 Found 59 hits with Last Name = 'matsumizu' and Initial = 'm'
Found 59 hits with Last Name = 'matsumizu' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50348115
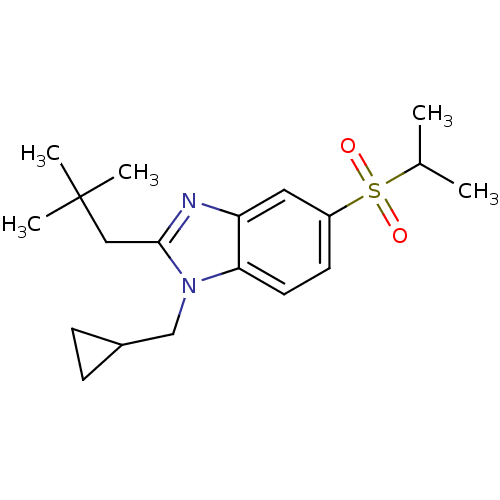
(CHEMBL1800654)Show SMILES CC(C)S(=O)(=O)c1ccc2n(CC3CC3)c(CC(C)(C)C)nc2c1 Show InChI InChI=1S/C19H28N2O2S/c1-13(2)24(22,23)15-8-9-17-16(10-15)20-18(11-19(3,4)5)21(17)12-14-6-7-14/h8-10,13-14H,6-7,11-12H2,1-5H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity at human CB1 receptor |
Bioorg Med Chem Lett 21: 4284-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.063
BindingDB Entry DOI: 10.7270/Q2959HXV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50348114
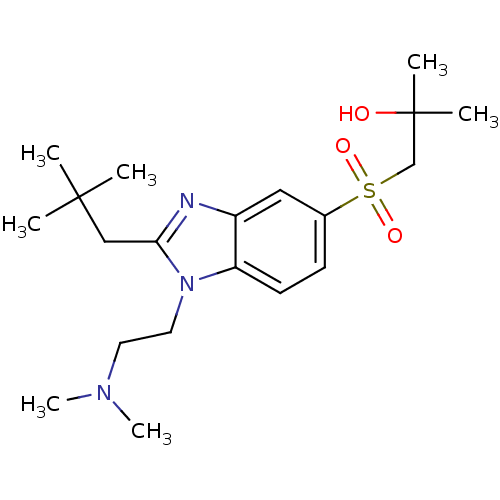
(CHEMBL1800653)Show SMILES CN(C)CCn1c(CC(C)(C)C)nc2cc(ccc12)S(=O)(=O)CC(C)(C)O Show InChI InChI=1S/C20H33N3O3S/c1-19(2,3)13-18-21-16-12-15(27(25,26)14-20(4,5)24)8-9-17(16)23(18)11-10-22(6)7/h8-9,12,24H,10-11,13-14H2,1-7H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity at human CB1 receptor |
Bioorg Med Chem Lett 21: 4284-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.063
BindingDB Entry DOI: 10.7270/Q2959HXV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50348122

