 Found 148 hits with Last Name = 'mavandadi' and Initial = 'f'
Found 148 hits with Last Name = 'mavandadi' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Pneumocystis carinii) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of Pneumocystis carinii (pc) Dihydrofolate reductase |
J Med Chem 38: 2158-65 (1995)
BindingDB Entry DOI: 10.7270/Q2DB80V1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Pneumocystis carinii) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition against Dihydrofolate reductase in Pneumocystis carinii |
J Med Chem 40: 1173-7 (1997)
Article DOI: 10.1021/jm960717q
BindingDB Entry DOI: 10.7270/Q2QR4W74 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM18224

(6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...)Show InChI InChI=1S/C17H19N5O2/c1-9-11(6-10-7-12(23-2)4-5-13(10)24-3)8-20-16-14(9)15(18)21-17(19)22-16/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition against Dihydrofolate reductase in rat liver |
J Med Chem 40: 1173-7 (1997)
Article DOI: 10.1021/jm960717q
BindingDB Entry DOI: 10.7270/Q2QR4W74 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM18224

(6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...)Show InChI InChI=1S/C17H19N5O2/c1-9-11(6-10-7-12(23-2)4-5-13(10)24-3)8-20-16-14(9)15(18)21-17(19)22-16/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of rat liver Dihydrofolate reductase |
J Med Chem 38: 2158-65 (1995)
BindingDB Entry DOI: 10.7270/Q2DB80V1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of rat liver Dihydrofolate reductase |
J Med Chem 38: 2158-65 (1995)
BindingDB Entry DOI: 10.7270/Q2DB80V1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition against Dihydrofolate reductase in rat liver |
J Med Chem 40: 1173-7 (1997)
Article DOI: 10.1021/jm960717q
BindingDB Entry DOI: 10.7270/Q2QR4W74 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition against Dihydrofolate reductase in rat liver |
J Med Chem 40: 1173-7 (1997)
Article DOI: 10.1021/jm960717q
BindingDB Entry DOI: 10.7270/Q2QR4W74 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of rat liver Dihydrofolate reductase |
J Med Chem 38: 2158-65 (1995)
BindingDB Entry DOI: 10.7270/Q2DB80V1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of Toxoplasma gondii (tc) Dihydrofolate reductase |
J Med Chem 38: 2158-65 (1995)
BindingDB Entry DOI: 10.7270/Q2DB80V1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM18224

(6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...)Show InChI InChI=1S/C17H19N5O2/c1-9-11(6-10-7-12(23-2)4-5-13(10)24-3)8-20-16-14(9)15(18)21-17(19)22-16/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of Toxoplasma gondii (tc) Dihydrofolate reductase |
J Med Chem 38: 2158-65 (1995)
BindingDB Entry DOI: 10.7270/Q2DB80V1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM18224

(6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...)Show InChI InChI=1S/C17H19N5O2/c1-9-11(6-10-7-12(23-2)4-5-13(10)24-3)8-20-16-14(9)15(18)21-17(19)22-16/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition against Dihydrofolate reductase in Toxoplasma gondii |
J Med Chem 40: 1173-7 (1997)
Article DOI: 10.1021/jm960717q
BindingDB Entry DOI: 10.7270/Q2QR4W74 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition against Dihydrofolate reductase in Toxoplasma gondii |
J Med Chem 40: 1173-7 (1997)
Article DOI: 10.1021/jm960717q
BindingDB Entry DOI: 10.7270/Q2QR4W74 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of Toxoplasma gondii (tc) Dihydrofolate reductase |
J Med Chem 38: 2158-65 (1995)
BindingDB Entry DOI: 10.7270/Q2DB80V1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition against Dihydrofolate reductase in Toxoplasma gondii |
J Med Chem 40: 1173-7 (1997)
Article DOI: 10.1021/jm960717q
BindingDB Entry DOI: 10.7270/Q2QR4W74 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50078072
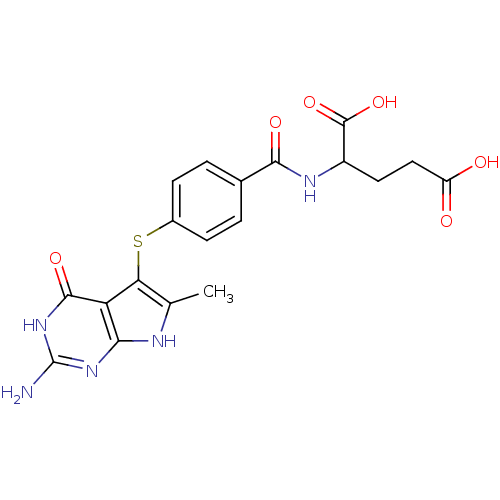
((S)-4-(4-(2-amino-6-methyl-4-oxo-4,7-dihydro-3H-py...)Show SMILES Cc1[nH]c2nc(N)[nH]c(=O)c2c1Sc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C19H19N5O6S/c1-8-14(13-15(21-8)23-19(20)24-17(13)28)31-10-4-2-9(3-5-10)16(27)22-11(18(29)30)6-7-12(25)26/h2-5,11H,6-7H2,1H3,(H,22,27)(H,25,26)(H,29,30)(H4,20,21,23,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition at a given concentration against Lactobacillus casei Thymidylate synthase |
J Med Chem 42: 2272-9 (1999)
Article DOI: 10.1021/jm980586o
BindingDB Entry DOI: 10.7270/Q29Z9421 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18771

