6]-[#7](-[#6]-[#6]-3)-[#6](=O)-c3ccc4-[#8]-[#6]-[#8]-c4c3)ccc2c1 |r| Show InChI InChI=1S/C30H30FN3O4/c31-25-5-3-22-13-21(1-2-23(22)15-25)17-33-10-9-26(18-33)32-29(35)14-20-7-11-34(12-8-20)30(36)24-4-6-27-28(16-24)38-19-37-27/h1-6,13-16,26H,7-12,17-19H2,(H,32,35)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50394470

(CHEMBL2160099)Show SMILES OCCOc1ccccc1NC(=O)N[C@@H]1CCN(Cc2ccc3cc(F)ccc3c2)C1 |r| Show InChI InChI=1S/C24H26FN3O3/c25-20-8-7-18-13-17(5-6-19(18)14-20)15-28-10-9-21(16-28)26-24(30)27-22-3-1-2-4-23(22)31-12-11-29/h1-8,13-14,21,29H,9-12,15-16H2,(H2,26,27,30)/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR3 receptor |
Bioorg Med Chem Lett 22: 6876-81 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.035
BindingDB Entry DOI: 10.7270/Q2QC04MN |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50183273

((S)-6-(3-chloro-2-fluorobenzyl)-1-(1-hydroxy-3-met...)Show SMILES COc1cc2n(cc(C(O)=O)c(=O)c2cc1Cc1cccc(Cl)c1F)[C@H](CO)C(C)C |r| Show InChI InChI=1S/C23H23ClFNO5/c1-12(2)19(11-27)26-10-16(23(29)30)22(28)15-8-14(20(31-3)9-18(15)26)7-13-5-4-6-17(24)21(13)25/h4-6,8-10,12,19,27H,7,11H2,1-3H3,(H,29,30)/t19-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 integrase |
Bioorg Med Chem 23: 3860-8 (2015)
Article DOI: 10.1016/j.bmc.2015.03.037
BindingDB Entry DOI: 10.7270/Q2FX7DFD |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297183

(CHEMBL551738 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...)Show SMILES [#6]-[#8]-c1ccc(cc1)-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]/[#6](=O)-[#7]-[#6@@H]-1-[#6]-[#6]-[#7](-[#6]-c2ccc3cc(F)ccc3c2)-[#6]-1 |r| Show InChI InChI=1S/C30H32FN3O3/c1-37-28-8-5-23(6-9-28)30(36)34-14-10-21(11-15-34)17-29(35)32-27-12-13-33(20-27)19-22-2-3-25-18-26(31)7-4-24(25)16-22/h2-9,16-18,27H,10-15,19-20H2,1H3,(H,32,35)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase A-Raf
(Homo sapiens (Human)) | BDBM50396483
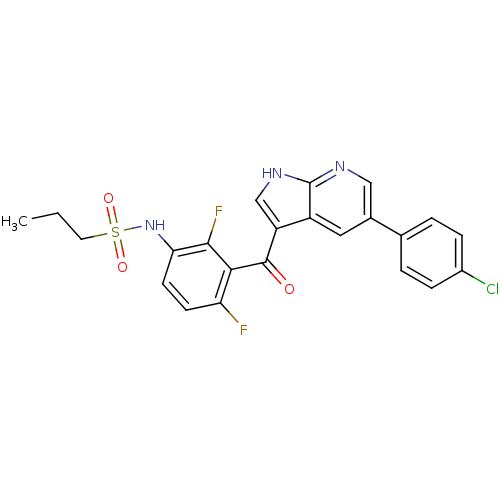