(CHEMBL1800168)Show InChI InChI=1S/C17H24N2O2S/c1-5-22(20,21)13-8-9-15-14(10-13)18-16(17(2,3)4)19(15)11-12-6-7-12/h8-10,12H,5-7,11H2,1-4H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity at human CB1 receptor |
Bioorg Med Chem Lett 21: 4284-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.063
BindingDB Entry DOI: 10.7270/Q2959HXV |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220714
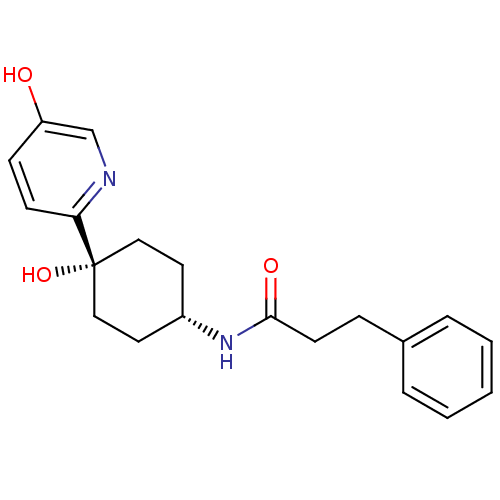
(CHEMBL237751 | N-((1s,4s)-4-hydroxy-4-(5-hydroxypy...)Show SMILES Oc1ccc(nc1)[C@@]1(O)CC[C@@H](CC1)NC(=O)CCc1ccccc1 |wD:7.8,11.15,(-10.78,-55,;-9.45,-54.23,;-9.45,-52.68,;-8.13,-51.91,;-6.79,-52.68,;-6.78,-54.21,;-8.1,-54.99,;-5.45,-51.91,;-5.46,-53.44,;-5.45,-50.37,;-4.13,-49.58,;-2.79,-50.37,;-2.79,-51.91,;-4.13,-52.68,;-1.45,-49.6,;-.12,-50.37,;-.12,-51.91,;1.23,-49.61,;2.55,-50.39,;3.89,-49.63,;5.21,-50.4,;6.55,-49.65,;6.56,-48.11,;5.24,-47.33,;3.9,-48.09,)| Show InChI InChI=1S/C20H24N2O3/c23-17-7-8-18(21-14-17)20(25)12-10-16(11-13-20)22-19(24)9-6-15-4-2-1-3-5-15/h1-5,7-8,14,16,23,25H,6,9-13H2,(H,22,24)/t16-,20+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101,606 from NR2B in rat forebrain P2 membrane |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220726
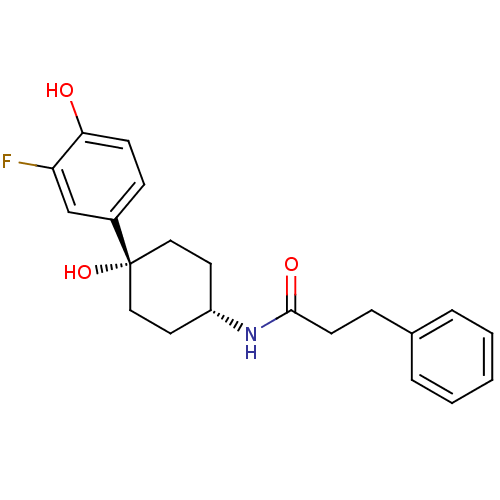
(CHEMBL237533 | N-((1s,4s)-4-(3-fluoro-4-hydroxyphe...)Show SMILES Oc1ccc(cc1F)[C@@]1(O)CC[C@@H](CC1)NC(=O)CCc1ccccc1 |wD:8.9,12.16,(12.39,-46.09,;13.72,-45.33,;15.07,-46.09,;16.39,-45.31,;16.38,-43.78,;15.04,-43.01,;13.72,-43.77,;12.38,-43,;17.72,-43,;17.71,-44.53,;17.72,-41.46,;19.04,-40.67,;20.38,-41.46,;20.38,-43,;19.04,-43.77,;21.72,-40.69,;23.05,-41.46,;23.05,-43.01,;24.4,-40.7,;25.72,-41.49,;27.06,-40.72,;28.37,-41.5,;29.72,-40.75,;29.73,-39.2,;28.41,-38.43,;27.07,-39.19,)| Show InChI InChI=1S/C21H24FNO3/c22-18-14-16(7-8-19(18)24)21(26)12-10-17(11-13-21)23-20(25)9-6-15-4-2-1-3-5-15/h1-5,7-8,14,17,24,26H,6,9-13H2,(H,23,25)/t17-,21+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101,606 from NR2B in rat forebrain P2 membrane |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220729
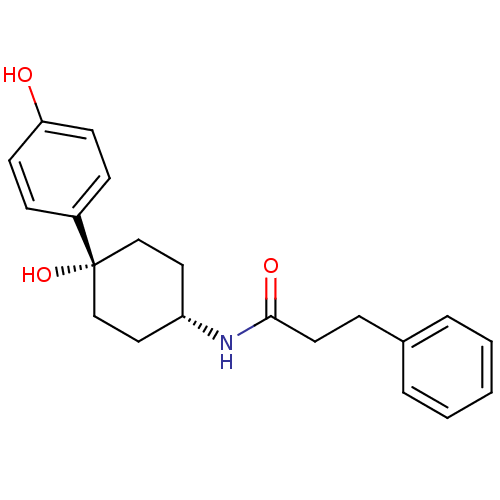
(CHEMBL237320 | N-((1s,4s)-4-hydroxy-4-(4-hydroxyph...)Show SMILES Oc1ccc(cc1)[C@@]1(O)CC[C@@H](CC1)NC(=O)CCc1ccccc1 |wD:7.8,11.15,(10.84,-24.52,;12.17,-23.76,;12.17,-22.2,;13.49,-21.43,;14.83,-22.2,;14.84,-23.74,;13.52,-24.52,;16.17,-21.43,;16.15,-22.96,;16.17,-19.89,;17.49,-19.1,;18.83,-19.89,;18.83,-21.43,;17.49,-22.2,;20.17,-19.12,;21.5,-19.89,;21.5,-21.44,;22.85,-19.13,;24.17,-19.91,;25.51,-19.15,;26.82,-19.93,;28.17,-19.18,;28.17,-17.63,;26.86,-16.86,;25.52,-17.61,)| Show InChI InChI=1S/C21H25NO3/c23-19-9-7-17(8-10-19)21(25)14-12-18(13-15-21)22-20(24)11-6-16-4-2-1-3-5-16/h1-5,7-10,18,23,25H,6,11-15H2,(H,22,24)/t18-,21+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101,606 from NR2B in rat forebrain P2 membrane |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220712
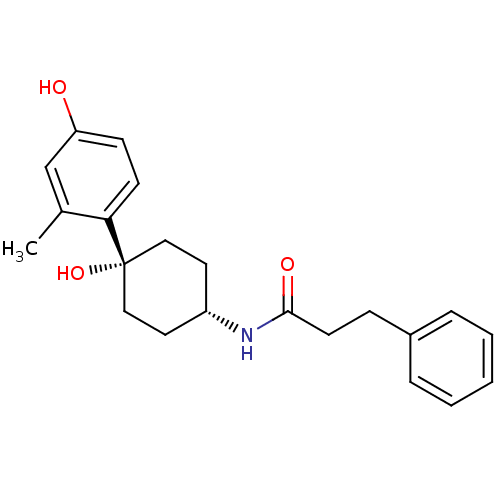
(CHEMBL395904 | N-((1s,4s)-4-hydroxy-4-(4-hydroxy-2...)Show SMILES Cc1cc(O)ccc1[C@@]1(O)CC[C@@H](CC1)NC(=O)CCc1ccccc1 |wD:8.9,12.16,(34.08,-28.89,;34.09,-30.43,;32.77,-31.2,;32.76,-32.76,;31.44,-33.52,;34.11,-33.52,;35.44,-32.74,;35.42,-31.2,;36.76,-30.43,;36.75,-31.96,;36.76,-28.89,;38.08,-28.1,;39.43,-28.89,;39.43,-30.43,;38.08,-31.2,;40.76,-28.12,;42.1,-28.89,;42.1,-30.44,;43.44,-28.13,;44.76,-28.91,;46.1,-28.15,;47.42,-28.93,;48.77,-28.18,;48.77,-26.63,;47.45,-25.85,;46.12,-26.61,)| Show InChI InChI=1S/C22H27NO3/c1-16-15-19(24)8-9-20(16)22(26)13-11-18(12-14-22)23-21(25)10-7-17-5-3-2-4-6-17/h2-6,8-9,15,18,24,26H,7,10-14H2,1H3,(H,23,25)/t18-,22+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101,606 from NR2B in rat forebrain P2 membrane |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220716
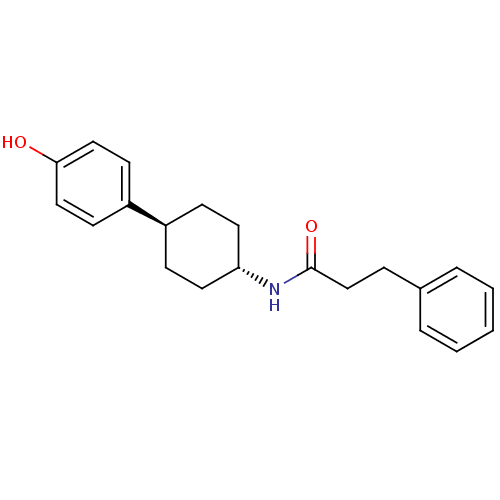
(CHEMBL236893 | N-((1r,4r)-4-(4-hydroxyphenyl)cyclo...)Show SMILES Oc1ccc(cc1)[C@H]1CC[C@@H](CC1)NC(=O)CCc1ccccc1 |wU:7.7,wD:10.14,(-10.14,-2.71,;-8.81,-1.94,;-8.81,-.39,;-7.49,.38,;-6.15,-.39,;-6.14,-1.92,;-7.46,-2.7,;-4.81,.38,;-4.81,1.92,;-3.49,2.71,;-2.14,1.92,;-2.14,.38,;-3.49,-.39,;-.81,2.69,;.52,1.92,;.52,.38,;1.87,2.68,;3.19,1.9,;4.54,2.66,;5.86,1.89,;7.19,2.65,;7.21,4.19,;5.87,4.97,;4.54,4.2,)| Show InChI InChI=1S/C21H25NO2/c23-20-13-9-18(10-14-20)17-7-11-19(12-8-17)22-21(24)15-6-16-4-2-1-3-5-16/h1-5,9-10,13-14,17,19,23H,6-8,11-12,15H2,(H,22,24)/t17-,19- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101,606 from NR2B in rat forebrain P2 membrane |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220727
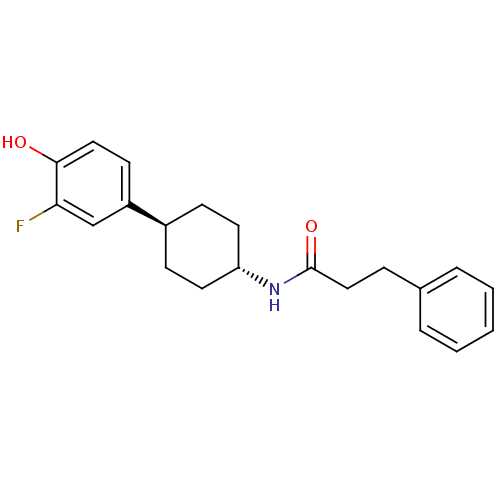
(CHEMBL237534 | N-((1r,4r)-4-(3-fluoro-4-hydroxyphe...)Show SMILES Oc1ccc(cc1F)[C@H]1CC[C@@H](CC1)NC(=O)CCc1ccccc1 |wU:8.8,wD:11.15,(31.75,-43.6,;33.08,-42.83,;34.43,-43.59,;35.75,-42.81,;35.74,-41.28,;34.4,-40.51,;33.08,-41.28,;31.74,-40.51,;37.08,-40.5,;37.08,-38.96,;38.4,-38.18,;39.74,-38.96,;39.74,-40.5,;38.4,-41.27,;41.08,-38.2,;42.41,-38.97,;42.41,-40.51,;43.76,-38.21,;45.08,-38.99,;46.42,-38.23,;47.74,-39,;49.08,-38.25,;49.09,-36.7,;47.77,-35.93,;46.43,-36.69,)| Show InChI InChI=1S/C21H24FNO2/c22-19-14-17(9-12-20(19)24)16-7-10-18(11-8-16)23-21(25)13-6-15-4-2-1-3-5-15/h1-5,9,12,14,16,18,24H,6-8,10-11,13H2,(H,23,25)/t16-,18- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101,606 from NR2B in rat forebrain P2 membrane |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220720
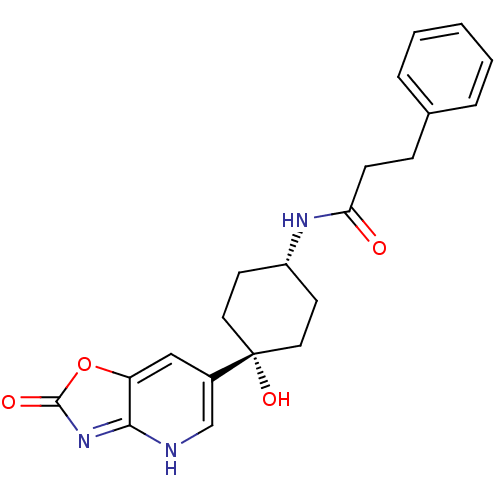
(CHEMBL237752 | N-((1s,4s)-4-hydroxy-4-(2-oxo-2,3-d...)Show SMILES O[C@@]1(CC[C@@H](CC1)NC(=O)CCc1ccccc1)c1c[nH]c2nc(=O)oc2c1 |wD:1.0,4.7,(16.47,-54.83,;16.48,-53.3,;16.48,-51.76,;17.81,-50.98,;19.14,-51.76,;19.14,-53.3,;17.81,-54.07,;20.48,-51,;21.81,-51.77,;21.81,-53.31,;23.16,-51.01,;24.48,-51.79,;25.81,-51.02,;27.13,-51.8,;28.47,-51.05,;28.49,-49.51,;27.17,-48.73,;25.83,-49.48,;15.14,-54.08,;15.16,-55.61,;13.83,-56.39,;12.47,-55.63,;11.01,-56.1,;10.1,-54.85,;8.55,-54.85,;11.01,-53.6,;12.48,-54.08,;13.81,-53.31,)| Show InChI InChI=1S/C21H23N3O4/c25-18(7-6-14-4-2-1-3-5-14)23-16-8-10-21(27,11-9-16)15-12-17-19(22-13-15)24-20(26)28-17/h1-5,12-13,16,27H,6-11H2,(H,23,25)(H,22,24,26)/t16-,21+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101,606 from NR2B in rat forebrain P2 membrane |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220725
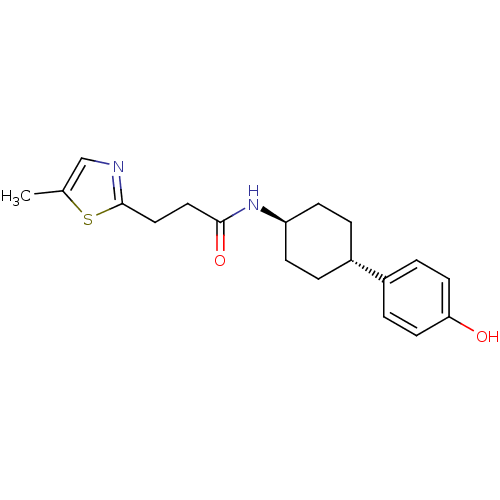
(CHEMBL237101 | N-((1r,4r)-4-(4-hydroxyphenyl)cyclo...)Show SMILES Cc1cnc(CCC(=O)N[C@H]2CC[C@@H](CC2)c2ccc(O)cc2)s1 |wU:13.16,wD:10.9,(9.08,-7.45,;7.55,-7.45,;6.65,-6.21,;5.19,-6.67,;5.18,-8.21,;3.84,-8.97,;2.51,-8.19,;1.18,-8.95,;1.18,-10.5,;-.16,-8.18,;-1.49,-8.96,;-2.83,-8.17,;-4.16,-8.96,;-4.16,-10.5,;-2.83,-11.27,;-1.49,-10.5,;-5.49,-11.27,;-6.83,-10.5,;-8.15,-11.26,;-8.16,-12.82,;-9.48,-13.59,;-6.81,-13.58,;-5.48,-12.81,;6.64,-8.7,)| Show InChI InChI=1S/C19H24N2O2S/c1-13-12-20-19(24-13)11-10-18(23)21-16-6-2-14(3-7-16)15-4-8-17(22)9-5-15/h4-5,8-9,12,14,16,22H,2-3,6-7,10-11H2,1H3,(H,21,23)/t14-,16- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101,606 from NR2B in rat forebrain P2 membrane |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220728
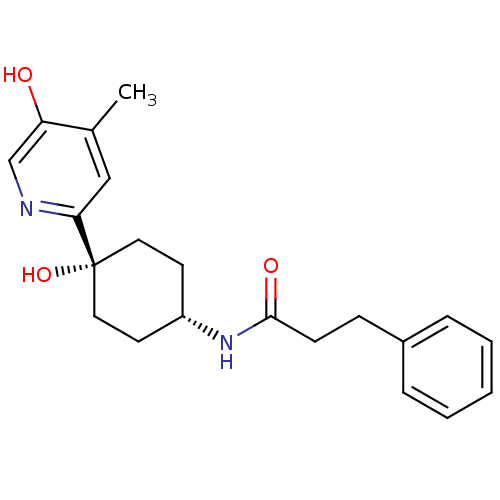
(CHEMBL237962 | N-((1s,4s)-4-hydroxy-4-(5-hydroxy-4...)Show SMILES Cc1cc(ncc1O)[C@@]1(O)CC[C@@H](CC1)NC(=O)CCc1ccccc1 |wD:8.9,12.16,(30.85,-52.95,;32.18,-53.72,;33.5,-52.95,;34.84,-53.72,;34.85,-55.26,;33.53,-56.04,;32.18,-55.27,;30.85,-56.04,;36.18,-52.95,;36.17,-54.48,;36.18,-51.41,;37.5,-50.62,;38.85,-51.41,;38.85,-52.95,;37.5,-53.72,;40.18,-50.64,;41.51,-51.41,;41.52,-52.95,;42.86,-50.65,;44.18,-51.43,;45.52,-50.67,;46.84,-51.45,;48.19,-50.69,;48.19,-49.15,;46.87,-48.37,;45.54,-49.13,)| Show InChI InChI=1S/C21H26N2O3/c1-15-13-19(22-14-18(15)24)21(26)11-9-17(10-12-21)23-20(25)8-7-16-5-3-2-4-6-16/h2-6,13-14,17,24,26H,7-12H2,1H3,(H,23,25)/t17-,21+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101,606 from NR2B in rat forebrain P2 membrane |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220715
