 Found 1376 hits with Last Name = 'masuda' and Initial = 'e'
Found 1376 hits with Last Name = 'masuda' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50396071
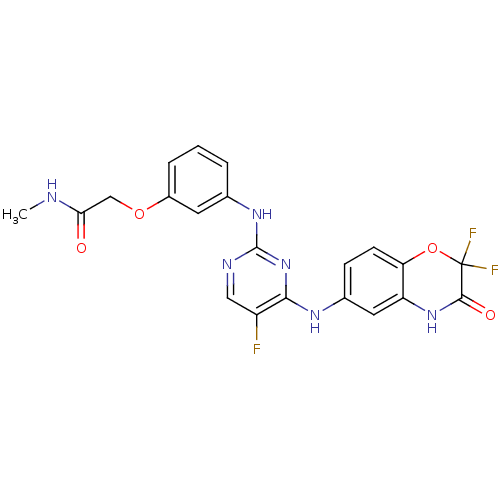
(CHEMBL2170582 | US10828301, Compound R921303)Show SMILES CNC(=O)COc1cccc(Nc2ncc(F)c(Nc3ccc4OC(F)(F)C(=O)Nc4c3)n2)c1 Show InChI InChI=1S/C21H17F3N6O4/c1-25-17(31)10-33-13-4-2-3-11(7-13)28-20-26-9-14(22)18(30-20)27-12-5-6-16-15(8-12)29-19(32)21(23,24)34-16/h2-9H,10H2,1H3,(H,25,31)(H,29,32)(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rigel, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK |
J Med Chem 55: 3614-43 (2012)
Article DOI: 10.1021/jm201271b
BindingDB Entry DOI: 10.7270/Q2NZ88RC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50325991
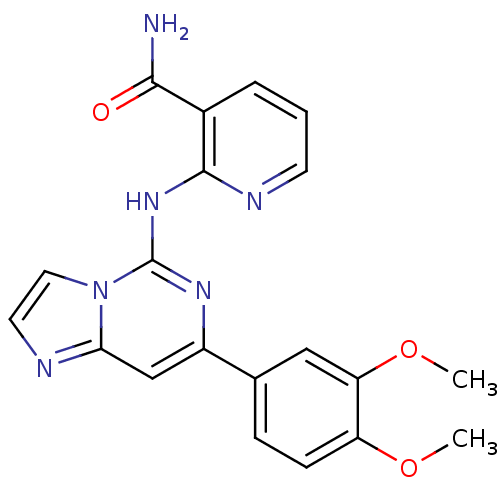
(2-(7-(3,4-dimethoxyphenyl)imidazo[1,2-c]pyrimidin-...)Show SMILES COc1ccc(cc1OC)-c1cc2nccn2c(Nc2ncccc2C(N)=O)n1 Show InChI InChI=1S/C20H18N6O3/c1-28-15-6-5-12(10-16(15)29-2)14-11-17-22-8-9-26(17)20(24-14)25-19-13(18(21)27)4-3-7-23-19/h3-11H,1-2H3,(H2,21,27)(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rigel, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK |
J Med Chem 55: 3614-43 (2012)
Article DOI: 10.1021/jm201271b
BindingDB Entry DOI: 10.7270/Q2NZ88RC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50396074
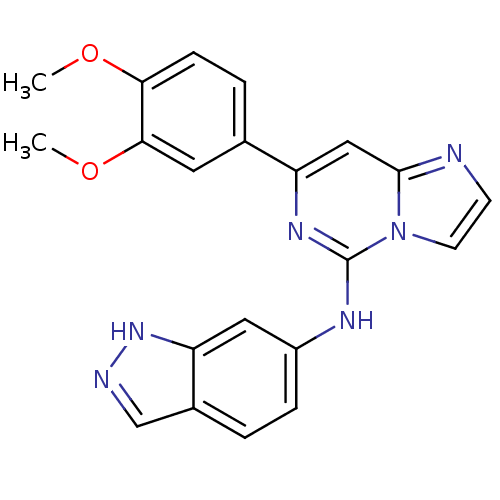
(CHEMBL2170585)Show SMILES COc1ccc(cc1OC)-c1cc2nccn2c(Nc2ccc3cn[nH]c3c2)n1 Show InChI InChI=1S/C21H18N6O2/c1-28-18-6-4-13(9-19(18)29-2)16-11-20-22-7-8-27(20)21(25-16)24-15-5-3-14-12-23-26-17(14)10-15/h3-12H,1-2H3,(H,23,26)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rigel, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK |
J Med Chem 55: 3614-43 (2012)
Article DOI: 10.1021/jm201271b
BindingDB Entry DOI: 10.7270/Q2NZ88RC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50396071

(CHEMBL2170582 | US10828301, Compound R921303)Show SMILES CNC(=O)COc1cccc(Nc2ncc(F)c(Nc3ccc4OC(F)(F)C(=O)Nc4c3)n2)c1 Show InChI InChI=1S/C21H17F3N6O4/c1-25-17(31)10-33-13-4-2-3-11(7-13)28-20-26-9-14(22)18(30-20)27-12-5-6-16-15(8-12)29-19(32)21(23,24)34-16/h2-9H,10H2,1H3,(H,25,31)(H,29,32)(H2,26,27,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rigel, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Lyn |
J Med Chem 55: 3614-43 (2012)
Article DOI: 10.1021/jm201271b
BindingDB Entry DOI: 10.7270/Q2NZ88RC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM60665
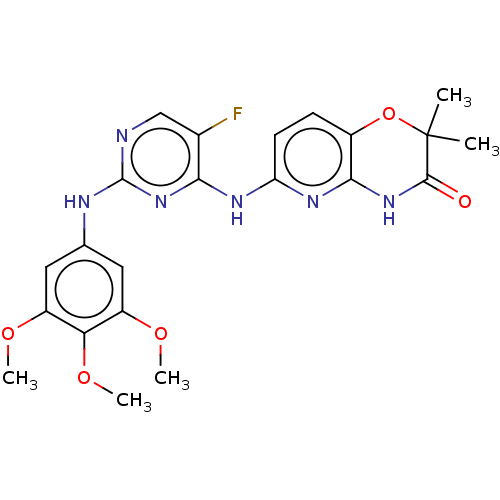
(BDBM50249542 | US9145414, R406 | US9212178, R406)Show SMILES COc1cc(Nc2ncc(F)c(Nc3ccc4OC(C)(C)C(=O)Nc4n3)n2)cc(OC)c1OC Show InChI InChI=1S/C22H23FN6O5/c1-22(2)20(30)28-19-13(34-22)6-7-16(27-19)26-18-12(23)10-24-21(29-18)25-11-8-14(31-3)17(33-5)15(9-11)32-4/h6-10H,1-5H3,(H3,24,25,26,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rigel, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK |
J Med Chem 55: 3614-43 (2012)
Article DOI: 10.1021/jm201271b
BindingDB Entry DOI: 10.7270/Q2NZ88RC |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50396071