((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...)Show SMILES Nc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C24H23N5O6/c1-2-11-29(13-14-3-8-18-17(12-14)22(33)28-24(25)27-18)16-6-4-15(5-7-16)21(32)26-19(23(34)35)9-10-20(30)31/h1,3-8,12,19H,9-11,13H2,(H,26,32)(H,30,31)(H,34,35)(H3,25,27,28,33)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition at a given concentration against recombinant human TS |
J Med Chem 42: 2272-9 (1999)
Article DOI: 10.1021/jm980586o
BindingDB Entry DOI: 10.7270/Q29Z9421 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18771

((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...)Show SMILES Nc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C24H23N5O6/c1-2-11-29(13-14-3-8-18-17(12-14)22(33)28-24(25)27-18)16-6-4-15(5-7-16)21(32)26-19(23(34)35)9-10-20(30)31/h1,3-8,12,19H,9-11,13H2,(H,26,32)(H,30,31)(H,34,35)(H3,25,27,28,33)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition at a given concentration against Lactobacillus casei TS |
J Med Chem 42: 2272-9 (1999)
Article DOI: 10.1021/jm980586o
BindingDB Entry DOI: 10.7270/Q29Z9421 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Pneumocystis carinii) | BDBM50035269

(2-{4-[(2,4-Diamino-7H-pyrrolo[2,3-d]pyrimidin-5-yl...)Show SMILES Nc1nc(N)c2c(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)c[nH]c2n1 Show InChI InChI=1S/C19H21N7O5/c20-15-14-10(8-23-16(14)26-19(21)25-15)7-22-11-3-1-9(2-4-11)17(29)24-12(18(30)31)5-6-13(27)28/h1-4,8,12,22H,5-7H2,(H,24,29)(H,27,28)(H,30,31)(H5,20,21,23,25,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of Pneumocystis carinii (pc) Dihydrofolate reductase |
J Med Chem 38: 2158-65 (1995)
BindingDB Entry DOI: 10.7270/Q2DB80V1 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Pneumocystis carinii) | BDBM50035269

(2-{4-[(2,4-Diamino-7H-pyrrolo[2,3-d]pyrimidin-5-yl...)Show SMILES Nc1nc(N)c2c(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)c[nH]c2n1 Show InChI InChI=1S/C19H21N7O5/c20-15-14-10(8-23-16(14)26-19(21)25-15)7-22-11-3-1-9(2-4-11)17(29)24-12(18(30)31)5-6-13(27)28/h1-4,8,12,22H,5-7H2,(H,24,29)(H,27,28)(H,30,31)(H5,20,21,23,25,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition against Dihydrofolate reductase in Pneumocystis carinii |
J Med Chem 40: 1173-7 (1997)
Article DOI: 10.1021/jm960717q
BindingDB Entry DOI: 10.7270/Q2QR4W74 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Pneumocystis carinii) | BDBM18224

(6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...)Show InChI InChI=1S/C17H19N5O2/c1-9-11(6-10-7-12(23-2)4-5-13(10)24-3)8-20-16-14(9)15(18)21-17(19)22-16/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,21,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of Pneumocystis carinii (pc) Dihydrofolate reductase |
J Med Chem 38: 2158-65 (1995)
BindingDB Entry DOI: 10.7270/Q2DB80V1 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Pneumocystis carinii) | BDBM18224

(6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...)Show InChI InChI=1S/C17H19N5O2/c1-9-11(6-10-7-12(23-2)4-5-13(10)24-3)8-20-16-14(9)15(18)21-17(19)22-16/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,21,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition against Dihydrofolate reductase in Pneumocystis carinii |
J Med Chem 40: 1173-7 (1997)
Article DOI: 10.1021/jm960717q
BindingDB Entry DOI: 10.7270/Q2QR4W74 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50078072