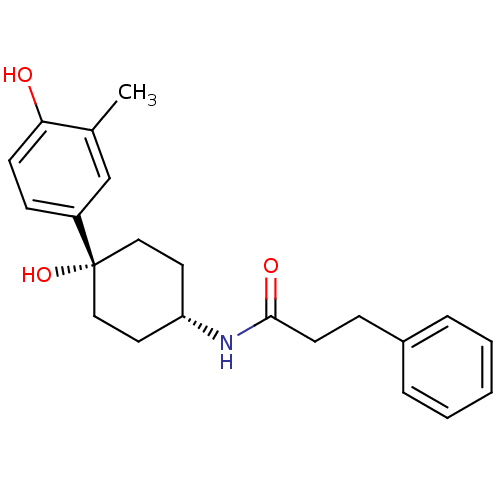
(CHEMBL237321 | N-((1s,4s)-4-hydroxy-4-(4-hydroxy-3...)Show SMILES Cc1cc(ccc1O)[C@@]1(O)CC[C@@H](CC1)NC(=O)CCc1ccccc1 |wD:8.9,12.16,(-9.08,-31.78,;-7.75,-32.55,;-6.43,-31.78,;-5.09,-32.56,;-5.08,-34.09,;-6.4,-34.87,;-7.75,-34.11,;-9.08,-34.87,;-3.75,-31.78,;-3.76,-33.31,;-3.75,-30.24,;-2.43,-29.45,;-1.08,-30.24,;-1.08,-31.78,;-2.43,-32.55,;.25,-29.47,;1.58,-30.24,;1.59,-31.79,;2.93,-29.48,;4.25,-30.27,;5.59,-29.5,;6.91,-30.28,;8.26,-29.53,;8.26,-27.98,;6.94,-27.21,;5.61,-27.97,)| Show InChI InChI=1S/C22H27NO3/c1-16-15-18(8-9-20(16)24)22(26)13-11-19(12-14-22)23-21(25)10-7-17-5-3-2-4-6-17/h2-6,8-9,15,19,24,26H,7,10-14H2,1H3,(H,23,25)/t19-,22+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 189 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101,606 from NR2B in rat forebrain P2 membrane |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220713
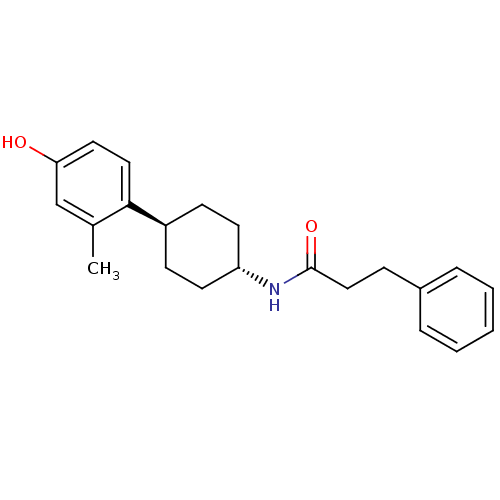
(CHEMBL237323 | N-((1r,4r)-4-(4-hydroxy-2-methylphe...)Show SMILES Cc1cc(O)ccc1[C@H]1CC[C@@H](CC1)NC(=O)CCc1ccccc1 |wU:8.8,wD:11.15,(-7.33,-40.77,;-7.32,-42.31,;-8.64,-43.08,;-8.65,-44.63,;-9.97,-45.4,;-7.3,-45.4,;-5.97,-44.62,;-5.99,-43.08,;-4.65,-42.31,;-4.65,-40.77,;-3.32,-39.98,;-1.98,-40.77,;-1.98,-42.31,;-3.32,-43.08,;-.65,-40,;.69,-40.77,;.69,-42.32,;2.03,-40.01,;3.35,-40.79,;4.69,-40.03,;6.01,-40.81,;7.36,-40.05,;7.36,-38.51,;6.04,-37.73,;4.71,-38.49,)| Show InChI InChI=1S/C22H27NO2/c1-16-15-20(24)12-13-21(16)18-8-10-19(11-9-18)23-22(25)14-7-17-5-3-2-4-6-17/h2-6,12-13,15,18-19,24H,7-11,14H2,1H3,(H,23,25)/t18-,19- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 722 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101,606 from NR2B in rat forebrain P2 membrane |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220718
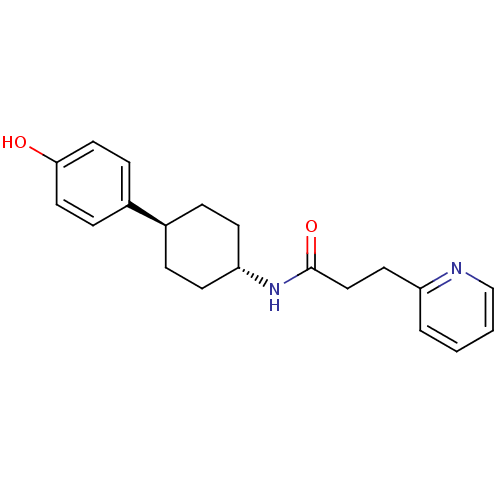
(CHEMBL236895 | N-((1r,4r)-4-(4-hydroxyphenyl)cyclo...)Show SMILES Oc1ccc(cc1)[C@H]1CC[C@@H](CC1)NC(=O)CCc1ccccn1 |wU:7.7,wD:10.14,(30.49,-.92,;31.82,-.15,;31.82,1.4,;33.14,2.17,;34.48,1.4,;34.49,-.14,;33.17,-.92,;35.82,2.17,;35.82,3.71,;37.14,4.5,;38.48,3.71,;38.48,2.17,;37.14,1.4,;39.82,4.48,;41.15,3.71,;41.15,2.16,;42.5,4.47,;43.82,3.69,;45.16,4.45,;45.17,5.99,;46.5,6.75,;47.83,5.97,;47.82,4.43,;46.48,3.67,)| Show InChI InChI=1S/C20H24N2O2/c23-19-11-6-16(7-12-19)15-4-8-18(9-5-15)22-20(24)13-10-17-3-1-2-14-21-17/h1-3,6-7,11-12,14-15,18,23H,4-5,8-10,13H2,(H,22,24)/t15-,18- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 856 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101,606 from NR2B in rat forebrain P2 membrane |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220723
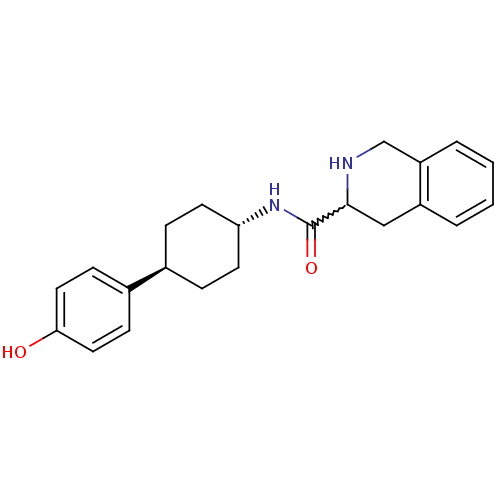
(CHEMBL237102 | N-((1r,4r)-4-(4-hydroxyphenyl)cyclo...)Show SMILES Oc1ccc(cc1)[C@H]1CC[C@@H](CC1)NC(=O)C1Cc2ccccc2CN1 |w:16.17,wU:7.7,wD:10.14,(35.06,-11.3,;36.39,-10.54,;36.39,-8.98,;37.71,-8.22,;39.05,-8.99,;39.06,-10.52,;37.74,-11.3,;40.39,-8.21,;40.39,-6.67,;41.71,-5.88,;43.05,-6.67,;43.05,-8.21,;41.71,-8.98,;44.39,-5.9,;45.72,-6.67,;45.72,-8.22,;47.07,-5.91,;48.39,-6.69,;49.73,-5.93,;51.05,-6.71,;52.39,-5.96,;52.4,-4.41,;51.08,-3.64,;49.74,-4.39,;48.41,-3.61,;47.07,-4.37,)| Show InChI InChI=1S/C22H26N2O2/c25-20-11-7-16(8-12-20)15-5-9-19(10-6-15)24-22(26)21-13-17-3-1-2-4-18(17)14-23-21/h1-4,7-8,11-12,15,19,21,23,25H,5-6,9-10,13-14H2,(H,24,26)/t15-,19-,21? | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101,606 from NR2B in rat forebrain P2 membrane |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220731
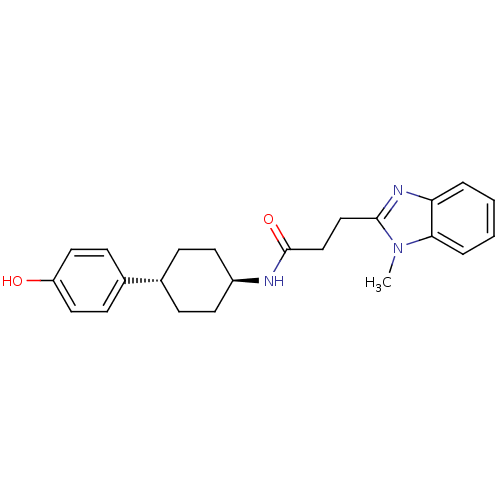
(CHEMBL393609 | N-((1r,4r)-4-(4-hydroxyphenyl)cyclo...)Show SMILES Cn1c(CCC(=O)N[C@H]2CC[C@@H](CC2)c2ccc(O)cc2)nc2ccccc12 |wU:11.14,wD:8.7,(27.83,-9.24,;27.07,-7.91,;25.61,-7.43,;24.27,-8.19,;22.94,-7.41,;21.6,-8.17,;21.6,-9.72,;20.26,-7.4,;18.93,-8.17,;17.58,-7.38,;16.25,-8.17,;16.25,-9.71,;17.58,-10.48,;18.93,-9.71,;14.92,-10.49,;13.58,-9.71,;12.24,-10.49,;12.24,-12.04,;10.91,-12.81,;13.59,-12.8,;14.93,-12.03,;25.61,-5.89,;27.08,-5.42,;27.84,-3.92,;29.37,-3.9,;30.15,-5.23,;29.39,-6.56,;27.85,-6.57,)| Show InChI InChI=1S/C23H27N3O2/c1-26-21-5-3-2-4-20(21)25-22(26)14-15-23(28)24-18-10-6-16(7-11-18)17-8-12-19(27)13-9-17/h2-5,8-9,12-13,16,18,27H,6-7,10-11,14-15H2,1H3,(H,24,28)/t16-,18- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101,606 from NR2B in rat forebrain P2 membrane |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220734
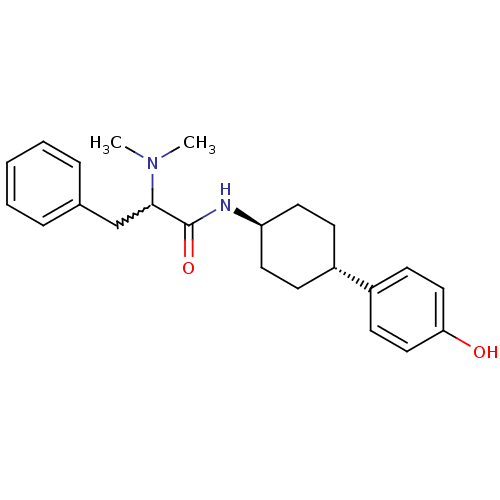
(2-(dimethylamino)-N-((1r,4r)-4-(4-hydroxyphenyl)cy...)Show SMILES CN(C)C(Cc1ccccc1)C(=O)N[C@H]1CC[C@@H](CC1)c1ccc(O)cc1 |w:3.3,wU:17.21,wD:14.14,(2.93,-17.32,;1.59,-18.09,;.25,-17.31,;1.59,-19.63,;2.91,-20.4,;4.25,-19.64,;5.57,-20.43,;6.91,-19.67,;6.92,-18.13,;5.6,-17.35,;4.26,-18.1,;.24,-20.39,;.24,-21.94,;-1.09,-19.62,;-2.43,-20.39,;-3.77,-19.6,;-5.09,-20.39,;-5.09,-21.93,;-3.77,-22.7,;-2.43,-21.93,;-6.43,-22.7,;-7.77,-21.93,;-9.09,-22.7,;-9.09,-24.25,;-10.42,-25.01,;-7.74,-25.02,;-6.42,-24.24,)| Show InChI InChI=1S/C23H30N2O2/c1-25(2)22(16-17-6-4-3-5-7-17)23(27)24-20-12-8-18(9-13-20)19-10-14-21(26)15-11-19/h3-7,10-11,14-15,18,20,22,26H,8-9,12-13,16H2,1-2H3,(H,24,27)/t18-,20-,22? | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101,606 from NR2B in rat forebrain P2 membrane |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220719
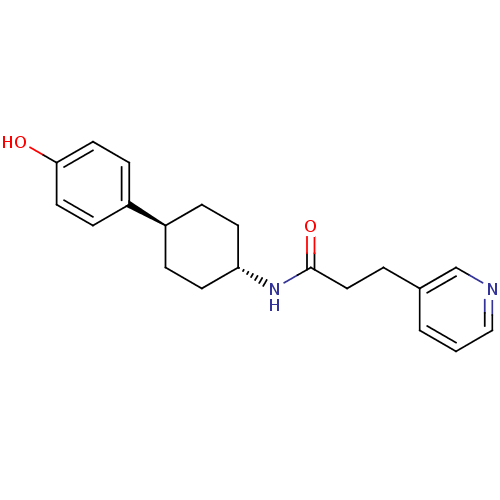
(CHEMBL236894 | N-((1r,4r)-4-(4-hydroxyphenyl)cyclo...)Show SMILES Oc1ccc(cc1)[C@H]1CC[C@@H](CC1)NC(=O)CCc1cccnc1 |wU:7.7,wD:10.14,(10.18,-1.13,;11.51,-.36,;11.51,1.19,;12.83,1.96,;14.17,1.19,;14.18,-.34,;12.86,-1.12,;15.51,1.97,;15.51,3.51,;16.83,4.29,;18.17,3.51,;18.17,1.97,;16.83,1.2,;19.51,4.27,;20.84,3.51,;20.84,1.96,;22.18,4.27,;23.51,3.49,;24.85,4.25,;24.85,5.79,;26.18,6.55,;27.52,5.77,;27.51,4.23,;26.17,3.47,)| Show InChI InChI=1S/C20H24N2O2/c23-19-10-6-17(7-11-19)16-4-8-18(9-5-16)22-20(24)12-3-15-2-1-13-21-14-15/h1-2,6-7,10-11,13-14,16,18,23H,3-5,8-9,12H2,(H,22,24)/t16-,18- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101,606 from NR2B in rat forebrain P2 membrane |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50220730
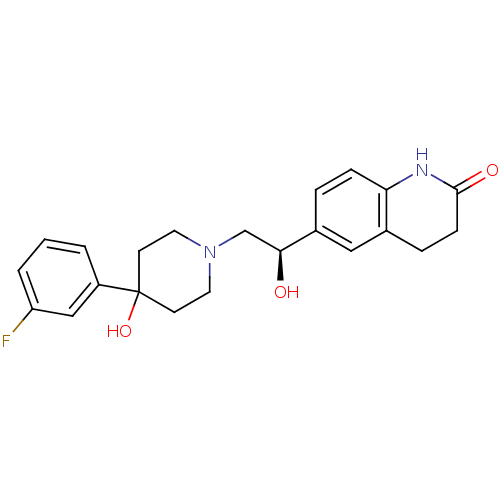
((R)-6-(2-(4-(3-fluorophenyl)-4-hydroxypiperidin-1-...)Show SMILES O[C@@H](CN1CCC(O)(CC1)c1cccc(F)c1)c1ccc2NC(=O)CCc2c1 Show InChI InChI=1S/C22H25FN2O3/c23-18-3-1-2-17(13-18)22(28)8-10-25(11-9-22)14-20(26)16-4-6-19-15(12-16)5-7-21(27)24-19/h1-4,6,12-13,20,26,28H,5,7-11,14H2,(H,24,27)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human HERG expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220732
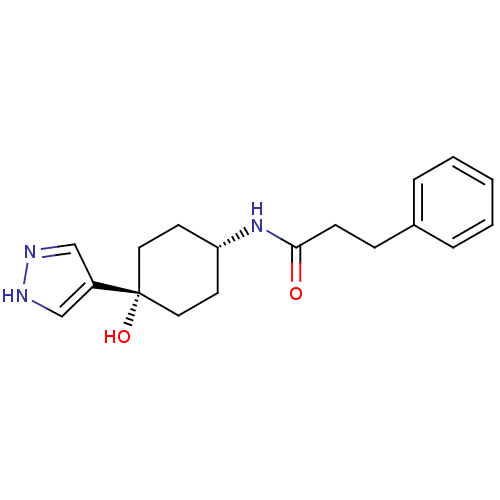
(CHEMBL237965 | N-((1s,4s)-4-hydroxy-4-(1H-pyrazol-...)Show SMILES O[C@@]1(CC[C@@H](CC1)NC(=O)CCc1ccccc1)c1cn[nH]c1 |wD:1.0,4.7,(16.71,-11.57,;16.72,-10.04,;16.72,-8.5,;18.05,-7.71,;19.38,-8.5,;19.38,-10.04,;18.05,-10.81,;20.72,-7.73,;22.05,-8.51,;22.05,-10.05,;23.39,-7.74,;24.72,-8.53,;26.05,-7.77,;27.38,-8.55,;28.72,-7.79,;28.73,-6.25,;27.4,-5.47,;26.07,-6.23,;15.39,-10.81,;13.92,-10.35,;13.02,-11.59,;13.93,-12.83,;15.39,-12.36,)| Show InChI InChI=1S/C18H23N3O2/c22-17(7-6-14-4-2-1-3-5-14)21-16-8-10-18(23,11-9-16)15-12-19-20-13-15/h1-5,12-13,16,23H,6-11H2,(H,19,20)(H,21,22)/t16-,18+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101,606 from NR2B in rat forebrain P2 membrane |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50348122