(CHEMBL2170582 | US10828301, Compound R921303)Show SMILES CNC(=O)COc1cccc(Nc2ncc(F)c(Nc3ccc4OC(F)(F)C(=O)Nc4c3)n2)c1 Show InChI InChI=1S/C21H17F3N6O4/c1-25-17(31)10-33-13-4-2-3-11(7-13)28-20-26-9-14(22)18(30-20)27-12-5-6-16-15(8-12)29-19(32)21(23,24)34-16/h2-9H,10H2,1H3,(H,25,31)(H,29,32)(H2,26,27,28,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rigel, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 55: 3614-43 (2012)
Article DOI: 10.1021/jm201271b
BindingDB Entry DOI: 10.7270/Q2NZ88RC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50249090
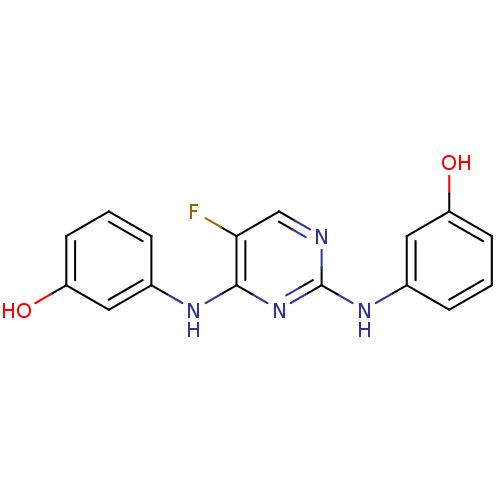
(3,3'-(5-fluoropyrimidine-2,4-diyl)bis(azanediyl)di...)Show InChI InChI=1S/C16H13FN4O2/c17-14-9-18-16(20-11-4-2-6-13(23)8-11)21-15(14)19-10-3-1-5-12(22)7-10/h1-9,22-23H,(H2,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amarit Bioscience, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 25: 2117-21 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.075
BindingDB Entry DOI: 10.7270/Q2R49SFK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50249090

(3,3'-(5-fluoropyrimidine-2,4-diyl)bis(azanediyl)di...)Show InChI InChI=1S/C16H13FN4O2/c17-14-9-18-16(20-11-4-2-6-13(23)8-11)21-15(14)19-10-3-1-5-12(22)7-10/h1-9,22-23H,(H2,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rigel, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK |
J Med Chem 55: 3614-43 (2012)
Article DOI: 10.1021/jm201271b
BindingDB Entry DOI: 10.7270/Q2NZ88RC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50396071

(CHEMBL2170582 | US10828301, Compound R921303)Show SMILES CNC(=O)COc1cccc(Nc2ncc(F)c(Nc3ccc4OC(F)(F)C(=O)Nc4c3)n2)c1 Show InChI InChI=1S/C21H17F3N6O4/c1-25-17(31)10-33-13-4-2-3-11(7-13)28-20-26-9-14(22)18(30-20)27-12-5-6-16-15(8-12)29-19(32)21(23,24)34-16/h2-9H,10H2,1H3,(H,25,31)(H,29,32)(H2,26,27,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 116 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rigel, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
J Med Chem 55: 3614-43 (2012)
Article DOI: 10.1021/jm201271b
BindingDB Entry DOI: 10.7270/Q2NZ88RC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50396071

(CHEMBL2170582 | US10828301, Compound R921303)Show SMILES CNC(=O)COc1cccc(Nc2ncc(F)c(Nc3ccc4OC(F)(F)C(=O)Nc4c3)n2)c1 Show InChI InChI=1S/C21H17F3N6O4/c1-25-17(31)10-33-13-4-2-3-11(7-13)28-20-26-9-14(22)18(30-20)27-12-5-6-16-15(8-12)29-19(32)21(23,24)34-16/h2-9H,10H2,1H3,(H,25,31)(H,29,32)(H2,26,27,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rigel, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
J Med Chem 55: 3614-43 (2012)
Article DOI: 10.1021/jm201271b
BindingDB Entry DOI: 10.7270/Q2NZ88RC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50290422

(4-(4-Methyl-piperazin-1-ylmethyl)-N-[3-(4-pyridin-...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2cccc(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C28H29N7O/c1-34-14-16-35(17-15-34)20-21-7-9-22(10-8-21)27(36)31-24-5-2-6-25(18-24)32-28-30-13-11-26(33-28)23-4-3-12-29-19-23/h2-13,18-19H,14-17,20H2,1H3,(H,31,36)(H,30,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rigel, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK |
J Med Chem 55: 3614-43 (2012)
Article DOI: 10.1021/jm201271b
BindingDB Entry DOI: 10.7270/Q2NZ88RC |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 4
(Homo sapiens (Human)) | BDBM50396071