((S)-4-(4-(2-amino-6-methyl-4-oxo-4,7-dihydro-3H-py...)Show SMILES Cc1[nH]c2nc(N)[nH]c(=O)c2c1Sc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C19H19N5O6S/c1-8-14(13-15(21-8)23-19(20)24-17(13)28)31-10-4-2-9(3-5-10)16(27)22-11(18(29)30)6-7-12(25)26/h2-5,11H,6-7H2,1H3,(H,22,27)(H,25,26)(H,29,30)(H4,20,21,23,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition at a given concentration against recombinant human Thymidylate synthase |
J Med Chem 42: 2272-9 (1999)
Article DOI: 10.1021/jm980586o
BindingDB Entry DOI: 10.7270/Q29Z9421 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Pneumocystis carinii) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of Pneumocystis carinii (pc) Dihydrofolate reductase |
J Med Chem 38: 2158-65 (1995)
BindingDB Entry DOI: 10.7270/Q2DB80V1 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Pneumocystis carinii) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition against Dihydrofolate reductase in Pneumocystis carinii |
J Med Chem 40: 1173-7 (1997)
Article DOI: 10.1021/jm960717q
BindingDB Entry DOI: 10.7270/Q2QR4W74 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM50035269

(2-{4-[(2,4-Diamino-7H-pyrrolo[2,3-d]pyrimidin-5-yl...)Show SMILES Nc1nc(N)c2c(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)c[nH]c2n1 Show InChI InChI=1S/C19H21N7O5/c20-15-14-10(8-23-16(14)26-19(21)25-15)7-22-11-3-1-9(2-4-11)17(29)24-12(18(30)31)5-6-13(27)28/h1-4,8,12,22H,5-7H2,(H,24,29)(H,27,28)(H,30,31)(H5,20,21,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition against Dihydrofolate reductase in rat liver |
J Med Chem 40: 1173-7 (1997)
Article DOI: 10.1021/jm960717q
BindingDB Entry DOI: 10.7270/Q2QR4W74 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM50035269

(2-{4-[(2,4-Diamino-7H-pyrrolo[2,3-d]pyrimidin-5-yl...)Show SMILES Nc1nc(N)c2c(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)c[nH]c2n1 Show InChI InChI=1S/C19H21N7O5/c20-15-14-10(8-23-16(14)26-19(21)25-15)7-22-11-3-1-9(2-4-11)17(29)24-12(18(30)31)5-6-13(27)28/h1-4,8,12,22H,5-7H2,(H,24,29)(H,27,28)(H,30,31)(H5,20,21,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of rat liver Dihydrofolate reductase |
J Med Chem 38: 2158-65 (1995)
BindingDB Entry DOI: 10.7270/Q2DB80V1 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Pneumocystis carinii) | BDBM50057314
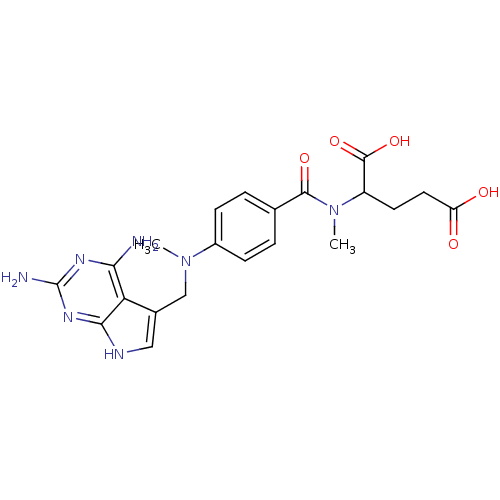
(2-({4-[(2,4-Diamino-7H-pyrrolo[2,3-d]pyrimidin-5-y...)Show SMILES CN(Cc1c[nH]c2nc(N)nc(N)c12)c1ccc(cc1)C(=O)N(C)C(CCC(O)=O)C(O)=O Show InChI InChI=1S/C21H25N7O5/c1-27(10-12-9-24-18-16(12)17(22)25-21(23)26-18)13-5-3-11(4-6-13)19(31)28(2)14(20(32)33)7-8-15(29)30/h3-6,9,14H,7-8,10H2,1-2H3,(H,29,30)(H,32,33)(H5,22,23,24,25,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition against Dihydrofolate reductase in Pneumocystis carinii |
J Med Chem 40: 1173-7 (1997)
Article DOI: 10.1021/jm960717q
BindingDB Entry DOI: 10.7270/Q2QR4W74 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM50057314