(CHEMBL1800168)Show InChI InChI=1S/C17H24N2O2S/c1-5-22(20,21)13-8-9-15-14(10-13)18-16(17(2,3)4)19(15)11-12-6-7-12/h8-10,12H,5-7,11H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 21: 4284-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.063
BindingDB Entry DOI: 10.7270/Q2959HXV |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220735
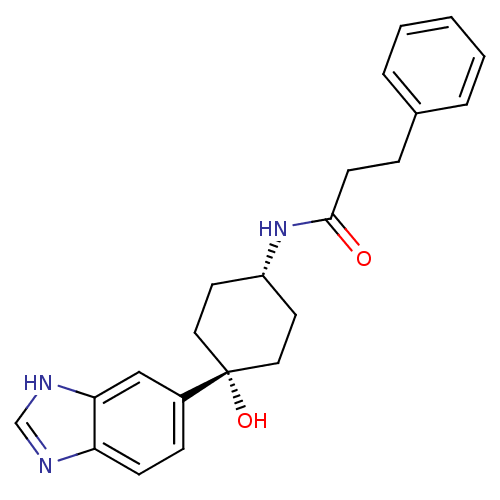
(CHEMBL238184 | N-((1s,4s)-4-(1H-benzo[d]imidazol-5...)Show SMILES O[C@@]1(CC[C@@H](CC1)NC(=O)CCc1ccccc1)c1ccc2nc[nH]c2c1 |wD:1.0,4.7,(39.84,-12.54,;39.85,-11.01,;39.85,-9.47,;41.17,-8.69,;42.52,-9.47,;42.52,-11.01,;41.17,-11.78,;43.85,-8.7,;45.19,-9.47,;45.19,-11.01,;46.52,-8.7,;47.84,-9.49,;49.18,-8.73,;50.5,-9.51,;51.85,-8.76,;51.85,-7.21,;50.53,-6.44,;49.21,-7.19,;38.51,-11.78,;38.52,-13.33,;37.2,-14.11,;35.86,-13.36,;34.4,-13.86,;33.47,-12.62,;34.35,-11.35,;35.83,-11.81,;37.17,-11.02,)| Show InChI InChI=1S/C22H25N3O2/c26-21(9-6-16-4-2-1-3-5-16)25-18-10-12-22(27,13-11-18)17-7-8-19-20(14-17)24-15-23-19/h1-5,7-8,14-15,18,27H,6,9-13H2,(H,23,24)(H,25,26)/t18-,22+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101,606 from NR2B in rat forebrain P2 membrane |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220722
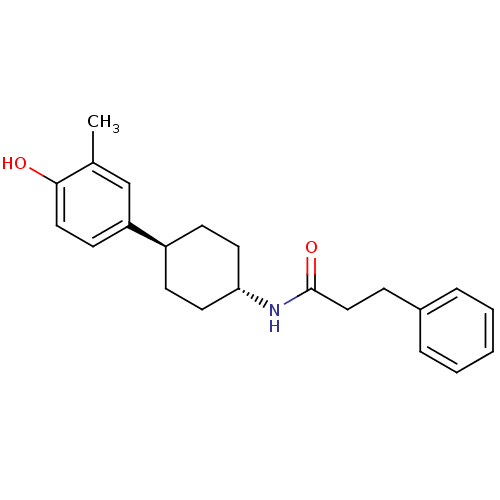
(CHEMBL237322 | N-((1r,4r)-4-(4-hydroxy-3-methylphe...)Show SMILES Cc1cc(ccc1O)[C@H]1CC[C@@H](CC1)NC(=O)CCc1ccccc1 |wU:8.8,wD:11.15,(10.84,-32.43,;12.18,-33.2,;13.5,-32.43,;14.84,-33.21,;14.85,-34.74,;13.53,-35.52,;12.18,-34.76,;10.85,-35.52,;16.18,-32.43,;16.18,-30.89,;17.5,-30.1,;18.84,-30.89,;18.84,-32.43,;17.5,-33.2,;20.18,-30.12,;21.51,-30.89,;21.51,-32.44,;22.86,-30.13,;24.18,-30.91,;25.52,-30.15,;26.84,-30.93,;28.18,-30.18,;28.19,-28.63,;26.87,-27.86,;25.53,-28.62,)| Show InChI InChI=1S/C22H27NO2/c1-16-15-19(10-13-21(16)24)18-8-11-20(12-9-18)23-22(25)14-7-17-5-3-2-4-6-17/h2-6,10,13,15,18,20,24H,7-9,11-12,14H2,1H3,(H,23,25)/t18-,20- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101,606 from NR2B in rat forebrain P2 membrane |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220717
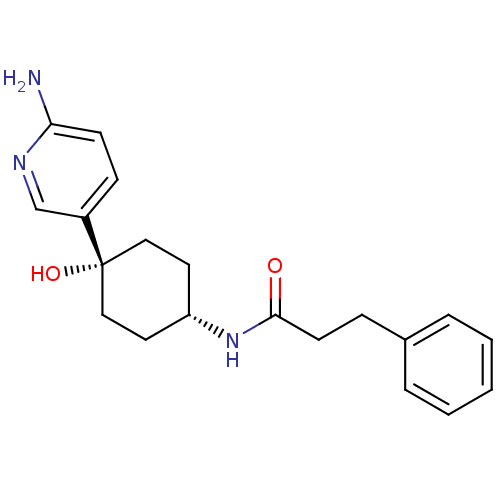
(CHEMBL237964 | N-((1s,4s)-4-(6-aminopyridin-3-yl)-...)Show SMILES Nc1ccc(cn1)[C@@]1(O)CC[C@@H](CC1)NC(=O)CCc1ccccc1 |wD:7.8,11.15,(16.02,-1.74,;17.5,-1.27,;17.5,.28,;18.82,1.06,;20.17,.28,;20.18,-1.25,;18.85,-2.03,;21.5,1.06,;21.49,-.47,;21.5,2.6,;22.83,3.39,;24.16,2.6,;24.16,1.06,;22.83,.29,;25.5,3.37,;26.83,2.6,;26.83,1.06,;28.17,3.36,;29.5,2.58,;30.83,3.35,;32.16,2.56,;33.49,3.32,;33.51,4.86,;32.18,5.64,;30.85,4.89,)| Show InChI InChI=1S/C20H25N3O2/c21-18-8-7-16(14-22-18)20(25)12-10-17(11-13-20)23-19(24)9-6-15-4-2-1-3-5-15/h1-5,7-8,14,17,25H,6,9-13H2,(H2,21,22)(H,23,24)/t17-,20+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101,606 from NR2B in rat forebrain P2 membrane |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
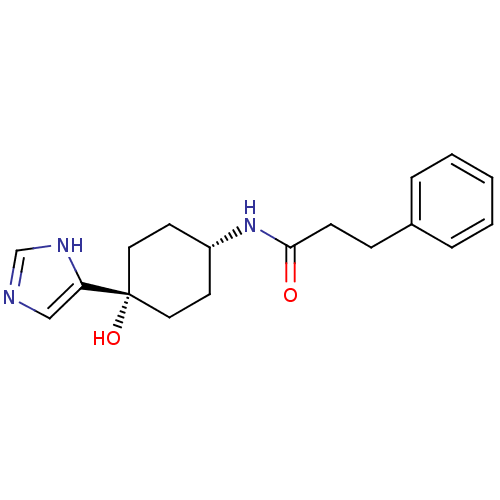
(Rattus norvegicus (Rat)) | BDBM50220721
(CHEMBL398127 | N-((1s,4s)-4-hydroxy-4-(1H-imidazol...)Show SMILES O[C@@]1(CC[C@@H](CC1)NC(=O)CCc1ccccc1)c1cnc[nH]1 |wD:1.0,4.7,(-5.23,-14.74,;-5.22,-13.21,;-5.22,-11.67,;-3.9,-10.88,;-2.57,-11.67,;-2.57,-13.21,;-3.9,-13.98,;-1.23,-10.9,;.1,-11.67,;.11,-13.22,;1.45,-10.91,;2.77,-11.69,;4.11,-10.93,;5.43,-11.72,;6.77,-10.96,;6.78,-9.42,;5.46,-8.64,;4.13,-9.39,;-6.56,-13.98,;-6.56,-15.52,;-8.02,-16,;-8.93,-14.76,;-8.03,-13.52,)| Show InChI InChI=1S/C18H23N3O2/c22-17(7-6-14-4-2-1-3-5-14)21-15-8-10-18(23,11-9-15)16-12-19-13-20-16/h1-5,12-13,15,23H,6-11H2,(H,19,20)(H,21,22)/t15-,18+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101,606 from NR2B in rat forebrain P2 membrane |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50220724
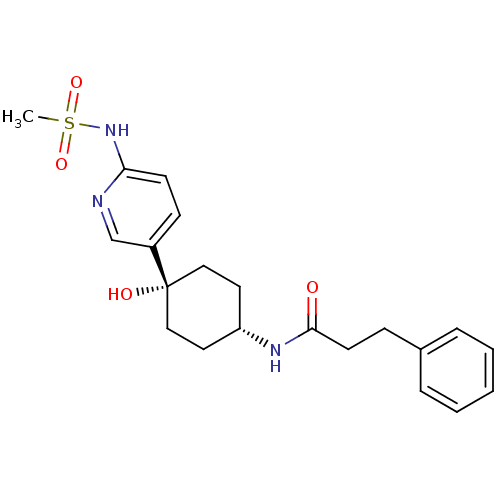
(CHEMBL237963 | N-((1s,4s)-4-hydroxy-4-(6-(methylsu...)Show SMILES CS(=O)(=O)Nc1ccc(cn1)[C@@]1(O)CC[C@@H](CC1)NC(=O)CCc1ccccc1 |wD:11.12,15.19,(-9.1,-.63,;-8.01,-1.73,;-9.07,-2.85,;-6.94,-.62,;-6.86,-2.76,;-5.39,-2.29,;-5.39,-.74,;-4.06,.03,;-2.72,-.74,;-2.71,-2.27,;-4.03,-3.05,;-1.38,.04,;-1.39,-1.5,;-1.38,1.58,;-.06,2.37,;1.28,1.58,;1.28,.04,;-.06,-.73,;2.61,2.34,;3.95,1.57,;3.95,.03,;5.29,2.33,;6.61,1.55,;7.95,2.31,;9.27,1.53,;10.61,2.29,;10.62,3.83,;9.31,4.6,;7.97,3.85,)| Show InChI InChI=1S/C21H27N3O4S/c1-29(27,28)24-19-9-8-17(15-22-19)21(26)13-11-18(12-14-21)23-20(25)10-7-16-5-3-2-4-6-16/h2-6,8-9,15,18,26H,7,10-14H2,1H3,(H,22,24)(H,23,25)/t18-,21+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP101,606 from NR2B in rat forebrain P2 membrane |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50220725