(CHEMBL2170582 | US10828301, Compound R921303)Show SMILES CNC(=O)COc1cccc(Nc2ncc(F)c(Nc3ccc4OC(F)(F)C(=O)Nc4c3)n2)c1 Show InChI InChI=1S/C21H17F3N6O4/c1-25-17(31)10-33-13-4-2-3-11(7-13)28-20-26-9-14(22)18(30-20)27-12-5-6-16-15(8-12)29-19(32)21(23,24)34-16/h2-9H,10H2,1H3,(H,25,31)(H,29,32)(H2,26,27,28,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 217 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rigel, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR4 |
J Med Chem 55: 3614-43 (2012)
Article DOI: 10.1021/jm201271b
BindingDB Entry DOI: 10.7270/Q2NZ88RC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50593320
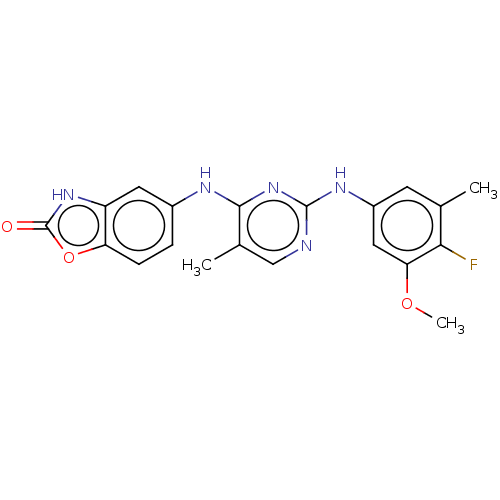
(A-301 | ATI-502 | Ati-502 | IFIDANCITINIB | Ifidan...)Show SMILES COc1cc(Nc2ncc(C)c(Nc3ccc4oc(=O)[nH]c4c3)n2)cc(C)c1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00411
BindingDB Entry DOI: 10.7270/Q2QR523S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM13530

(4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rigel, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK |
J Med Chem 55: 3614-43 (2012)
Article DOI: 10.1021/jm201271b
BindingDB Entry DOI: 10.7270/Q2NZ88RC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50593319

(CHEMBL5188333)Show SMILES COc1cc(Nc2ncc(C)c(Nc3ccc4oc(=O)[nH]c4c3)n2)cc(C)c1C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00411
BindingDB Entry DOI: 10.7270/Q2QR523S |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM639195
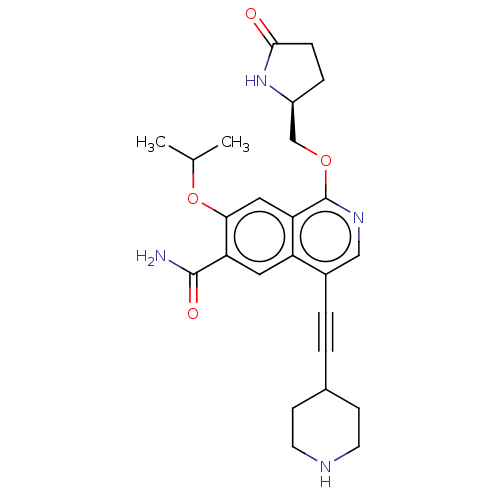
((S)-7-isopropoxy-1-((5-oxopyrrolidin-2-yl)methoxy)...)Show SMILES CC(C)Oc1cc2c(OC[C@@H]3CCC(=O)N3)ncc(C#CC3CCNCC3)c2cc1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM639244
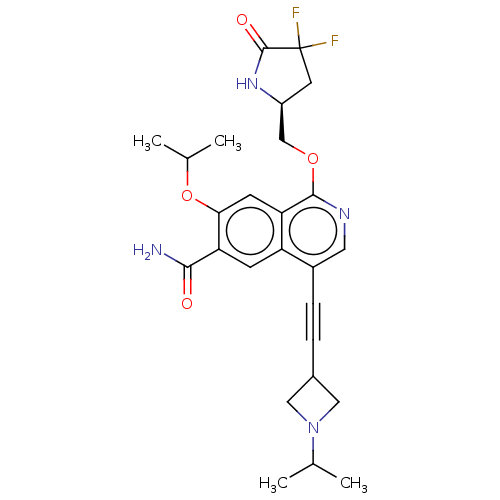
(1-(((S)-4,4-difluoro-5-oxopyrrolidin-2-yl)methoxy)...)Show SMILES CC(C)Oc1cc2c(OC[C@@H]3CC(F)(F)C(=O)N3)ncc(C#CC3CN(C3)C(C)C)c2cc1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50589401

(CHEMBL5169945)Show SMILES [H][C@@]12C[C@@]([H])(C=C1)[C@@H]([C@@H]2Nc1nc(Nc2cc(F)cc(c2)C(=O)N2CCCC2)nc2ccsc12)C(N)=O |r,c:5| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128900
BindingDB Entry DOI: 10.7270/Q21J9FR3 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50589421

(CHEMBL5187995)Show SMILES [H][C@@]12C[C@@]([H])(C=C1)[C@@H]([C@@H]2Nc1nc(Nc2cccc(c2)C(=O)N2CCCC2)nc2ccn(C(F)F)c12)C(N)=O |r,c:5| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128900
BindingDB Entry DOI: 10.7270/Q21J9FR3 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50582250

(CHEMBL5075301)Show SMILES [H][C@@]12C[C@@]([H])(C=C1)[C@@H]([C@@H]2Nc1nc(Nc2cccc(c2)C(=O)N2CCCC2)ncc1-c1ccoc1)C(N)=O |r,c:5| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IRAK4 (unknown origin) in presence of MBP as substrate by ADP-Glo assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00056
BindingDB Entry DOI: 10.7270/Q2028WDG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50593320