(PLX-4032 | RG 7204 | Ro 5185426 | US10570155, Vemu...)Show SMILES CCCS(=O)(=O)Nc1ccc(F)c(C(=O)c2c[nH]c3ncc(cc23)-c2ccc(Cl)cc2)c1F Show InChI InChI=1S/C23H18ClF2N3O3S/c1-2-9-33(31,32)29-19-8-7-18(25)20(21(19)26)22(30)17-12-28-23-16(17)10-14(11-27-23)13-3-5-15(24)6-4-13/h3-8,10-12,29H,2,9H2,1H3,(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of ARAF (unknown origin) using human His6-tagged MEK1 K97R mutant as substrate pretreated for 20 mins followed by [33P]-ATP addition measu... |
Eur J Med Chem 130: 86-106 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.041
BindingDB Entry DOI: 10.7270/Q2DN47JG |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50394487

(CHEMBL2160111)Show SMILES Oc1ccccc1S(=O)(=O)N1CCC(CC1)NC(=O)N[C@@H]1CCN(Cc2ccc3cc(F)ccc3c2)C1 |r| Show InChI InChI=1S/C27H31FN4O4S/c28-22-8-7-20-15-19(5-6-21(20)16-22)17-31-12-9-24(18-31)30-27(34)29-23-10-13-32(14-11-23)37(35,36)26-4-2-1-3-25(26)33/h1-8,15-16,23-24,33H,9-14,17-18H2,(H2,29,30,34)/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR3 receptor |
Bioorg Med Chem Lett 22: 6876-81 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.035
BindingDB Entry DOI: 10.7270/Q2QC04MN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fer
(Homo sapiens (Human)) | BDBM50501965
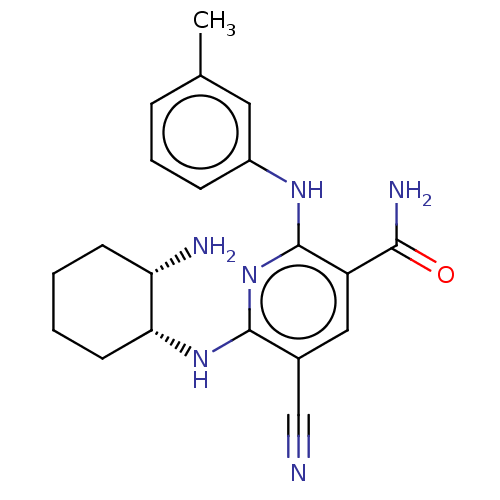
(CHEMBL4457164)Show SMILES Cc1cccc(Nc2nc(N[C@@H]3CCCC[C@@H]3N)c(cc2C(N)=O)C#N)c1 |r| Show InChI InChI=1S/C20H24N6O/c1-12-5-4-6-14(9-12)24-20-15(18(23)27)10-13(11-21)19(26-20)25-17-8-3-2-7-16(17)22/h4-6,9-10,16-17H,2-3,7-8,22H2,1H3,(H2,23,27)(H2,24,25,26)/t16-,17+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His-tagged human FER (SH2 domain to C-terminal) expressed in Escherichia coli assessed as decrease in FL-Peptide... |
ACS Med Chem Lett 10: 737-742 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00631
BindingDB Entry DOI: 10.7270/Q2WS8XG3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fer
(Homo sapiens (Human)) | BDBM50501961
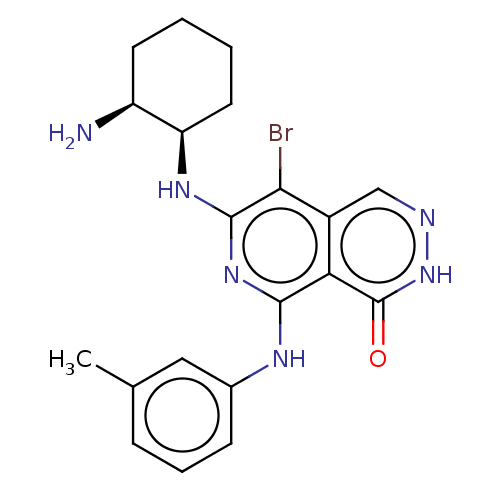