(2-({4-[(2,4-Diamino-7H-pyrrolo[2,3-d]pyrimidin-5-y...)Show SMILES CN(Cc1c[nH]c2nc(N)nc(N)c12)c1ccc(cc1)C(=O)N(C)C(CCC(O)=O)C(O)=O Show InChI InChI=1S/C21H25N7O5/c1-27(10-12-9-24-18-16(12)17(22)25-21(23)26-18)13-5-3-11(4-6-13)19(31)28(2)14(20(32)33)7-8-15(29)30/h3-6,9,14H,7-8,10H2,1-2H3,(H,29,30)(H,32,33)(H5,22,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition against Dihydrofolate reductase in rat liver |
J Med Chem 40: 1173-7 (1997)
Article DOI: 10.1021/jm960717q
BindingDB Entry DOI: 10.7270/Q2QR4W74 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18808
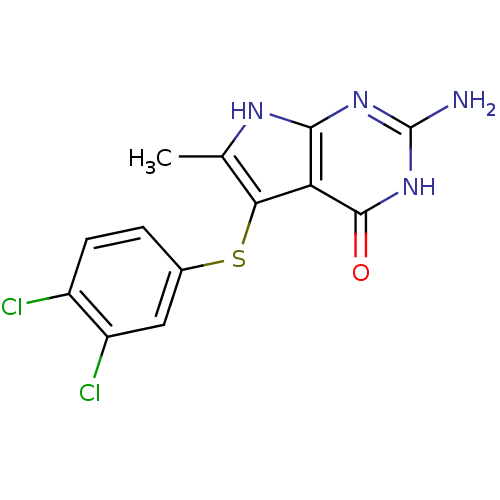
(2-amino-5-[(3,4-dichlorophenyl)sulfanyl]-6-methyl-...)Show SMILES Cc1[nH]c2nc(N)[nH]c(=O)c2c1Sc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C13H10Cl2N4OS/c1-5-10(21-6-2-3-7(14)8(15)4-6)9-11(17-5)18-13(16)19-12(9)20/h2-4H,1H3,(H4,16,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
The inhibitory concentration of compound was evaluated on Human Thymidylate synthase |
J Med Chem 39: 4563-8 (1996)
Article DOI: 10.1021/jm960097t
BindingDB Entry DOI: 10.7270/Q27W6CVS |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18807
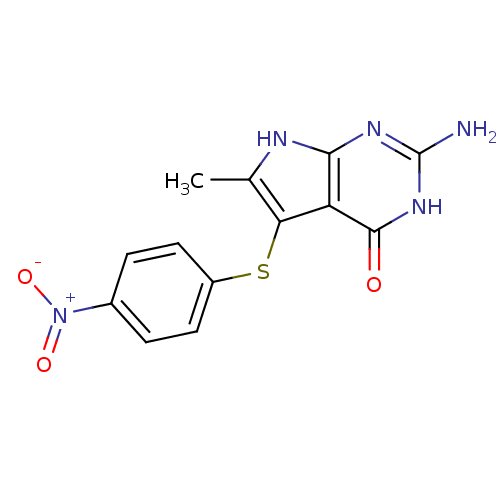
(2-amino-6-methyl-5-[(4-nitrophenyl)sulfanyl]-3H,4H...)Show SMILES Cc1[nH]c2nc(N)[nH]c(=O)c2c1Sc1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C13H11N5O3S/c1-6-10(9-11(15-6)16-13(14)17-12(9)19)22-8-4-2-7(3-5-8)18(20)21/h2-5H,1H3,(H4,14,15,16,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
The inhibitory concentration of compound was evaluated on Human Thymidylate synthase |
J Med Chem 39: 4563-8 (1996)
Article DOI: 10.1021/jm960097t
BindingDB Entry DOI: 10.7270/Q27W6CVS |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50057314

(2-({4-[(2,4-Diamino-7H-pyrrolo[2,3-d]pyrimidin-5-y...)Show SMILES CN(Cc1c[nH]c2nc(N)nc(N)c12)c1ccc(cc1)C(=O)N(C)C(CCC(O)=O)C(O)=O Show InChI InChI=1S/C21H25N7O5/c1-27(10-12-9-24-18-16(12)17(22)25-21(23)26-18)13-5-3-11(4-6-13)19(31)28(2)14(20(32)33)7-8-15(29)30/h3-6,9,14H,7-8,10H2,1-2H3,(H,29,30)(H,32,33)(H5,22,23,24,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition against Dihydrofolate reductase in Toxoplasma gondii |
J Med Chem 40: 1173-7 (1997)
Article DOI: 10.1021/jm960717q
BindingDB Entry DOI: 10.7270/Q2QR4W74 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50035269

(2-{4-[(2,4-Diamino-7H-pyrrolo[2,3-d]pyrimidin-5-yl...)Show SMILES Nc1nc(N)c2c(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)c[nH]c2n1 Show InChI InChI=1S/C19H21N7O5/c20-15-14-10(8-23-16(14)26-19(21)25-15)7-22-11-3-1-9(2-4-11)17(29)24-12(18(30)31)5-6-13(27)28/h1-4,8,12,22H,5-7H2,(H,24,29)(H,27,28)(H,30,31)(H5,20,21,23,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition against Dihydrofolate reductase in Toxoplasma gondii |
J Med Chem 40: 1173-7 (1997)
Article DOI: 10.1021/jm960717q
BindingDB Entry DOI: 10.7270/Q2QR4W74 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50035269