(A-301 | ATI-502 | Ati-502 | IFIDANCITINIB | Ifidan...)Show SMILES COc1cc(Nc2ncc(C)c(Nc3ccc4oc(=O)[nH]c4c3)n2)cc(C)c1F | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00411
BindingDB Entry DOI: 10.7270/Q2QR523S |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM639196
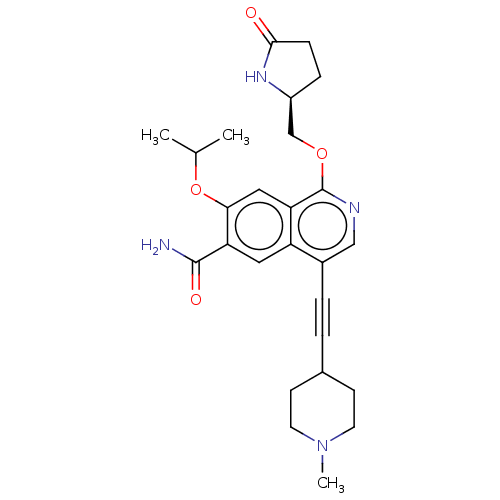
((S)-7-isopropoxy-4-((1-methylpiperidin-4-yl)ethyny...)Show SMILES CC(C)Oc1cc2c(OC[C@@H]3CCC(=O)N3)ncc(C#CC3CCN(C)CC3)c2cc1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM639254
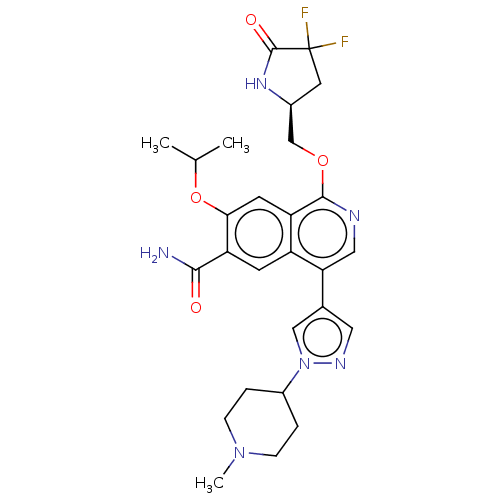
((S)-1-((4,4-difluoro-5-oxopyrrolidin-2-yl)methoxy)...)Show SMILES CC(C)Oc1cc2c(OC[C@@H]3CC(F)(F)C(=O)N3)ncc(-c3cnn(c3)C3CCN(C)CC3)c2cc1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM639204
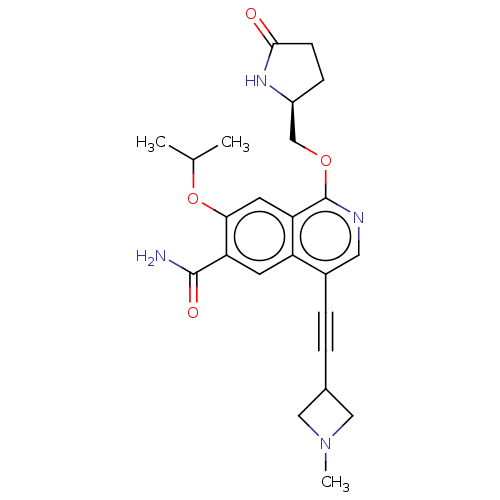
((S)-7-isopropoxy-4-((1-methylazetidin-3-yl)ethynyl...)Show SMILES CC(C)Oc1cc2c(OC[C@@H]3CCC(=O)N3)ncc(C#CC3CN(C)C3)c2cc1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50589432

(CHEMBL5178313)Show SMILES [H][C@@]12C[C@@]([H])(C=C1)[C@@H]([C@@H]2Nc1nc(Nc2ccc3cnn(C(C)C)c3c2)nc2ccn(C(C)C)c12)C(N)=O |r,c:5| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128900
BindingDB Entry DOI: 10.7270/Q21J9FR3 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM639241
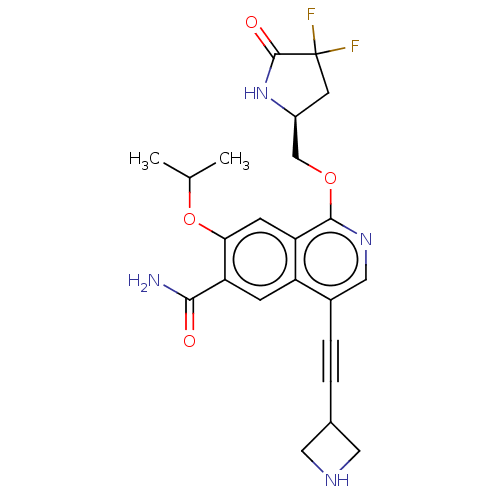
((S)-4-(azetidin-3-ylethynyl)-1-((4,4-difluoro-5-ox...)Show SMILES CC(C)Oc1cc2c(OC[C@@H]3CC(F)(F)C(=O)N3)ncc(C#CC3CNC3)c2cc1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM639251
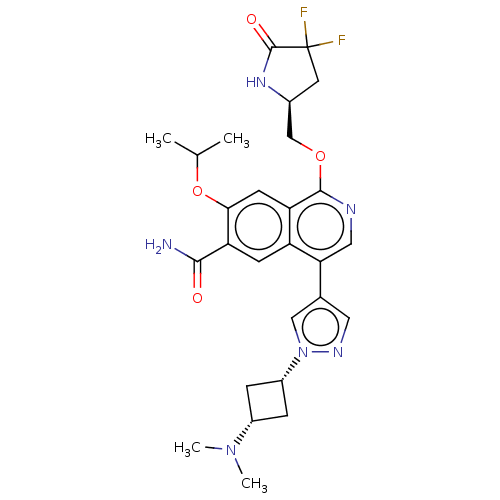
(1-(((S)-4,4-difluoro-5-oxopyrrolidin-2-yl)methoxy)...)Show SMILES CC(C)Oc1cc2c(OC[C@@H]3CC(F)(F)C(=O)N3)ncc(-c3cnn(c3)[C@@H]3C[C@@H](C3)N(C)C)c2cc1C(N)=O |r,wU:10.9,26.27,28.32,(4.85,5.94,;4.08,4.6,;4.85,3.27,;2.54,4.6,;1.77,3.27,;2.54,1.94,;1.77,.6,;2.54,-.73,;4.08,-.73,;4.85,-2.07,;6.39,-2.07,;7.29,-3.31,;8.76,-2.84,;10.25,-3.23,;9.53,-4.17,;8.76,-1.3,;10.09,-.53,;7.29,-.82,;1.77,-2.07,;.23,-2.07,;-.54,-.73,;-2.08,-.73,;-2.99,.51,;-4.45,.04,;-4.45,-1.5,;-2.99,-1.98,;-5.78,-2.27,;-7.27,-1.87,;-7.67,-3.36,;-6.18,-3.76,;-8.76,-4.45,;-10.25,-4.05,;-8.36,-5.94,;.23,.6,;-.54,1.94,;.23,3.27,;-.54,4.6,;.23,5.94,;-2.08,4.6,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50589397
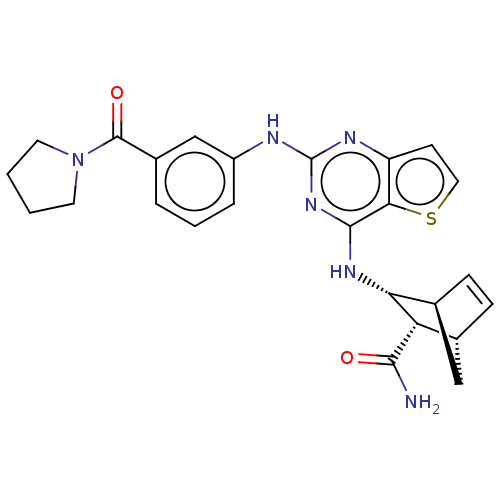
(CHEMBL5185144)Show SMILES [H][C@@]12C[C@@]([H])(C=C1)[C@@H]([C@@H]2Nc1nc(Nc2cccc(c2)C(=O)N2CCCC2)nc2ccsc12)C(N)=O |r,c:5| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128900
BindingDB Entry DOI: 10.7270/Q21J9FR3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50589400