(CHEMBL4456804)Show SMILES Cc1cccc(Nc2nc(N[C@@H]3CCCC[C@@H]3N)c(Br)c3cn[nH]c(=O)c23)c1 |r| Show InChI InChI=1S/C20H23BrN6O/c1-11-5-4-6-12(9-11)24-18-16-13(10-23-27-20(16)28)17(21)19(26-18)25-15-8-3-2-7-14(15)22/h4-6,9-10,14-15H,2-3,7-8,22H2,1H3,(H,27,28)(H2,24,25,26)/t14-,15+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His-tagged human FER (SH2 domain to C-terminal) expressed in Escherichia coli assessed as decrease in FL-Peptide... |
ACS Med Chem Lett 10: 737-742 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00631
BindingDB Entry DOI: 10.7270/Q2WS8XG3 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297177

(CHEMBL561535 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...)Show SMILES [#6]-[#8]-c1ccc(-[#6](=O)-[#7]-2-[#6]-[#6]\[#6](-[#6]-[#6]-2)=[#6]/[#6](=O)-[#7]-[#6@@H]-2-[#6]-[#6]-[#7](-[#6]-c3ccc4cc(F)ccc4c3)-[#6]-2)c(-[#8])c1 |r| Show InChI InChI=1S/C30H32FN3O4/c1-38-26-6-7-27(28(35)17-26)30(37)34-12-8-20(9-13-34)15-29(36)32-25-10-11-33(19-25)18-21-2-3-23-16-24(31)5-4-22(23)14-21/h2-7,14-17,25,35H,8-13,18-19H2,1H3,(H,32,36)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297182

(2-[1-(3,4-Dimethoxybenzoyl)piperidin-4-ylidene]-N-...)Show SMILES [#6]-[#8]-c1ccc(cc1-[#8]-[#6])-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]/[#6](=O)-[#7]-[#6@@H]-1-[#6]-[#6]-[#7](-[#6]-c2ccc3cc(F)ccc3c2)-[#6]-1 |r| Show InChI InChI=1S/C31H34FN3O4/c1-38-28-8-6-25(18-29(28)39-2)31(37)35-13-9-21(10-14-35)16-30(36)33-27-11-12-34(20-27)19-22-3-4-24-17-26(32)7-5-23(24)15-22/h3-8,15-18,27H,9-14,19-20H2,1-2H3,(H,33,36)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297190
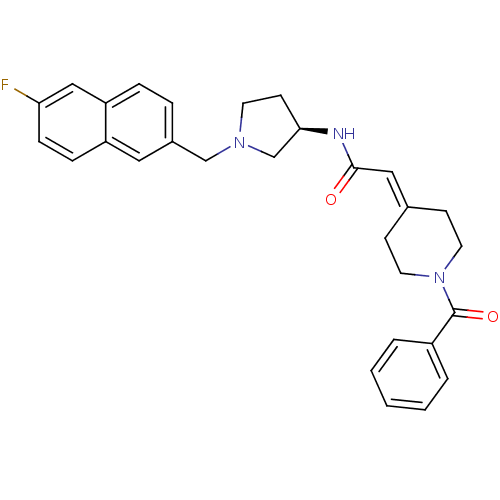
(2-(1-Benzoylpiperidin-4-ylidene)-N-{(3R)-1-[(6-flu...)Show SMILES Fc1ccc2cc(-[#6]-[#7]-3-[#6]-[#6]-[#6@H](-[#6]-3)-[#7]-[#6](=O)\[#6]=[#6]-3/[#6]-[#6]-[#7](-[#6]-[#6]-3)-[#6](=O)-c3ccccc3)ccc2c1 |r| Show InChI InChI=1S/C29H30FN3O2/c30-26-9-8-24-16-22(6-7-25(24)18-26)19-32-13-12-27(20-32)31-28(34)17-21-10-14-33(15-11-21)29(35)23-4-2-1-3-5-23/h1-9,16-18,27H,10-15,19-20H2,(H,31,34)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fer
(Homo sapiens (Human)) | BDBM50501963
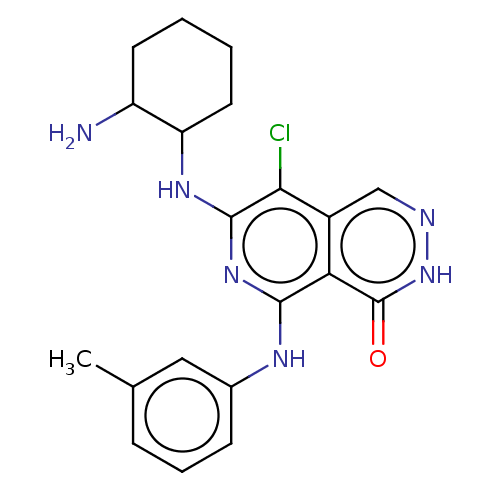