(2-{4-[(2,4-Diamino-7H-pyrrolo[2,3-d]pyrimidin-5-yl...)Show SMILES Nc1nc(N)c2c(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)c[nH]c2n1 Show InChI InChI=1S/C19H21N7O5/c20-15-14-10(8-23-16(14)26-19(21)25-15)7-22-11-3-1-9(2-4-11)17(29)24-12(18(30)31)5-6-13(27)28/h1-4,8,12,22H,5-7H2,(H,24,29)(H,27,28)(H,30,31)(H5,20,21,23,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of Toxoplasma gondii (tc) Dihydrofolate reductase |
J Med Chem 38: 2158-65 (1995)
BindingDB Entry DOI: 10.7270/Q2DB80V1 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50054514

(2-Amino-6-methyl-5-(pyridin-4-ylsulfanyl)-3,7-dihy...)Show InChI InChI=1S/C12H11N5OS/c1-6-9(19-7-2-4-14-5-3-7)8-10(15-6)16-12(13)17-11(8)18/h2-5H,1H3,(H4,13,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
The inhibitory concentration of compound was evaluated on Human Thymidylate synthase |
J Med Chem 39: 4563-8 (1996)
Article DOI: 10.1021/jm960097t
BindingDB Entry DOI: 10.7270/Q27W6CVS |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50054514

(2-Amino-6-methyl-5-(pyridin-4-ylsulfanyl)-3,7-dihy...)Show InChI InChI=1S/C12H11N5OS/c1-6-9(19-7-2-4-14-5-3-7)8-10(15-6)16-12(13)17-11(8)18/h2-5H,1H3,(H4,13,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition at a given concentration against recombinant human Thymidylate synthase |
J Med Chem 42: 2272-9 (1999)
Article DOI: 10.1021/jm980586o
BindingDB Entry DOI: 10.7270/Q29Z9421 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18795
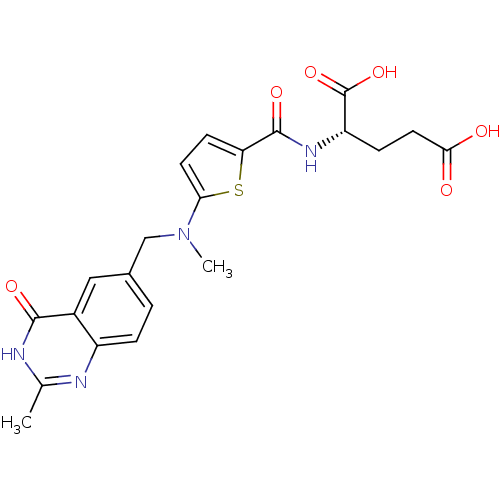
((2S)-2-[(5-{methyl[(2-methyl-4-oxo-1,4-dihydroquin...)Show SMILES CN(Cc1ccc2nc(C)[nH]c(=O)c2c1)c1ccc(s1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C21H22N4O6S/c1-11-22-14-4-3-12(9-13(14)19(28)23-11)10-25(2)17-7-6-16(32-17)20(29)24-15(21(30)31)5-8-18(26)27/h3-4,6-7,9,15H,5,8,10H2,1-2H3,(H,24,29)(H,26,27)(H,30,31)(H,22,23,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
The inhibitory concentration of compound was evaluated on Pneumocystis carini Thymidylate synthase |
J Med Chem 39: 4563-8 (1996)
Article DOI: 10.1021/jm960097t
BindingDB Entry DOI: 10.7270/Q27W6CVS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50057315
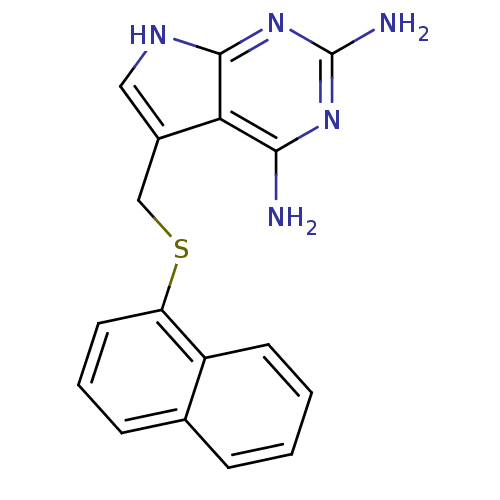
(5-(Naphthalen-1-ylsulfanylmethyl)-7H-pyrrolo[2,3-d...)Show InChI InChI=1S/C17H15N5S/c18-15-14-11(8-20-16(14)22-17(19)21-15)9-23-13-7-3-5-10-4-1-2-6-12(10)13/h1-8H,9H2,(H5,18,19,20,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition against Dihydrofolate reductase in Toxoplasma gondii |
J Med Chem 40: 1173-7 (1997)
Article DOI: 10.1021/jm960717q
BindingDB Entry DOI: 10.7270/Q2QR4W74 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50057312
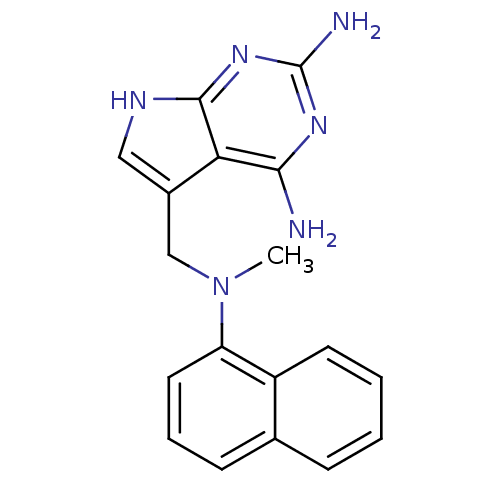
(5-[(Methyl-naphthalen-1-yl-amino)-methyl]-7H-pyrro...)Show InChI InChI=1S/C18H18N6/c1-24(14-8-4-6-11-5-2-3-7-13(11)14)10-12-9-21-17-15(12)16(19)22-18(20)23-17/h2-9H,10H2,1H3,(H5,19,20,21,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition against Dihydrofolate reductase in Toxoplasma gondii |
J Med Chem 40: 1173-7 (1997)
Article DOI: 10.1021/jm960717q
BindingDB Entry DOI: 10.7270/Q2QR4W74 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18795