(CHEMBL237101 | N-((1r,4r)-4-(4-hydroxyphenyl)cyclo...)Show SMILES Cc1cnc(CCC(=O)N[C@H]2CC[C@@H](CC2)c2ccc(O)cc2)s1 |wU:13.16,wD:10.9,(9.08,-7.45,;7.55,-7.45,;6.65,-6.21,;5.19,-6.67,;5.18,-8.21,;3.84,-8.97,;2.51,-8.19,;1.18,-8.95,;1.18,-10.5,;-.16,-8.18,;-1.49,-8.96,;-2.83,-8.17,;-4.16,-8.96,;-4.16,-10.5,;-2.83,-11.27,;-1.49,-10.5,;-5.49,-11.27,;-6.83,-10.5,;-8.15,-11.26,;-8.16,-12.82,;-9.48,-13.59,;-6.81,-13.58,;-5.48,-12.81,;6.64,-8.7,)| Show InChI InChI=1S/C19H24N2O2S/c1-13-12-20-19(24-13)11-10-18(23)21-16-6-2-14(3-7-16)15-4-8-17(22)9-5-15/h4-5,8-9,12,14,16,22H,2-3,6-7,10-11H2,1H3,(H,21,23)/t14-,16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human HERG expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50220716

(CHEMBL236893 | N-((1r,4r)-4-(4-hydroxyphenyl)cyclo...)Show SMILES Oc1ccc(cc1)[C@H]1CC[C@@H](CC1)NC(=O)CCc1ccccc1 |wU:7.7,wD:10.14,(-10.14,-2.71,;-8.81,-1.94,;-8.81,-.39,;-7.49,.38,;-6.15,-.39,;-6.14,-1.92,;-7.46,-2.7,;-4.81,.38,;-4.81,1.92,;-3.49,2.71,;-2.14,1.92,;-2.14,.38,;-3.49,-.39,;-.81,2.69,;.52,1.92,;.52,.38,;1.87,2.68,;3.19,1.9,;4.54,2.66,;5.86,1.89,;7.19,2.65,;7.21,4.19,;5.87,4.97,;4.54,4.2,)| Show InChI InChI=1S/C21H25NO2/c23-20-13-9-18(10-14-20)17-7-11-19(12-8-17)22-21(24)15-6-16-4-2-1-3-5-16/h1-5,9-10,13-14,17,19,23H,6-8,11-12,15H2,(H,22,24)/t17-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human HERG expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50220729