(CHEMBL5175590)Show SMILES [H][C@@]12C[C@@]([H])(C=C1)[C@@H]([C@@H]2Nc1nc(Nc2ccc(F)c(c2)C(=O)N2CCCC2)nc2ccsc12)C(N)=O |r,c:5| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128900
BindingDB Entry DOI: 10.7270/Q21J9FR3 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50589414

(CHEMBL5192205)Show SMILES [H][C@@]12C[C@@]([H])(C=C1)[C@@H]([C@@H]2Nc1nc(Nc2cccc(c2)C(=O)N2CCCC2)nc2ccoc12)C(N)=O |r,c:5| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128900
BindingDB Entry DOI: 10.7270/Q21J9FR3 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50589418
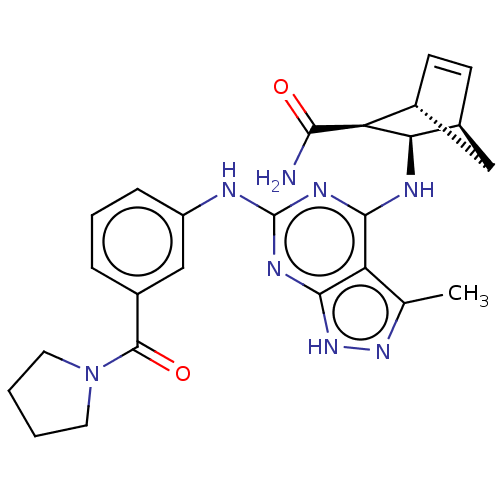
(CHEMBL5183959)Show SMILES [H][C@@]12C[C@@]([H])(C=C1)[C@@H]([C@@H]2Nc1nc(Nc2cccc(c2)C(=O)N2CCCC2)nc2[nH]nc(C)c12)C(N)=O |r,c:5| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128900
BindingDB Entry DOI: 10.7270/Q21J9FR3 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50589424

(CHEMBL5187663)Show SMILES [H][C@@]12C[C@@]([H])(C=C1)[C@@H]([C@@H]2Nc1nc(Nc2cccc(c2)C(=O)N2CCCC2)nc2ncsc12)C(N)=O |r,c:5| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128900
BindingDB Entry DOI: 10.7270/Q21J9FR3 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50582248
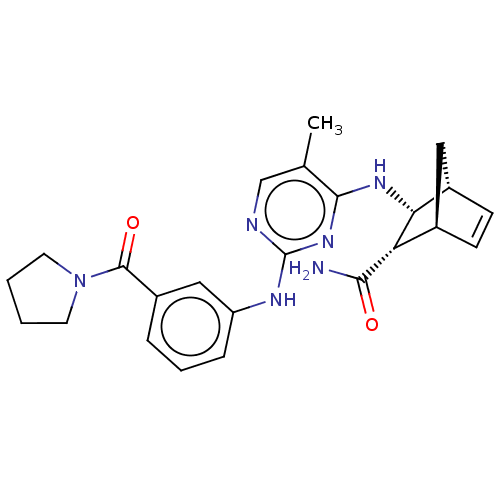
(CHEMBL5076447)Show SMILES [H][C@@]12C[C@@]([H])(C=C1)[C@@H]([C@@H]2Nc1nc(Nc2cccc(c2)C(=O)N2CCCC2)ncc1C)C(N)=O |r,c:5| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IRAK4 (unknown origin) in presence of MBP as substrate by ADP-Glo assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00056
BindingDB Entry DOI: 10.7270/Q2028WDG |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50582258