(CHEMBL4524587)Show SMILES Cc1cccc(Nc2nc(NC3CCCCC3N)c(Cl)c3cn[nH]c(=O)c23)c1 Show InChI InChI=1S/C20H23ClN6O/c1-11-5-4-6-12(9-11)24-18-16-13(10-23-27-20(16)28)17(21)19(26-18)25-15-8-3-2-7-14(15)22/h4-6,9-10,14-15H,2-3,7-8,22H2,1H3,(H,27,28)(H2,24,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His-tagged human FER (SH2 domain to C-terminal) expressed in Escherichia coli assessed as decrease in FL-Peptide... |
ACS Med Chem Lett 10: 737-742 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00631
BindingDB Entry DOI: 10.7270/Q2WS8XG3 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297188
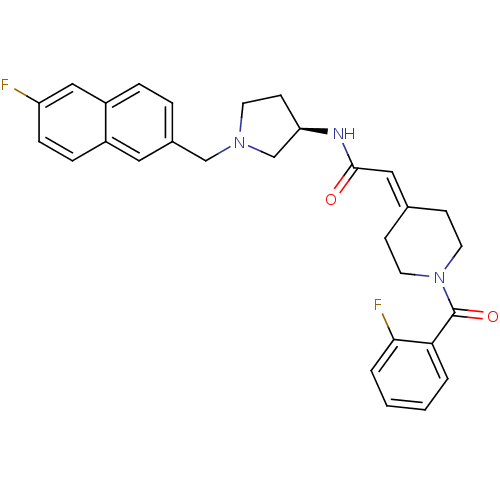
(2-[1-(2-Fluorobenzoyl)piperidin-4-ylidene]-N-{(3R)...)Show SMILES Fc1ccc2cc(-[#6]-[#7]-3-[#6]-[#6]-[#6@H](-[#6]-3)-[#7]-[#6](=O)\[#6]=[#6]-3/[#6]-[#6]-[#7](-[#6]-[#6]-3)-[#6](=O)-c3ccccc3F)ccc2c1 |r| Show InChI InChI=1S/C29H29F2N3O2/c30-24-8-7-22-15-21(5-6-23(22)17-24)18-33-12-11-25(19-33)32-28(35)16-20-9-13-34(14-10-20)29(36)26-3-1-2-4-27(26)31/h1-8,15-17,25H,9-14,18-19H2,(H,32,35)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50387669
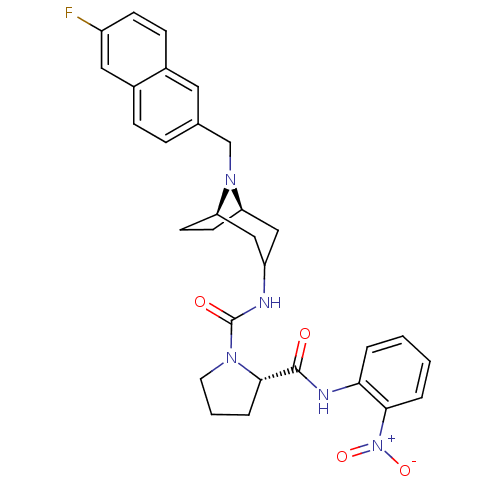
(CHEMBL2058071)Show SMILES [O-][N+](=O)c1ccccc1NC(=O)[C@@H]1CCCN1C(=O)NC1C[C@H]2CC[C@H](C1)N2Cc1ccc2cc(F)ccc2c1 |r,TLB:28:27:20.21.26:23.24,THB:19:20:27:23.24| Show InChI InChI=1S/C30H32FN5O4/c31-22-10-9-20-14-19(7-8-21(20)15-22)18-35-24-11-12-25(35)17-23(16-24)32-30(38)34-13-3-6-28(34)29(37)33-26-4-1-2-5-27(26)36(39)40/h1-2,4-5,7-10,14-15,23-25,28H,3,6,11-13,16-18H2,(H,32,38)(H,33,37)/t24-,25-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 |
Bioorg Med Chem Lett 22: 4951-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.042
BindingDB Entry DOI: 10.7270/Q2057H06 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297180
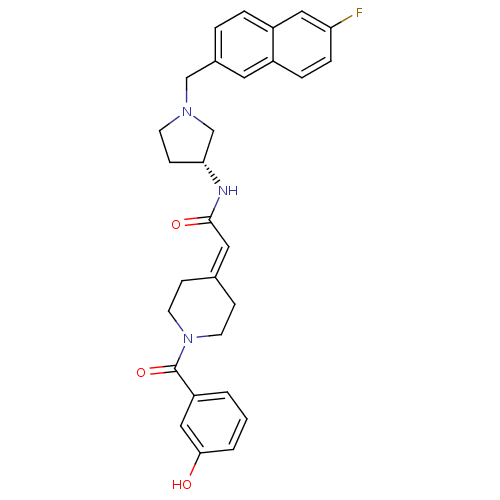
(CHEMBL556916 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...)Show SMILES [#8]-c1cccc(c1)-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]/[#6](=O)-[#7]-[#6@@H]-1-[#6]-[#6]-[#7](-[#6]-c2ccc3cc(F)ccc3c2)-[#6]-1 |r| Show InChI InChI=1S/C29H30FN3O3/c30-25-7-6-22-14-21(4-5-23(22)16-25)18-32-11-10-26(19-32)31-28(35)15-20-8-12-33(13-9-20)29(36)24-2-1-3-27(34)17-24/h1-7,14-17,26,34H,8-13,18-19H2,(H,31,35)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297185