((2S)-2-[(5-{methyl[(2-methyl-4-oxo-1,4-dihydroquin...)Show SMILES CN(Cc1ccc2nc(C)[nH]c(=O)c2c1)c1ccc(s1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C21H22N4O6S/c1-11-22-14-4-3-12(9-13(14)19(28)23-11)10-25(2)17-7-6-16(32-17)20(29)24-15(21(30)31)5-8-18(26)27/h3-4,6-7,9,15H,5,8,10H2,1-2H3,(H,24,29)(H,26,27)(H,30,31)(H,22,23,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
The inhibitory concentration of compound was evaluated on Human thymidylate synthase |
J Med Chem 39: 4563-8 (1996)
Article DOI: 10.1021/jm960097t
BindingDB Entry DOI: 10.7270/Q27W6CVS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50057313
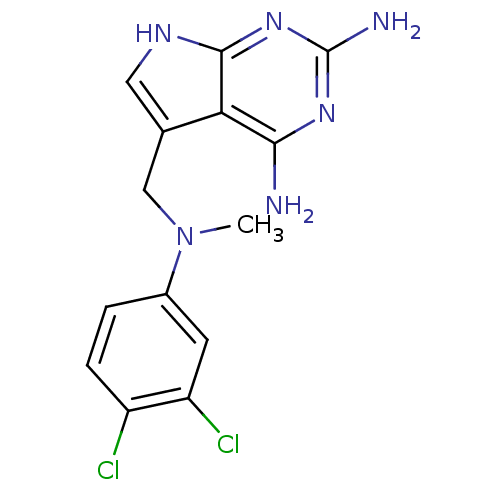
(5-(((3,4-dichlorophenyl)(methyl)amino)methyl)-7H-p...)Show SMILES CN(Cc1c[nH]c2nc(N)nc(N)c12)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C14H14Cl2N6/c1-22(8-2-3-9(15)10(16)4-8)6-7-5-19-13-11(7)12(17)20-14(18)21-13/h2-5H,6H2,1H3,(H5,17,18,19,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition against Dihydrofolate reductase in Toxoplasma gondii |
J Med Chem 40: 1173-7 (1997)
Article DOI: 10.1021/jm960717q
BindingDB Entry DOI: 10.7270/Q2QR4W74 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50054512
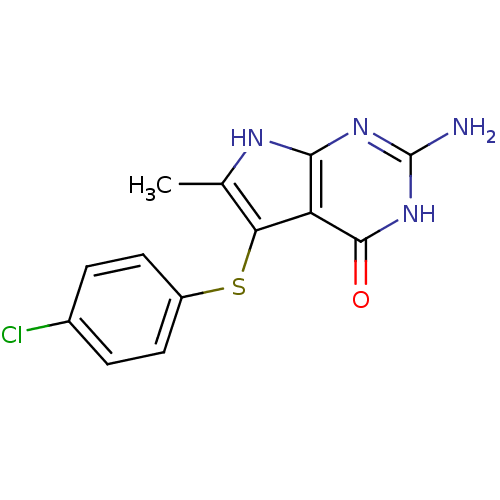
(2-Amino-5-(4-chloro-phenylsulfanyl)-6-methyl-3,7-d...)Show InChI InChI=1S/C13H11ClN4OS/c1-6-10(20-8-4-2-7(14)3-5-8)9-11(16-6)17-13(15)18-12(9)19/h2-5H,1H3,(H4,15,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
The inhibitory concentration of compound was evaluated on Human Thymidylate synthase |
J Med Chem 39: 4563-8 (1996)
Article DOI: 10.1021/jm960097t
BindingDB Entry DOI: 10.7270/Q27W6CVS |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50035274
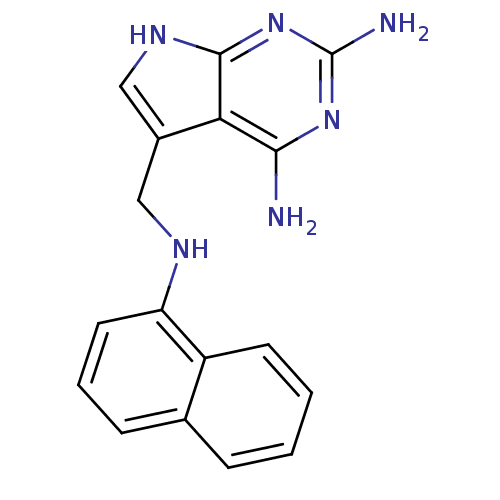
(5-(Naphthalen-1-ylaminomethyl)-7H-pyrrolo[2,3-d]py...)Show InChI InChI=1S/C17H16N6/c18-15-14-11(9-21-16(14)23-17(19)22-15)8-20-13-7-3-5-10-4-1-2-6-12(10)13/h1-7,9,20H,8H2,(H5,18,19,21,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of Toxoplasma gondii (tc) Dihydrofolate reductase |
J Med Chem 38: 2158-65 (1995)
BindingDB Entry DOI: 10.7270/Q2DB80V1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50035274