(CHEMBL237320 | N-((1s,4s)-4-hydroxy-4-(4-hydroxyph...)Show SMILES Oc1ccc(cc1)[C@@]1(O)CC[C@@H](CC1)NC(=O)CCc1ccccc1 |wD:7.8,11.15,(10.84,-24.52,;12.17,-23.76,;12.17,-22.2,;13.49,-21.43,;14.83,-22.2,;14.84,-23.74,;13.52,-24.52,;16.17,-21.43,;16.15,-22.96,;16.17,-19.89,;17.49,-19.1,;18.83,-19.89,;18.83,-21.43,;17.49,-22.2,;20.17,-19.12,;21.5,-19.89,;21.5,-21.44,;22.85,-19.13,;24.17,-19.91,;25.51,-19.15,;26.82,-19.93,;28.17,-19.18,;28.17,-17.63,;26.86,-16.86,;25.52,-17.61,)| Show InChI InChI=1S/C21H25NO3/c23-19-9-7-17(8-10-19)21(25)14-12-18(13-15-21)22-20(24)11-6-16-4-2-1-3-5-16/h1-5,7-10,18,23,25H,6,11-15H2,(H,22,24)/t18-,21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human HERG expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50220714

(CHEMBL237751 | N-((1s,4s)-4-hydroxy-4-(5-hydroxypy...)Show SMILES Oc1ccc(nc1)[C@@]1(O)CC[C@@H](CC1)NC(=O)CCc1ccccc1 |wD:7.8,11.15,(-10.78,-55,;-9.45,-54.23,;-9.45,-52.68,;-8.13,-51.91,;-6.79,-52.68,;-6.78,-54.21,;-8.1,-54.99,;-5.45,-51.91,;-5.46,-53.44,;-5.45,-50.37,;-4.13,-49.58,;-2.79,-50.37,;-2.79,-51.91,;-4.13,-52.68,;-1.45,-49.6,;-.12,-50.37,;-.12,-51.91,;1.23,-49.61,;2.55,-50.39,;3.89,-49.63,;5.21,-50.4,;6.55,-49.65,;6.56,-48.11,;5.24,-47.33,;3.9,-48.09,)| Show InChI InChI=1S/C20H24N2O3/c23-17-7-8-18(21-14-17)20(25)12-10-16(11-13-20)22-19(24)9-6-15-4-2-1-3-5-15/h1-5,7-8,14,16,23,25H,6,9-13H2,(H,22,24)/t16-,20+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human HERG expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50220733
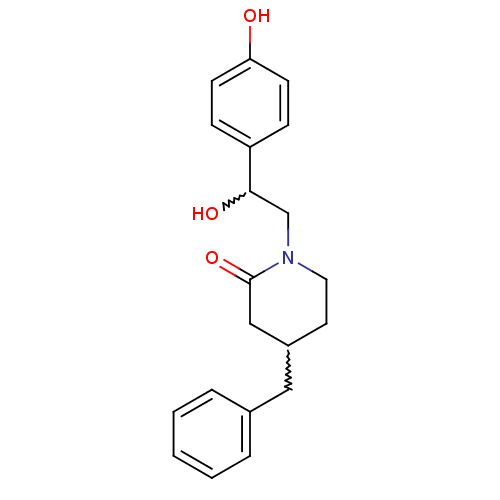
(4-benzyl-1-(2-hydroxy-2-(4-hydroxyphenyl)ethyl)pip...)Show SMILES OC(CN1CCC(Cc2ccccc2)CC1=O)c1ccc(O)cc1 |w:6.6,1.0| Show InChI InChI=1S/C20H23NO3/c22-18-8-6-17(7-9-18)19(23)14-21-11-10-16(13-20(21)24)12-15-4-2-1-3-5-15/h1-9,16,19,22-23H,10-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human HERG expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50220726