(CHEMBL556227 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...)Show SMILES [#6]-[#8]-c1ccccc1-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]/[#6](=O)-[#7]-[#6@@H]-1-[#6]-[#6]-[#7](-[#6]-c2ccc3cc(F)ccc3c2)-[#6]-1 |r| Show InChI InChI=1S/C30H32FN3O3/c1-37-28-5-3-2-4-27(28)30(36)34-14-10-21(11-15-34)17-29(35)32-26-12-13-33(20-26)19-22-6-7-24-18-25(31)9-8-23(24)16-22/h2-9,16-18,26H,10-15,19-20H2,1H3,(H,32,35)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297179

(CHEMBL562923 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...)Show SMILES [#8]-c1ccc(cc1)-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]/[#6](=O)-[#7]-[#6@@H]-1-[#6]-[#6]-[#7](-[#6]-c2ccc3cc(F)ccc3c2)-[#6]-1 |r| Show InChI InChI=1S/C29H30FN3O3/c30-25-6-3-23-15-21(1-2-24(23)17-25)18-32-12-11-26(19-32)31-28(35)16-20-9-13-33(14-10-20)29(36)22-4-7-27(34)8-5-22/h1-8,15-17,26,34H,9-14,18-19H2,(H,31,35)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297184
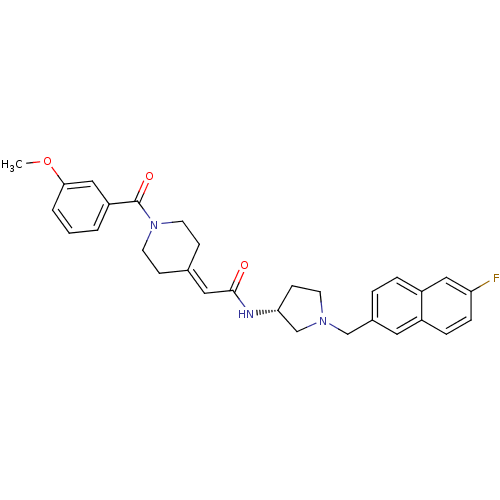
(CHEMBL557118 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...)Show SMILES [#6]-[#8]-c1cccc(c1)-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]/[#6](=O)-[#7]-[#6@@H]-1-[#6]-[#6]-[#7](-[#6]-c2ccc3cc(F)ccc3c2)-[#6]-1 |r| Show InChI InChI=1S/C30H32FN3O3/c1-37-28-4-2-3-25(18-28)30(36)34-13-9-21(10-14-34)16-29(35)32-27-11-12-33(20-27)19-22-5-6-24-17-26(31)8-7-23(24)15-22/h2-8,15-18,27H,9-14,19-20H2,1H3,(H,32,35)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297174

(2-[1-(4-Fluoro-2-hydroxybenzoyl)piperidin-4-yliden...)Show SMILES [#8]-c1cc(F)ccc1-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]/[#6](=O)-[#7]-[#6@@H]-1-[#6]-[#6]-[#7](-[#6]-c2ccc3cc(F)ccc3c2)-[#6]-1 |r| Show InChI InChI=1S/C29H29F2N3O3/c30-23-4-3-21-13-20(1-2-22(21)15-23)17-33-10-9-25(18-33)32-28(36)14-19-7-11-34(12-8-19)29(37)26-6-5-24(31)16-27(26)35/h1-6,13-16,25,35H,7-12,17-18H2,(H,32,36)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of CRAF (unknown origin) using human His6-tagged MEK1 K97R mutant as substrate pretreated for 20 mins followed by [33P]-ATP addition measu... |