(CHEMBL5094640)Show SMILES [H][C@@]12C[C@@]([H])(C=C1)[C@@H]([C@@H]2Nc1nc(Nc2cccc(c2)C(=O)N2CCCC2)ncc1-c1ccnc(F)c1)C(N)=O |r,c:5| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IRAK4 (unknown origin) in presence of MBP as substrate by ADP-Glo assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00056
BindingDB Entry DOI: 10.7270/Q2028WDG |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM639281
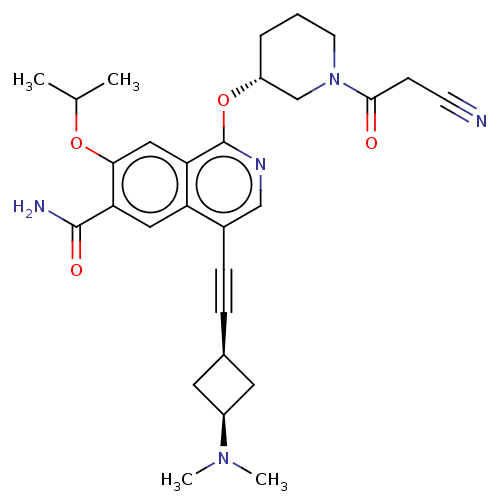
(1-(((R)-1-(2-cyanoacetyl)piperidin-3-yl)oxy)-4-((c...)Show SMILES CC(C)Oc1cc2c(O[C@@H]3CCCN(C3)C(=O)CC#N)ncc(C#C[C@H]3C[C@H](C3)N(C)C)c2cc1C(N)=O |r,wU:9.8,25.25,27.30,(3.4,5.33,;2.63,4,;3.4,2.67,;1.09,4,;.32,2.67,;1.09,1.33,;.32,,;1.09,-1.33,;2.63,-1.33,;3.4,-2.67,;2.63,-4,;3.4,-5.33,;4.94,-5.33,;5.71,-4,;4.94,-2.67,;7.25,-4,;8.02,-2.67,;8.02,-5.33,;9.56,-5.33,;11.1,-5.33,;.32,-2.67,;-1.22,-2.67,;-1.99,-1.33,;-3.53,-1.33,;-5.07,-1.33,;-6.61,-1.33,;-7.7,-2.42,;-8.79,-1.33,;-7.7,-.24,;-10.33,-1.33,;-11.1,-2.67,;-11.1,,;-1.22,,;-1.99,1.33,;-1.22,2.67,;-1.99,4,;-1.22,5.33,;-3.53,4,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM639234
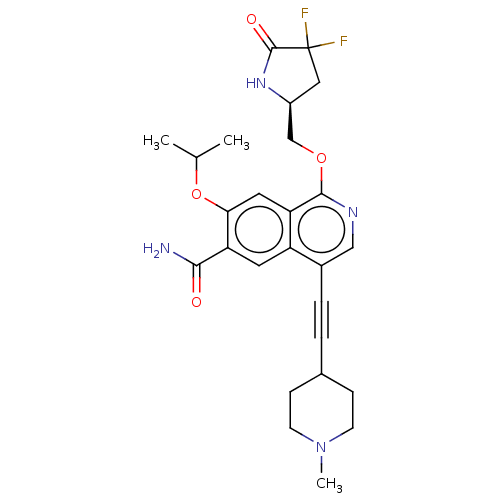
((S)-1-((4,4-difluoro-5-oxopyrrolidin-2-yl)methoxy)...)Show SMILES CC(C)Oc1cc2c(OC[C@@H]3CC(F)(F)C(=O)N3)ncc(C#CC3CCN(C)CC3)c2cc1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM639203
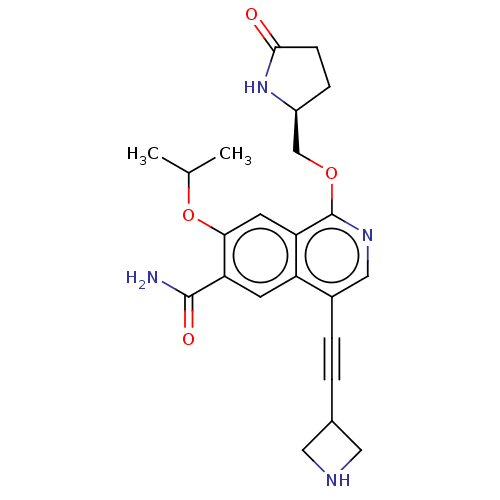
((S)-4-(azetidin-3-ylethynyl)-7-isopropoxy-1-((5-ox...)Show SMILES CC(C)Oc1cc2c(OC[C@@H]3CCC(=O)N3)ncc(C#CC3CNC3)c2cc1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM639209
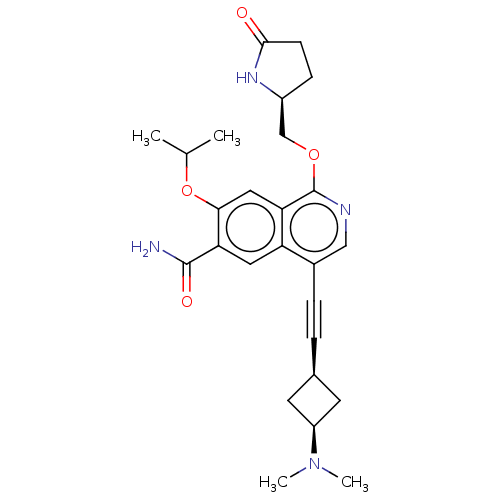
(4-((cis-3-(dimethylamino)cyclobutyl)ethynyl)-7-iso...)Show SMILES CC(C)Oc1cc2c(OC[C@@H]3CCC(=O)N3)ncc(C#C[C@H]3C[C@H](C3)N(C)C)c2cc1C(N)=O |r,wU:10.9,21.21,23.26,(4.63,4.62,;3.86,3.29,;4.63,1.96,;2.32,3.29,;1.55,1.96,;2.32,.62,;1.55,-.71,;2.32,-2.04,;3.86,-2.04,;4.63,-3.38,;6.17,-3.38,;7.07,-4.62,;8.54,-4.15,;8.54,-2.61,;9.87,-1.84,;7.07,-2.13,;1.55,-3.38,;.01,-3.38,;-.76,-2.04,;-2.3,-2.04,;-3.84,-2.04,;-5.38,-2.04,;-6.47,-3.13,;-7.56,-2.04,;-6.47,-.96,;-9.1,-2.04,;-9.87,-3.38,;-9.87,-.71,;.01,-.71,;-.76,.62,;.01,1.96,;-.76,3.29,;.01,4.62,;-2.3,3.29,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM639233
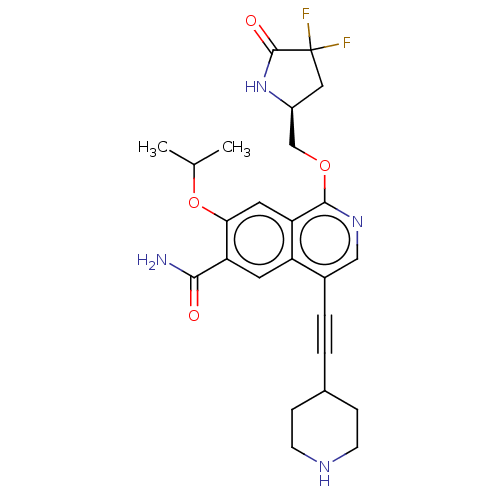