(5-(Naphthalen-1-ylaminomethyl)-7H-pyrrolo[2,3-d]py...)Show InChI InChI=1S/C17H16N6/c18-15-14-11(9-21-16(14)23-17(19)22-15)8-20-13-7-3-5-10-4-1-2-6-12(10)13/h1-7,9,20H,8H2,(H5,18,19,21,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition against Dihydrofolate reductase in Toxoplasma gondii |
J Med Chem 40: 1173-7 (1997)
Article DOI: 10.1021/jm960717q
BindingDB Entry DOI: 10.7270/Q2QR4W74 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50035273
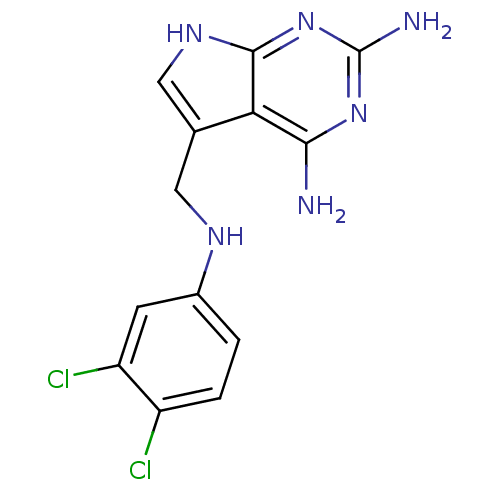
(5-((5,6,7,8-tetrahydronaphthalen-1-ylamino)methyl)...)Show InChI InChI=1S/C13H12Cl2N6/c14-8-2-1-7(3-9(8)15)18-4-6-5-19-12-10(6)11(16)20-13(17)21-12/h1-3,5,18H,4H2,(H5,16,17,19,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of Toxoplasma gondii (tc) Dihydrofolate reductase |
J Med Chem 38: 2158-65 (1995)
BindingDB Entry DOI: 10.7270/Q2DB80V1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50035273