Eur J Med Chem 130: 86-106 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.041
BindingDB Entry DOI: 10.7270/Q2DN47JG |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50264730
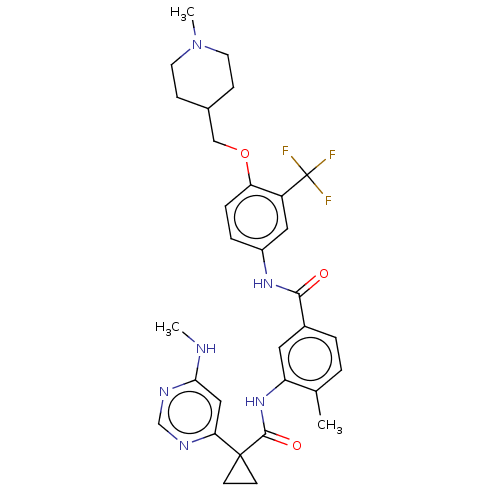
(CHEMBL4080362)Show SMILES CNc1cc(ncn1)C1(CC1)C(=O)Nc1cc(ccc1C)C(=O)Nc1ccc(OCC2CCN(C)CC2)c(c1)C(F)(F)F Show InChI InChI=1S/C31H35F3N6O3/c1-19-4-5-21(14-24(19)39-29(42)30(10-11-30)26-16-27(35-2)37-18-36-26)28(41)38-22-6-7-25(23(15-22)31(32,33)34)43-17-20-8-12-40(3)13-9-20/h4-7,14-16,18,20H,8-13,17H2,1-3H3,(H,38,41)(H,39,42)(H,35,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of CRAF (unknown origin) using human His6-tagged MEK1 K97R mutant as substrate pretreated for 20 mins followed by [33P]-ATP addition measu... |
Eur J Med Chem 130: 86-106 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.041
BindingDB Entry DOI: 10.7270/Q2DN47JG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50396483

(PLX-4032 | RG 7204 | Ro 5185426 | US10570155, Vemu...)Show SMILES CCCS(=O)(=O)Nc1ccc(F)c(C(=O)c2c[nH]c3ncc(cc23)-c2ccc(Cl)cc2)c1F Show InChI InChI=1S/C23H18ClF2N3O3S/c1-2-9-33(31,32)29-19-8-7-18(25)20(21(19)26)22(30)17-12-28-23-16(17)10-14(11-27-23)13-3-5-15(24)6-4-13/h3-8,10-12,29H,2,9H2,1H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of wild-type BRAF (unknown origin) using human His6-tagged MEK1 K97R mutant as substrate pretreated for 20 mins followed by [33P]-ATP addi... |
Eur J Med Chem 130: 86-106 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.041
BindingDB Entry DOI: 10.7270/Q2DN47JG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase A-Raf
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of ARAF (unknown origin) using human His6-tagged MEK1 K97R mutant as substrate pretreated for 20 mins followed by [33P]-ATP addition measu... |