((S)-1-((4,4-difluoro-5-oxopyrrolidin-2-yl)methoxy)...)Show SMILES CC(C)Oc1cc2c(OC[C@@H]3CC(F)(F)C(=O)N3)ncc(C#CC3CCNCC3)c2cc1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM639242
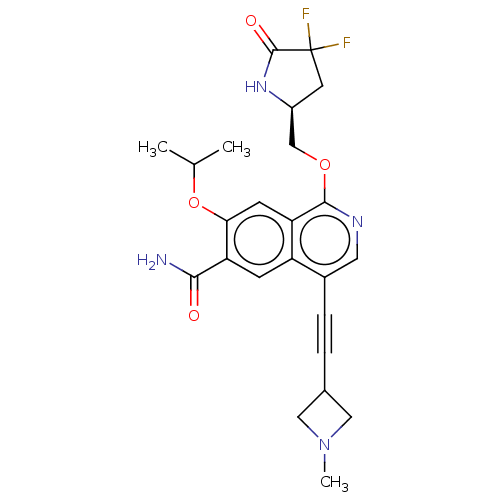
((S)-1-((4,4-difluoro-5-oxopyrrolidin-2-yl)methoxy)...)Show SMILES CC(C)Oc1cc2c(OC[C@@H]3CC(F)(F)C(=O)N3)ncc(C#CC3CN(C)C3)c2cc1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM639256
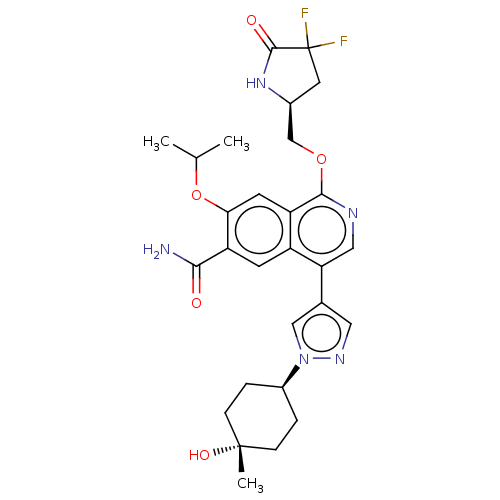
(1-(((S)-4,4-difluoro-5-oxopyrrolidin-2-yl)methoxy)...)Show SMILES CC(C)Oc1cc2c(OC[C@@H]3CC(F)(F)C(=O)N3)ncc(-c3cnn(c3)[C@H]3CC[C@](C)(O)CC3)c2cc1C(N)=O |r,wU:10.9,26.27,29.31,wD:29.32,(4.45,5.94,;3.68,4.6,;4.45,3.27,;2.14,4.6,;1.37,3.27,;2.14,1.94,;1.37,.6,;2.14,-.73,;3.68,-.73,;4.45,-2.07,;5.99,-2.07,;6.9,-3.31,;8.36,-2.84,;9.85,-3.23,;9.13,-4.17,;8.36,-1.3,;9.69,-.53,;6.9,-.82,;1.37,-2.07,;-.17,-2.07,;-.94,-.73,;-2.48,-.73,;-3.38,.51,;-4.85,.04,;-4.85,-1.5,;-3.38,-1.98,;-6.18,-2.27,;-5.78,-3.76,;-6.87,-4.85,;-8.36,-4.45,;-8.76,-5.94,;-9.85,-4.85,;-8.76,-2.96,;-7.67,-1.87,;-.17,.6,;-.94,1.94,;-.17,3.27,;-.94,4.6,;-.17,5.94,;-2.48,4.6,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM639237
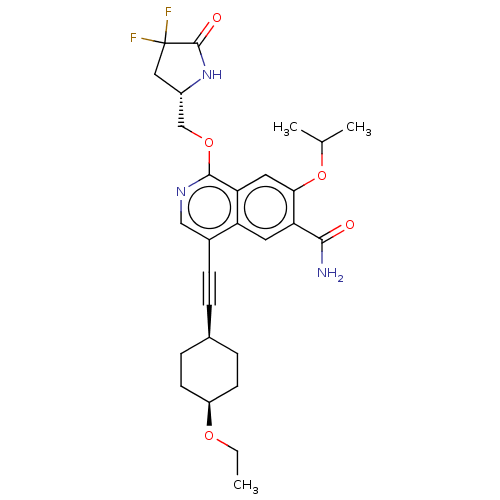
(1-(((S)-4,4-difluoro-5-oxopyrrolidin-2-yl)methoxy)...)Show SMILES CCO[C@H]1CC[C@H](CC1)C#Cc1cnc(OC[C@@H]2CC(F)(F)C(=O)N2)c2cc(OC(C)C)c(cc12)C(N)=O |r,wU:17.17,6.9,3.2,(-11.17,-.28,;-9.63,-.28,;-8.86,-1.62,;-7.32,-1.62,;-6.55,-.28,;-5.01,-.28,;-4.24,-1.62,;-5.01,-2.95,;-6.55,-2.95,;-2.7,-1.62,;-1.16,-1.62,;.38,-1.62,;1.15,-2.95,;2.69,-2.95,;3.46,-1.62,;5,-1.62,;5.77,-2.95,;7.31,-2.95,;8.22,-4.2,;9.68,-3.72,;11.17,-4.12,;10.45,-5.05,;9.68,-2.18,;11.02,-1.41,;8.22,-1.7,;2.69,-.28,;3.46,1.05,;2.69,2.39,;3.46,3.72,;5,3.72,;5.77,5.05,;5.77,2.39,;1.15,2.39,;.38,1.05,;1.15,-.28,;.38,3.72,;1.15,5.05,;-1.16,3.72,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM639253
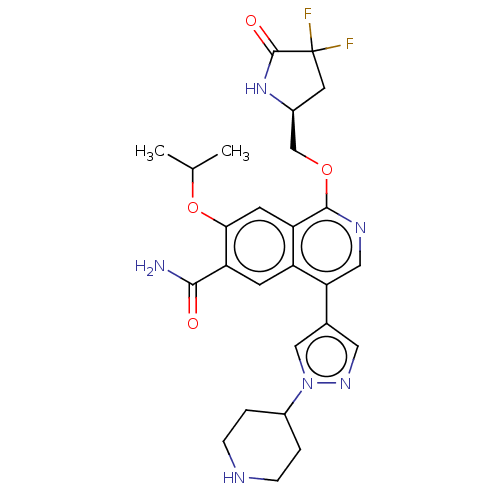
((S)-1-((4,4-difluoro-5-oxopyrrolidin-2-yl)methoxy)...)Show SMILES CC(C)Oc1cc2c(OC[C@@H]3CC(F)(F)C(=O)N3)ncc(-c3cnn(c3)C3CCNCC3)c2cc1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50589417
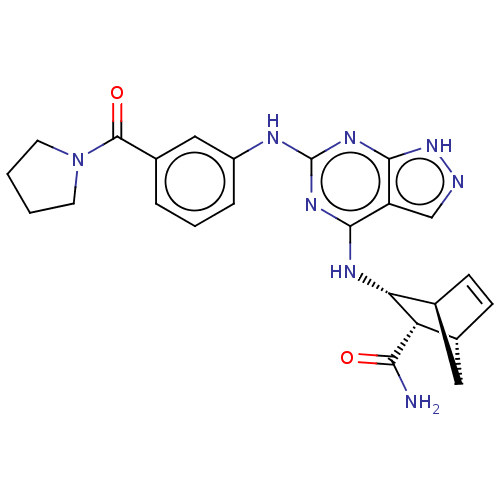
(CHEMBL5198583)Show SMILES [H][C@@]12C[C@@]([H])(C=C1)[C@@H]([C@@H]2Nc1nc(Nc2cccc(c2)C(=O)N2CCCC2)nc2[nH]ncc12)C(N)=O |r,c:5| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128900