(5-((5,6,7,8-tetrahydronaphthalen-1-ylamino)methyl)...)Show InChI InChI=1S/C13H12Cl2N6/c14-8-2-1-7(3-9(8)15)18-4-6-5-19-12-10(6)11(16)20-13(17)21-12/h1-3,5,18H,4H2,(H5,16,17,19,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition against Dihydrofolate reductase in Toxoplasma gondii |
J Med Chem 40: 1173-7 (1997)
Article DOI: 10.1021/jm960717q
BindingDB Entry DOI: 10.7270/Q2QR4W74 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50035271
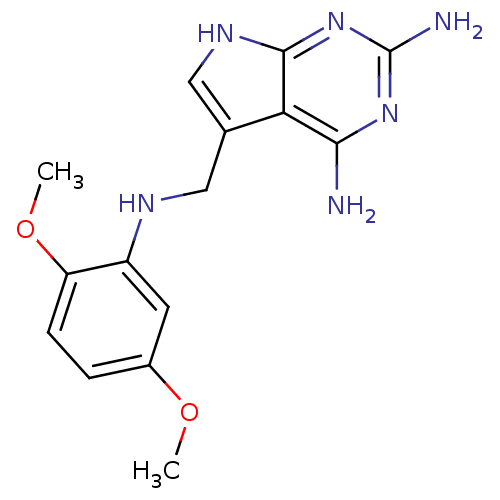
(5-((2,5-dimethoxyphenylamino)methyl)-7H-pyrrolo[2,...)Show InChI InChI=1S/C15H18N6O2/c1-22-9-3-4-11(23-2)10(5-9)18-6-8-7-19-14-12(8)13(16)20-15(17)21-14/h3-5,7,18H,6H2,1-2H3,(H5,16,17,19,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of Toxoplasma gondii (tc) Dihydrofolate reductase |
J Med Chem 38: 2158-65 (1995)
BindingDB Entry DOI: 10.7270/Q2DB80V1 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50035271

(5-((2,5-dimethoxyphenylamino)methyl)-7H-pyrrolo[2,...)Show InChI InChI=1S/C15H18N6O2/c1-22-9-3-4-11(23-2)10(5-9)18-6-8-7-19-14-12(8)13(16)20-15(17)21-14/h3-5,7,18H,6H2,1-2H3,(H5,16,17,19,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition against Dihydrofolate reductase in Toxoplasma gondii |
J Med Chem 40: 1173-7 (1997)
Article DOI: 10.1021/jm960717q
BindingDB Entry DOI: 10.7270/Q2QR4W74 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18807

(2-amino-6-methyl-5-[(4-nitrophenyl)sulfanyl]-3H,4H...)Show SMILES Cc1[nH]c2nc(N)[nH]c(=O)c2c1Sc1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C13H11N5O3S/c1-6-10(9-11(15-6)16-13(14)17-12(9)19)22-8-4-2-7(3-5-8)18(20)21/h2-5H,1H3,(H4,14,15,16,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
The inhibitory concentration of compound was evaluated on Pneumocystis carini Thymidylate synthase |
J Med Chem 39: 4563-8 (1996)
Article DOI: 10.1021/jm960097t
BindingDB Entry DOI: 10.7270/Q27W6CVS |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50054508
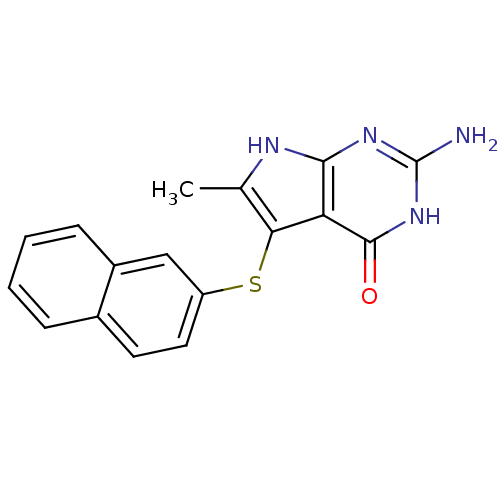
(2-Amino-6-methyl-5-(naphthalen-2-ylsulfanyl)-3,7-d...)Show InChI InChI=1S/C17H14N4OS/c1-9-14(13-15(19-9)20-17(18)21-16(13)22)23-12-7-6-10-4-2-3-5-11(10)8-12/h2-8H,1H3,(H4,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
The inhibitory concentration of compound was evaluated on Human Thymidylate synthase |
J Med Chem 39: 4563-8 (1996)
Article DOI: 10.1021/jm960097t
BindingDB Entry DOI: 10.7270/Q27W6CVS |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18808

(2-amino-5-[(3,4-dichlorophenyl)sulfanyl]-6-methyl-...)Show SMILES Cc1[nH]c2nc(N)[nH]c(=O)c2c1Sc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C13H10Cl2N4OS/c1-5-10(21-6-2-3-7(14)8(15)4-6)9-11(17-5)18-13(16)19-12(9)20/h2-4H,1H3,(H4,16,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
The inhibitory concentration of compound was evaluated on Pneumocystis carini Thymidylate synthase |
J Med Chem 39: 4563-8 (1996)
Article DOI: 10.1021/jm960097t
BindingDB Entry DOI: 10.7270/Q27W6CVS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data