Eur J Med Chem 130: 86-106 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.041
BindingDB Entry DOI: 10.7270/Q2DN47JG |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297173
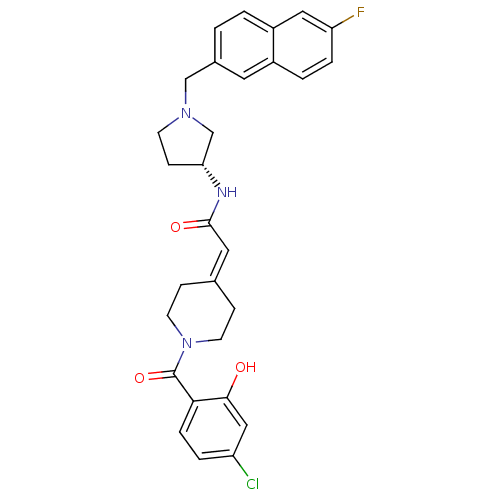
(2-[1-(4-Chloro-2-hydroxybenzoyl)piperidin-4-yliden...)Show SMILES [#8]-c1cc(Cl)ccc1-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]/[#6](=O)-[#7]-[#6@@H]-1-[#6]-[#6]-[#7](-[#6]-c2ccc3cc(F)ccc3c2)-[#6]-1 |r| Show InChI InChI=1S/C29H29ClFN3O3/c30-23-4-6-26(27(35)16-23)29(37)34-11-7-19(8-12-34)14-28(36)32-25-9-10-33(18-25)17-20-1-2-22-15-24(31)5-3-21(22)13-20/h1-6,13-16,25,35H,7-12,17-18H2,(H,32,36)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50387649

(CHEMBL2057751)Show SMILES Nc1ccc2n(C3CCN(CC3)C(=O)NC3C[C@H]4CC[C@H](C3)N4Cc3ccc4cc(F)ccc4c3)c(=O)[nH]c2c1 |r,TLB:23:22:15.16.21:18.19,THB:14:15:22:18.19| Show InChI InChI=1S/C31H35FN6O2/c32-22-4-3-20-13-19(1-2-21(20)14-22)18-37-26-6-7-27(37)17-24(16-26)34-30(39)36-11-9-25(10-12-36)38-29-8-5-23(33)15-28(29)35-31(38)40/h1-5,8,13-15,24-27H,6-7,9-12,16-18,33H2,(H,34,39)(H,35,40)/t26-,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 |
Bioorg Med Chem Lett 22: 4951-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.042
BindingDB Entry DOI: 10.7270/Q2057H06 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297171

(CHEMBL551735 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...)Show SMILES [#8]-c1ccccc1-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]/[#6](=O)-[#7]-[#6@@H]-1-[#6]-[#6]-[#7](-[#6]-c2ccc3cc(F)ccc3c2)-[#6]-1 |r| Show InChI InChI=1S/C29H30FN3O3/c30-24-8-7-22-15-21(5-6-23(22)17-24)18-32-12-11-25(19-32)31-28(35)16-20-9-13-33(14-10-20)29(36)26-3-1-2-4-27(26)34/h1-8,15-17,25,34H,9-14,18-19H2,(H,31,35)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50264690

(CHEMBL4066939)Show SMILES CNc1cc(ncn1)C1(CC1)C(=O)Nc1cc(ccc1C)C(=O)Nc1ccc(OC2CCN(C)CC2)c(c1)C(F)(F)F Show InChI InChI=1S/C30H33F3N6O3/c1-18-4-5-19(14-23(18)38-28(41)29(10-11-29)25-16-26(34-2)36-17-35-25)27(40)37-20-6-7-24(22(15-20)30(31,32)33)42-21-8-12-39(3)13-9-21/h4-7,14-17,21H,8-13H2,1-3H3,(H,37,40)(H,38,41)(H,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of CRAF (unknown origin) using human His6-tagged MEK1 K97R mutant as substrate pretreated for 20 mins followed by [33P]-ATP addition measu... |