BindingDB Entry DOI: 10.7270/Q21J9FR3 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50589420
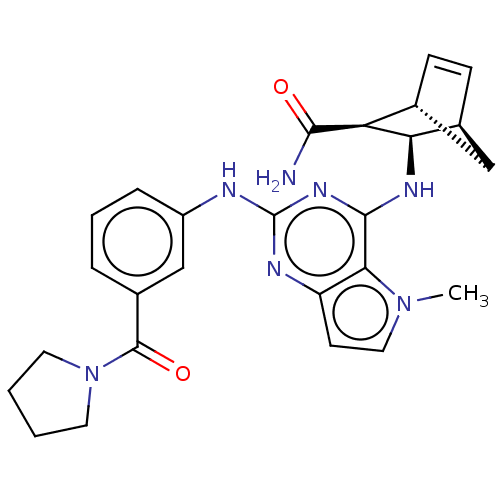
(CHEMBL5181320)Show SMILES [H][C@@]12C[C@@]([H])(C=C1)[C@@H]([C@@H]2Nc1nc(Nc2cccc(c2)C(=O)N2CCCC2)nc2ccn(C)c12)C(N)=O |r,c:5| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128900
BindingDB Entry DOI: 10.7270/Q21J9FR3 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50589423
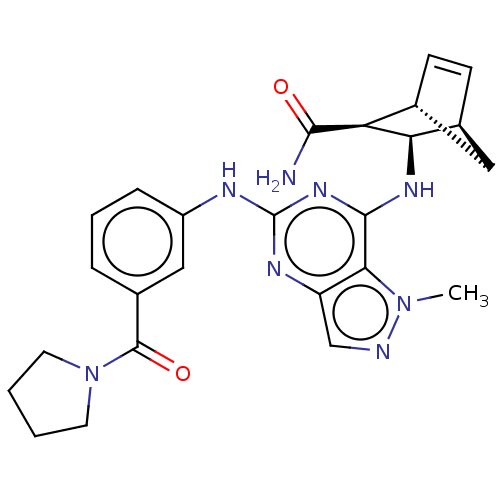
(CHEMBL5175829)Show SMILES [H][C@@]12C[C@@]([H])(C=C1)[C@@H]([C@@H]2Nc1nc(Nc2cccc(c2)C(=O)N2CCCC2)nc2cnn(C)c12)C(N)=O |r,c:5| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128900
BindingDB Entry DOI: 10.7270/Q21J9FR3 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50582254
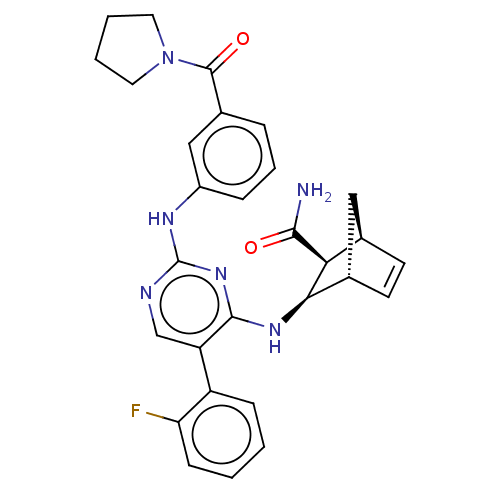
(CHEMBL5088588)Show SMILES [H][C@@]12C[C@@]([H])(C=C1)[C@@H]([C@@H]2Nc1nc(Nc2cccc(c2)C(=O)N2CCCC2)ncc1-c1ccccc1F)C(N)=O |r,c:5| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IRAK4 (unknown origin) in presence of MBP as substrate by ADP-Glo assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00056
BindingDB Entry DOI: 10.7270/Q2028WDG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50396073
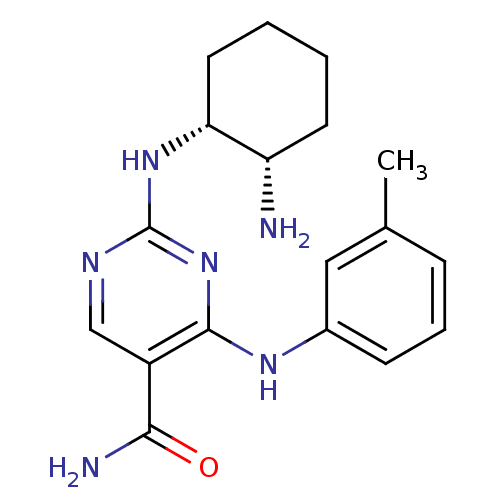
(CHEMBL1235110)Show SMILES Cc1cccc(Nc2nc(N[C@@H]3CCCC[C@@H]3N)ncc2C(N)=O)c1 |r| Show InChI InChI=1S/C18H24N6O/c1-11-5-4-6-12(9-11)22-17-13(16(20)25)10-21-18(24-17)23-15-8-3-2-7-14(15)19/h4-6,9-10,14-15H,2-3,7-8,19H2,1H3,(H2,20,25)(H2,21,22,23,24)/t14-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rigel, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK |
J Med Chem 55: 3614-43 (2012)
Article DOI: 10.1021/jm201271b
BindingDB Entry DOI: 10.7270/Q2NZ88RC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50589431

(CHEMBL5200765)Show SMILES [H][C@@]12C[C@@]([H])(C=C1)[C@@H]([C@@H]2Nc1nc(Nc2ccc3cnn(C(C)C)c3c2)nc2ccn(C)c12)C(N)=O |r,c:5| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128900
BindingDB Entry DOI: 10.7270/Q21J9FR3 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50589433

(CHEMBL5193503)Show SMILES [H][C@@]12C[C@@]([H])(C=C1)[C@@H]([C@@H]2Nc1nc(Nc2ccc3cnn(C(C)C)c3c2)nc2ccn(C(F)F)c12)C(N)=O |r,c:5| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128900
BindingDB Entry DOI: 10.7270/Q21J9FR3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data