(CHEMBL237533 | N-((1s,4s)-4-(3-fluoro-4-hydroxyphe...)Show SMILES Oc1ccc(cc1F)[C@@]1(O)CC[C@@H](CC1)NC(=O)CCc1ccccc1 |wD:8.9,12.16,(12.39,-46.09,;13.72,-45.33,;15.07,-46.09,;16.39,-45.31,;16.38,-43.78,;15.04,-43.01,;13.72,-43.77,;12.38,-43,;17.72,-43,;17.71,-44.53,;17.72,-41.46,;19.04,-40.67,;20.38,-41.46,;20.38,-43,;19.04,-43.77,;21.72,-40.69,;23.05,-41.46,;23.05,-43.01,;24.4,-40.7,;25.72,-41.49,;27.06,-40.72,;28.37,-41.5,;29.72,-40.75,;29.73,-39.2,;28.41,-38.43,;27.07,-39.19,)| Show InChI InChI=1S/C21H24FNO3/c22-18-14-16(7-8-19(18)24)21(26)12-10-17(11-13-21)23-20(25)9-6-15-4-2-1-3-5-15/h1-5,7-8,14,17,24,26H,6,9-13H2,(H,23,25)/t17-,21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human HERG expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50220723

(CHEMBL237102 | N-((1r,4r)-4-(4-hydroxyphenyl)cyclo...)Show SMILES Oc1ccc(cc1)[C@H]1CC[C@@H](CC1)NC(=O)C1Cc2ccccc2CN1 |w:16.17,wU:7.7,wD:10.14,(35.06,-11.3,;36.39,-10.54,;36.39,-8.98,;37.71,-8.22,;39.05,-8.99,;39.06,-10.52,;37.74,-11.3,;40.39,-8.21,;40.39,-6.67,;41.71,-5.88,;43.05,-6.67,;43.05,-8.21,;41.71,-8.98,;44.39,-5.9,;45.72,-6.67,;45.72,-8.22,;47.07,-5.91,;48.39,-6.69,;49.73,-5.93,;51.05,-6.71,;52.39,-5.96,;52.4,-4.41,;51.08,-3.64,;49.74,-4.39,;48.41,-3.61,;47.07,-4.37,)| Show InChI InChI=1S/C22H26N2O2/c25-20-11-7-16(8-12-20)15-5-9-19(10-6-15)24-22(26)21-13-17-3-1-2-4-18(17)14-23-21/h1-4,7-8,11-12,15,19,21,23,25H,5-6,9-10,13-14H2,(H,24,26)/t15-,19-,21? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human HERG expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50220719

(CHEMBL236894 | N-((1r,4r)-4-(4-hydroxyphenyl)cyclo...)Show SMILES Oc1ccc(cc1)[C@H]1CC[C@@H](CC1)NC(=O)CCc1cccnc1 |wU:7.7,wD:10.14,(10.18,-1.13,;11.51,-.36,;11.51,1.19,;12.83,1.96,;14.17,1.19,;14.18,-.34,;12.86,-1.12,;15.51,1.97,;15.51,3.51,;16.83,4.29,;18.17,3.51,;18.17,1.97,;16.83,1.2,;19.51,4.27,;20.84,3.51,;20.84,1.96,;22.18,4.27,;23.51,3.49,;24.85,4.25,;24.85,5.79,;26.18,6.55,;27.52,5.77,;27.51,4.23,;26.17,3.47,)| Show InChI InChI=1S/C20H24N2O2/c23-19-10-6-17(7-11-19)16-4-8-18(9-5-16)22-20(24)12-3-15-2-1-13-21-14-15/h1-2,6-7,10-11,13-14,16,18,23H,3-5,8-9,12H2,(H,22,24)/t16-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human HERG expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50220718

(CHEMBL236895 | N-((1r,4r)-4-(4-hydroxyphenyl)cyclo...)Show SMILES Oc1ccc(cc1)[C@H]1CC[C@@H](CC1)NC(=O)CCc1ccccn1 |wU:7.7,wD:10.14,(30.49,-.92,;31.82,-.15,;31.82,1.4,;33.14,2.17,;34.48,1.4,;34.49,-.14,;33.17,-.92,;35.82,2.17,;35.82,3.71,;37.14,4.5,;38.48,3.71,;38.48,2.17,;37.14,1.4,;39.82,4.48,;41.15,3.71,;41.15,2.16,;42.5,4.47,;43.82,3.69,;45.16,4.45,;45.17,5.99,;46.5,6.75,;47.83,5.97,;47.82,4.43,;46.48,3.67,)| Show InChI InChI=1S/C20H24N2O2/c23-19-11-6-16(7-12-19)15-4-8-18(9-5-15)22-20(24)13-10-17-3-1-2-14-21-17/h1-3,6-7,11-12,14-15,18,23H,4-5,8-10,13H2,(H,22,24)/t15-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human HERG expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50220713

(CHEMBL237323 | N-((1r,4r)-4-(4-hydroxy-2-methylphe...)Show SMILES Cc1cc(O)ccc1[C@H]1CC[C@@H](CC1)NC(=O)CCc1ccccc1 |wU:8.8,wD:11.15,(-7.33,-40.77,;-7.32,-42.31,;-8.64,-43.08,;-8.65,-44.63,;-9.97,-45.4,;-7.3,-45.4,;-5.97,-44.62,;-5.99,-43.08,;-4.65,-42.31,;-4.65,-40.77,;-3.32,-39.98,;-1.98,-40.77,;-1.98,-42.31,;-3.32,-43.08,;-.65,-40,;.69,-40.77,;.69,-42.32,;2.03,-40.01,;3.35,-40.79,;4.69,-40.03,;6.01,-40.81,;7.36,-40.05,;7.36,-38.51,;6.04,-37.73,;4.71,-38.49,)| Show InChI InChI=1S/C22H27NO2/c1-16-15-20(24)12-13-21(16)18-8-10-19(11-9-18)23-22(25)14-7-17-5-3-2-4-6-17/h2-6,12-13,15,18-19,24H,7-11,14H2,1H3,(H,23,25)/t18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human HERG expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 5533-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.039
BindingDB Entry DOI: 10.7270/Q23778FR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50348117
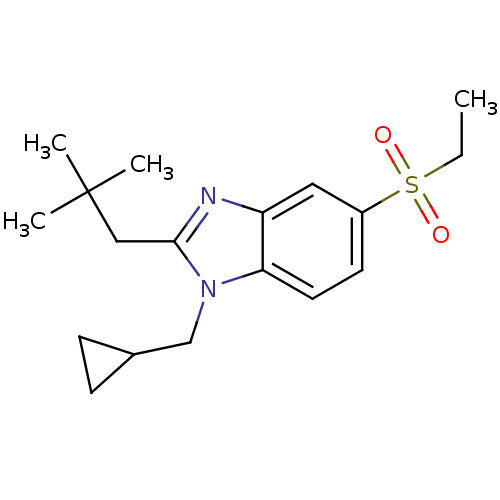
(CHEMBL1800163)Show SMILES CCS(=O)(=O)c1ccc2n(CC3CC3)c(CC(C)(C)C)nc2c1 Show InChI InChI=1S/C18H26N2O2S/c1-5-23(21,22)14-8-9-16-15(10-14)19-17(11-18(2,3)4)20(16)12-13-6-7-13/h8-10,13H,5-7,11-12H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release |
Bioorg Med Chem Lett 21: 4284-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.063
BindingDB Entry DOI: 10.7270/Q2959HXV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50348116
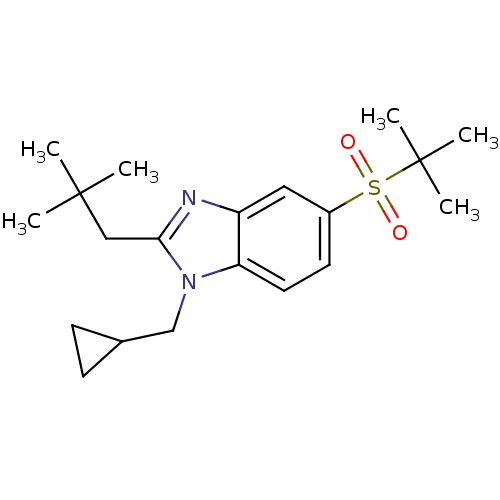
(CHEMBL1800655)Show SMILES CC(C)(C)Cc1nc2cc(ccc2n1CC1CC1)S(=O)(=O)C(C)(C)C Show InChI InChI=1S/C20H30N2O2S/c1-19(2,3)12-18-21-16-11-15(25(23,24)20(4,5)6)9-10-17(16)22(18)13-14-7-8-14/h9-11,14H,7-8,12-13H2,1-6H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release |
Bioorg Med Chem Lett 21: 4284-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.063
BindingDB Entry DOI: 10.7270/Q2959HXV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50348119
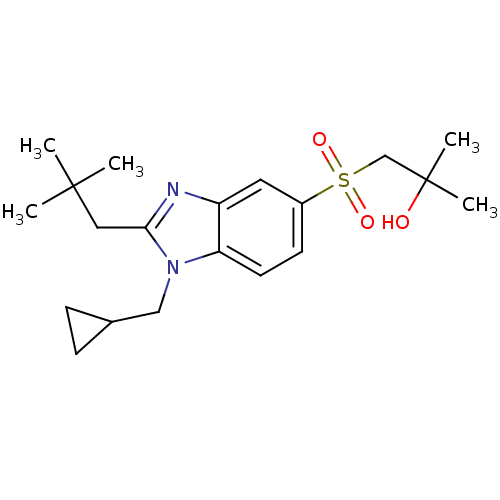
(CHEMBL1800165)Show SMILES CC(C)(C)Cc1nc2cc(ccc2n1CC1CC1)S(=O)(=O)CC(C)(C)O Show InChI InChI=1S/C20H30N2O3S/c1-19(2,3)11-18-21-16-10-15(26(24,25)13-20(4,5)23)8-9-17(16)22(18)12-14-6-7-14/h8-10,14,23H,6-7,11-13H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release |
Bioorg Med Chem Lett 21: 4284-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.063
BindingDB Entry DOI: 10.7270/Q2959HXV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50348120
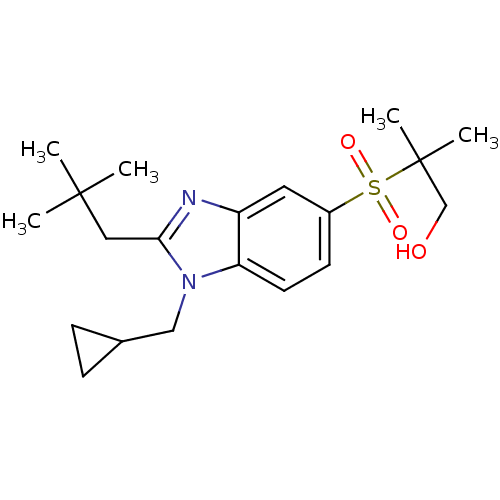
(CHEMBL1800166)Show SMILES CC(C)(C)Cc1nc2cc(ccc2n1CC1CC1)S(=O)(=O)C(C)(C)CO Show InChI InChI=1S/C20H30N2O3S/c1-19(2,3)11-18-21-16-10-15(26(24,25)20(4,5)13-23)8-9-17(16)22(18)12-14-6-7-14/h8-10,14,23H,6-7,11-13H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release |
Bioorg Med Chem Lett 21: 4284-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.063
BindingDB Entry DOI: 10.7270/Q2959HXV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50348121
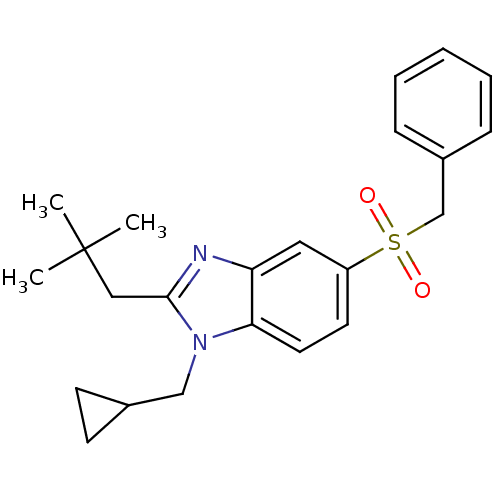
(CHEMBL1800167)Show SMILES CC(C)(C)Cc1nc2cc(ccc2n1CC1CC1)S(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C23H28N2O2S/c1-23(2,3)14-22-24-20-13-19(11-12-21(20)25(22)15-17-9-10-17)28(26,27)16-18-7-5-4-6-8-18/h4-8,11-13,17H,9-10,14-16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release |
Bioorg Med Chem Lett 21: 4284-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.063
BindingDB Entry DOI: 10.7270/Q2959HXV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50348122