Eur J Med Chem 130: 86-106 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.041
BindingDB Entry DOI: 10.7270/Q2DN47JG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116599
BindingDB Entry DOI: 10.7270/Q2P27363 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50394482

(CHEMBL2159867)Show SMILES OCCCCOc1ccccc1NC(=O)N[C@@H]1CCN(Cc2ccc3cc(F)ccc3c2)C1 |r| Show InChI InChI=1S/C26H30FN3O3/c27-22-10-9-20-15-19(7-8-21(20)16-22)17-30-12-11-23(18-30)28-26(32)29-24-5-1-2-6-25(24)33-14-4-3-13-31/h1-2,5-10,15-16,23,31H,3-4,11-14,17-18H2,(H2,28,29,32)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR3 receptor |
Bioorg Med Chem Lett 22: 6876-81 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.035
BindingDB Entry DOI: 10.7270/Q2QC04MN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116599
BindingDB Entry DOI: 10.7270/Q2P27363 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116599
BindingDB Entry DOI: 10.7270/Q2P27363 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116599
BindingDB Entry DOI: 10.7270/Q2P27363 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fer
(Homo sapiens (Human)) | BDBM50501959
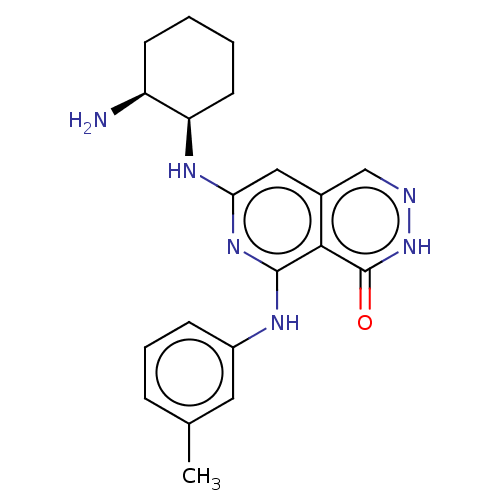
(CHEMBL4557212)Show SMILES Cc1cccc(Nc2nc(N[C@@H]3CCCC[C@@H]3N)cc3cn[nH]c(=O)c23)c1 |r| Show InChI InChI=1S/C20H24N6O/c1-12-5-4-6-14(9-12)23-19-18-13(11-22-26-20(18)27)10-17(25-19)24-16-8-3-2-7-15(16)21/h4-6,9-11,15-16H,2-3,7-8,21H2,1H3,(H,26,27)(H2,23,24,25)/t15-,16+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His-tagged human FER (SH2 domain to C-terminal) expressed in Escherichia coli assessed as decrease in FL-Peptide... |
ACS Med Chem Lett 10: 737-742 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00631
BindingDB Entry DOI: 10.7270/Q2WS8XG3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data