(CHEMBL1800168)Show InChI InChI=1S/C17H24N2O2S/c1-5-22(20,21)13-8-9-15-14(10-13)18-16(17(2,3)4)19(15)11-12-6-7-12/h8-10,12H,5-7,11H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release |
Bioorg Med Chem Lett 21: 4284-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.063
BindingDB Entry DOI: 10.7270/Q2959HXV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50348115

(CHEMBL1800654)Show SMILES CC(C)S(=O)(=O)c1ccc2n(CC3CC3)c(CC(C)(C)C)nc2c1 Show InChI InChI=1S/C19H28N2O2S/c1-13(2)24(22,23)15-8-9-17-16(10-15)20-18(11-19(3,4)5)21(17)12-14-6-7-14/h8-10,13-14H,6-7,11-12H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release |
Bioorg Med Chem Lett 21: 4284-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.063
BindingDB Entry DOI: 10.7270/Q2959HXV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50348114

(CHEMBL1800653)Show SMILES CN(C)CCn1c(CC(C)(C)C)nc2cc(ccc12)S(=O)(=O)CC(C)(C)O Show InChI InChI=1S/C20H33N3O3S/c1-19(2,3)13-18-21-16-12-15(27(25,26)14-20(4,5)24)8-9-17(16)23(18)11-10-22(6)7/h8-9,12,24H,10-11,13-14H2,1-7H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release |
Bioorg Med Chem Lett 21: 4284-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.063
BindingDB Entry DOI: 10.7270/Q2959HXV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50348123
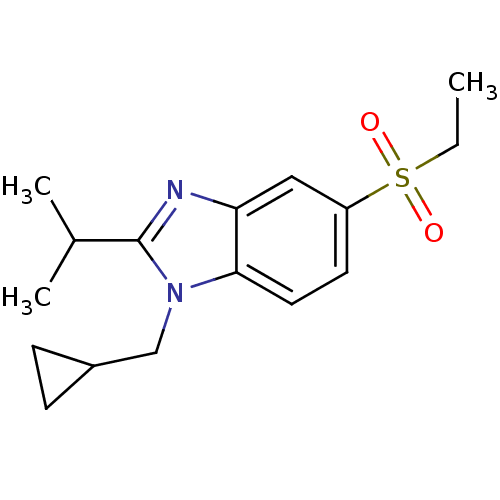
(CHEMBL1800169)Show InChI InChI=1S/C16H22N2O2S/c1-4-21(19,20)13-7-8-15-14(9-13)17-16(11(2)3)18(15)10-12-5-6-12/h7-9,11-12H,4-6,10H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 132 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release |
Bioorg Med Chem Lett 21: 4284-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.063
BindingDB Entry DOI: 10.7270/Q2959HXV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50348124
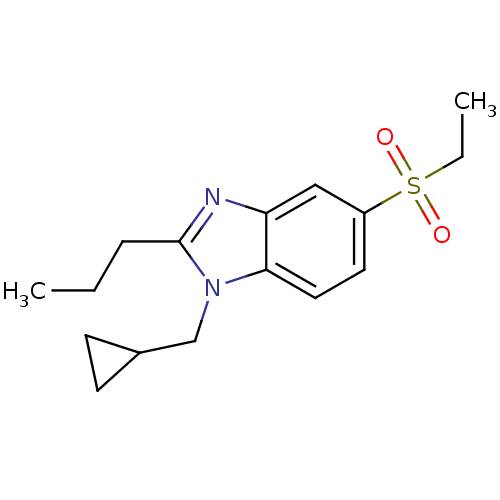
(CHEMBL1800170)Show InChI InChI=1S/C16H22N2O2S/c1-3-5-16-17-14-10-13(21(19,20)4-2)8-9-15(14)18(16)11-12-6-7-12/h8-10,12H,3-7,11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 199 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release |
Bioorg Med Chem Lett 21: 4284-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.063
BindingDB Entry DOI: 10.7270/Q2959HXV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50348125

(CHEMBL1800171)Show InChI InChI=1S/C17H22N2O2S/c1-2-22(20,21)14-7-8-16-15(10-14)18-17(9-12-3-4-12)19(16)11-13-5-6-13/h7-8,10,12-13H,2-6,9,11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 425 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release |
Bioorg Med Chem Lett 21: 4284-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.063
BindingDB Entry DOI: 10.7270/Q2959HXV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50348126
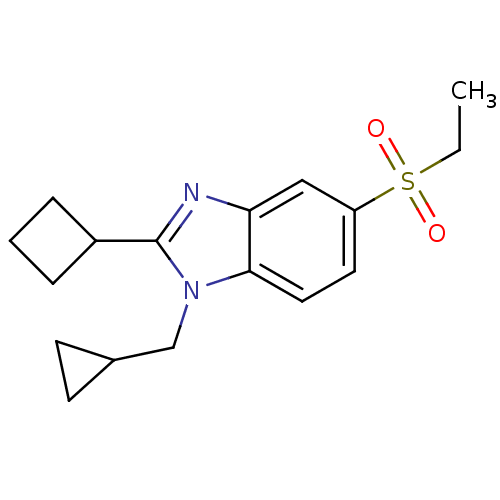
(CHEMBL1800172)Show InChI InChI=1S/C17H22N2O2S/c1-2-22(20,21)14-8-9-16-15(10-14)18-17(13-4-3-5-13)19(16)11-12-6-7-12/h8-10,12-13H,2-7,11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 82 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release |
Bioorg Med Chem Lett 21: 4284-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.063
BindingDB Entry DOI: 10.7270/Q2959HXV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50348127
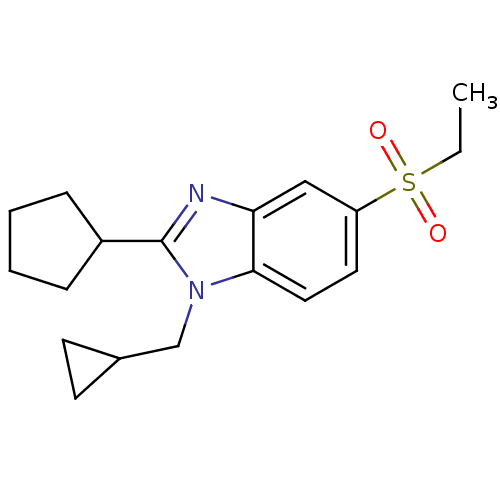
(CHEMBL1800173)Show InChI InChI=1S/C18H24N2O2S/c1-2-23(21,22)15-9-10-17-16(11-15)19-18(14-5-3-4-6-14)20(17)12-13-7-8-13/h9-11,13-14H,2-8,12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in CHO cells assessed as inhibition forskolin-induced cAMP release |
Bioorg Med Chem Lett 21: 4284-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.063
BindingDB Entry DOI: 10.7270/Q2959HXV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data