

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM41571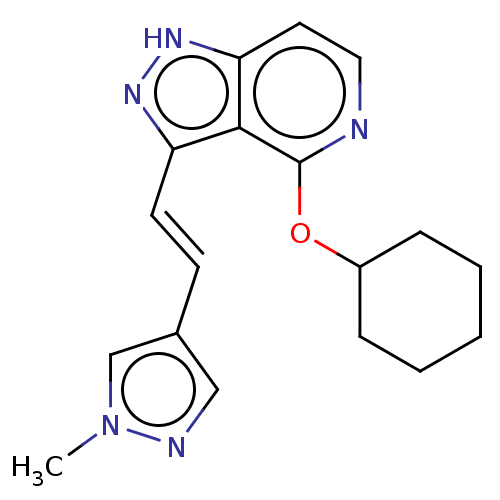 (US8569281, 52) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50507669 (CHEMBL4539170) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged LRRK2 G2019S mutant catalytic domain (970 to 2527 residues) expressed in insect cells using RtGWWRFYTLRRAR... | Bioorg Med Chem Lett 29: 674-680 (2019) Article DOI: 10.1016/j.bmcl.2018.10.017 BindingDB Entry DOI: 10.7270/Q2HH6PCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50507673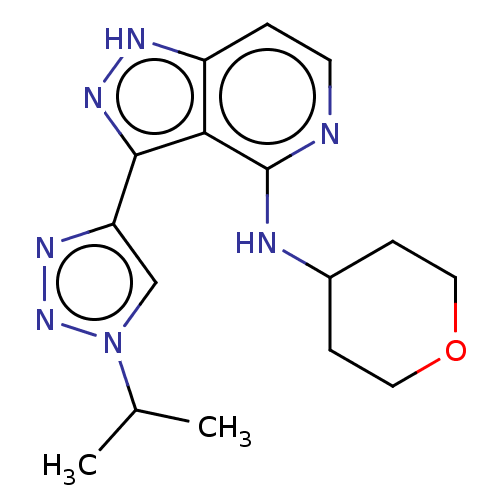 (CHEMBL4441650) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged LRRK2 G2019S mutant catalytic domain (970 to 2527 residues) expressed in insect cells using RtGWWRFYTLRRAR... | Bioorg Med Chem Lett 29: 674-680 (2019) Article DOI: 10.1016/j.bmcl.2018.10.017 BindingDB Entry DOI: 10.7270/Q2HH6PCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105075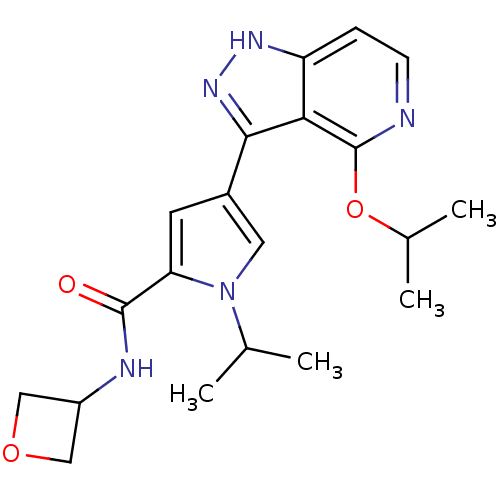 (CHEMBL2152708 | US8569281, 59) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105125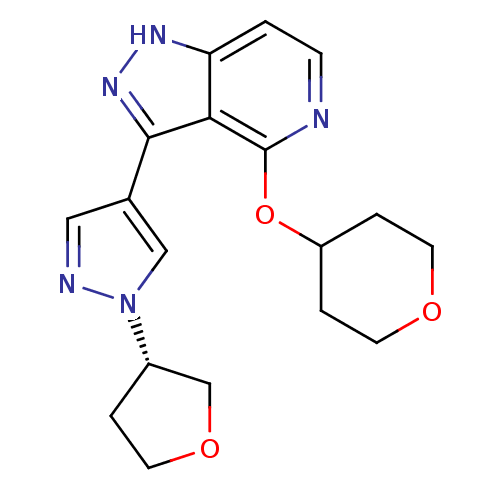 (US8569281, 147) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105100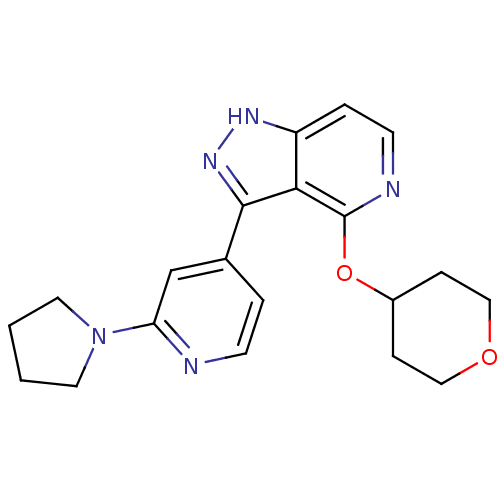 (US8569281, 110) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105080 (US8569281, 70) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50507671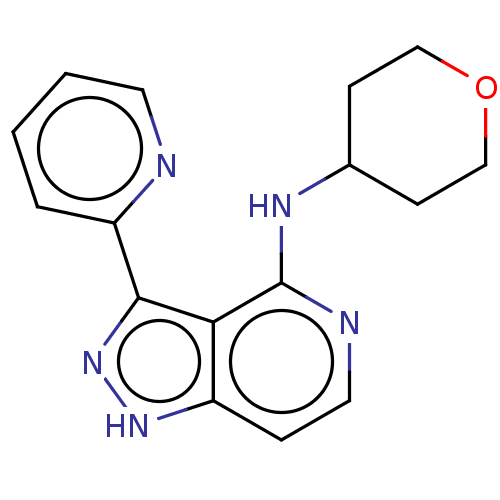 (CHEMBL4435096) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged LRRK2 G2019S mutant catalytic domain (970 to 2527 residues) expressed in insect cells using RtGWWRFYTLRRAR... | Bioorg Med Chem Lett 29: 674-680 (2019) Article DOI: 10.1016/j.bmcl.2018.10.017 BindingDB Entry DOI: 10.7270/Q2HH6PCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50507678 (CHEMBL4560272) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged LRRK2 G2019S mutant catalytic domain (970 to 2527 residues) expressed in insect cells using RtGWWRFYTLRRAR... | Bioorg Med Chem Lett 29: 674-680 (2019) Article DOI: 10.1016/j.bmcl.2018.10.017 BindingDB Entry DOI: 10.7270/Q2HH6PCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50507670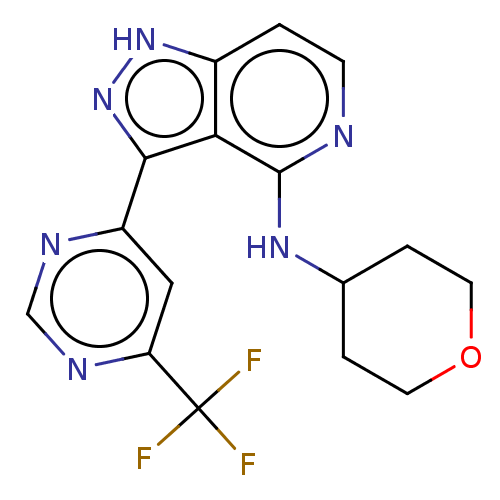 (CHEMBL4527314) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged LRRK2 G2019S mutant catalytic domain (970 to 2527 residues) expressed in insect cells using RtGWWRFYTLRRAR... | Bioorg Med Chem Lett 29: 674-680 (2019) Article DOI: 10.1016/j.bmcl.2018.10.017 BindingDB Entry DOI: 10.7270/Q2HH6PCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50507672 (CHEMBL4460272) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged LRRK2 G2019S mutant catalytic domain (970 to 2527 residues) expressed in insect cells using RtGWWRFYTLRRAR... | Bioorg Med Chem Lett 29: 674-680 (2019) Article DOI: 10.1016/j.bmcl.2018.10.017 BindingDB Entry DOI: 10.7270/Q2HH6PCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105124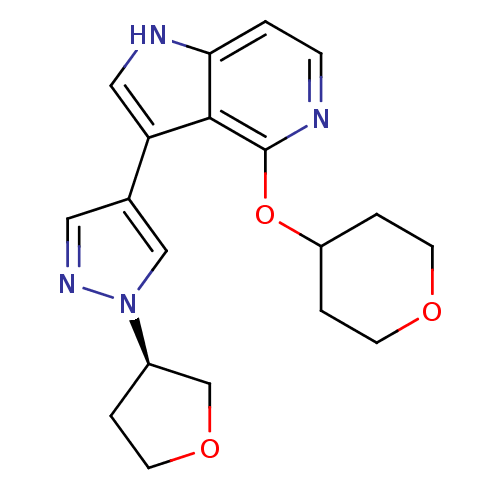 (US8569281, 146) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105092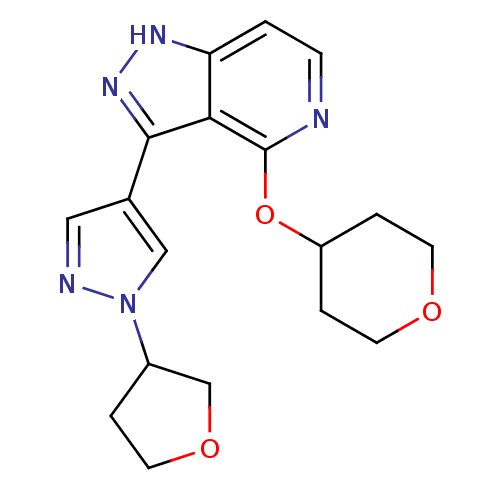 (US8569281, 98) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105093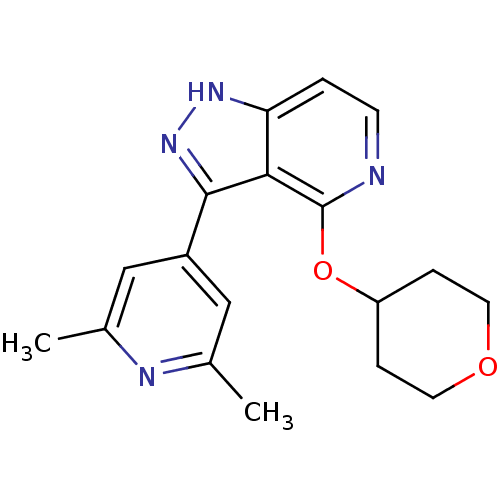 (US8569281, 100) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105115 (US8569281, 132) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105071 (US8569281, 39) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105065 (US8569281, 14) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105176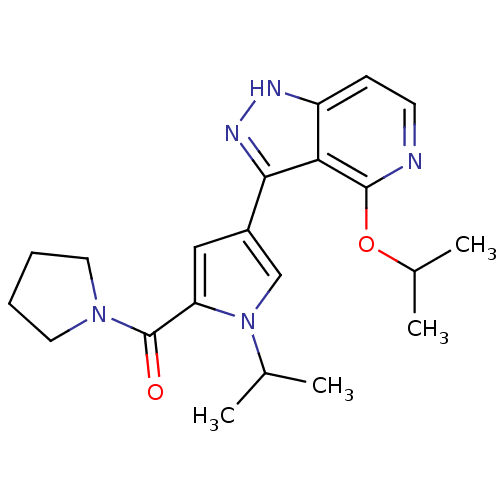 (US8569281, 216) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105101 (US8569281, 111) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105091 (US8569281, 96) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105143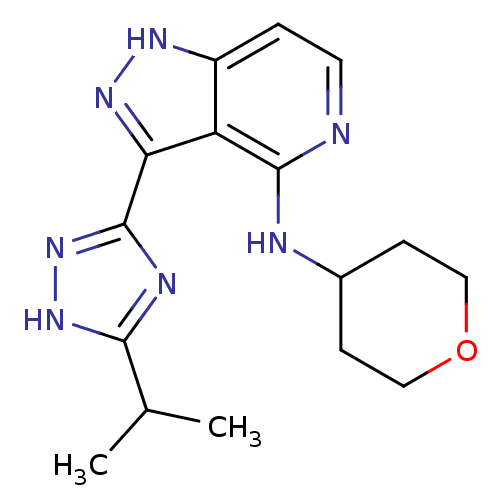 (US8569281, 168) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105148 (US8569281, 175) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105180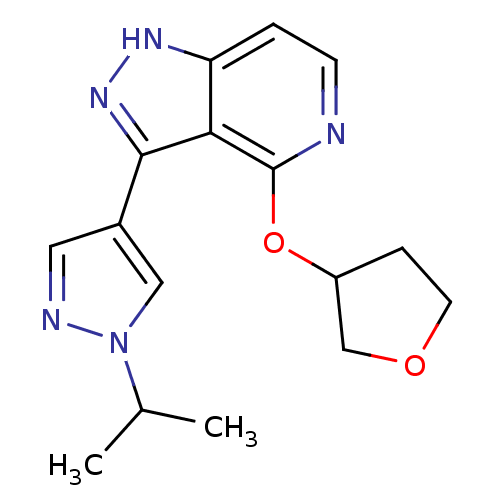 (US8569281, 224) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105143 (US8569281, 168) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged LRRK2 G2019S mutant catalytic domain (970 to 2527 residues) expressed in insect cells using RtGWWRFYTLRRAR... | Bioorg Med Chem Lett 29: 674-680 (2019) Article DOI: 10.1016/j.bmcl.2018.10.017 BindingDB Entry DOI: 10.7270/Q2HH6PCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105177 (US8569281, 218) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105103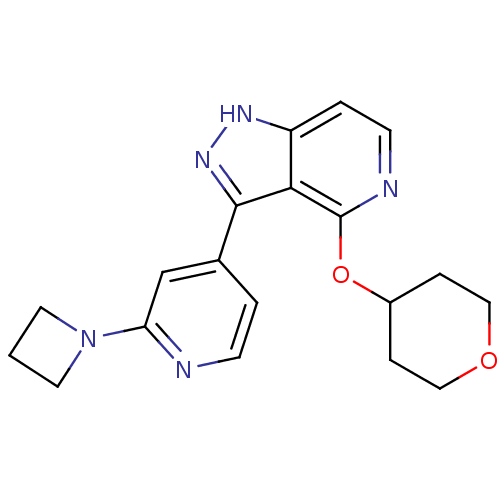 (US8569281, 114) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105174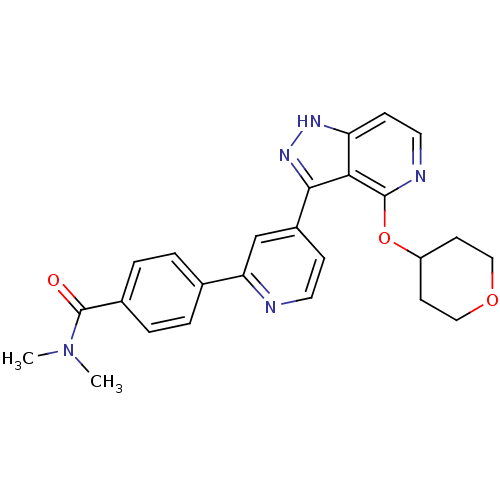 (US8569281, 212) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105163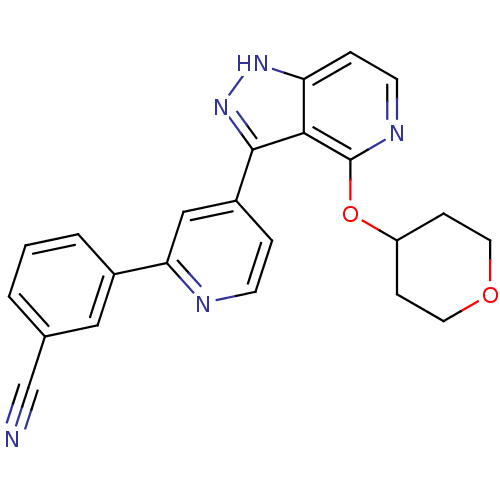 (US8569281, 201) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105104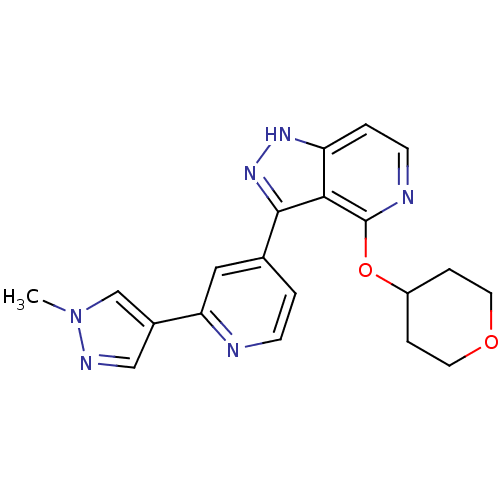 (US8569281, 116) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105171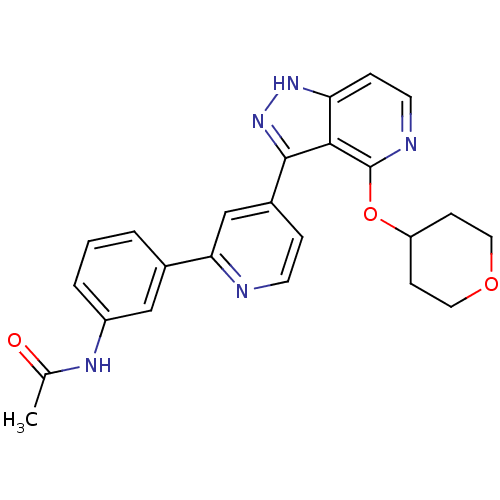 (US8569281, 209) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105114 (US8569281, 129) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105172 (US8569281, 210) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105090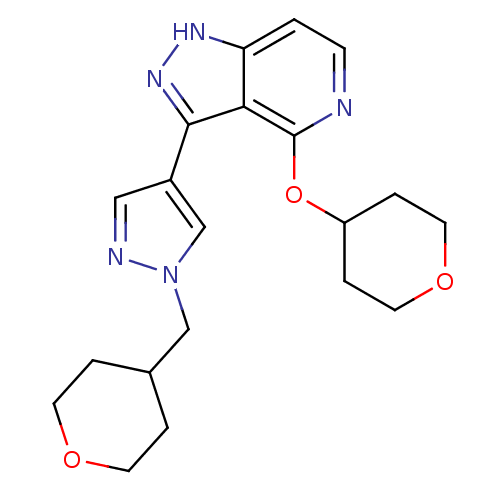 (US8569281, 93) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105128 (US8569281, 150) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105175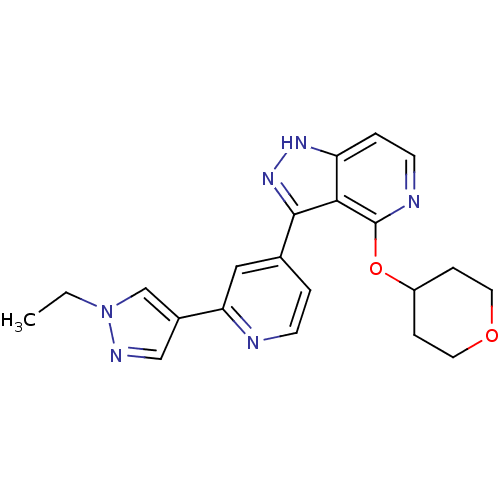 (US8569281, 213) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105166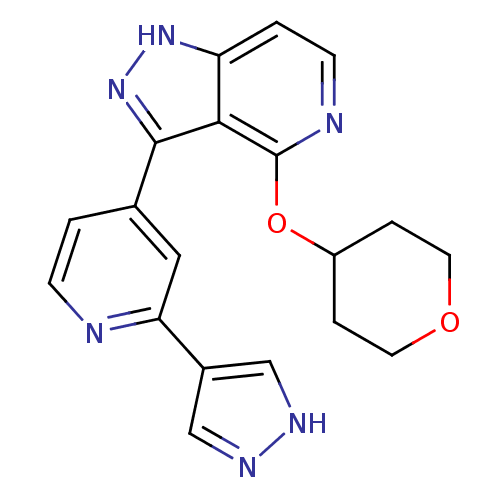 (US8569281, 204) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105084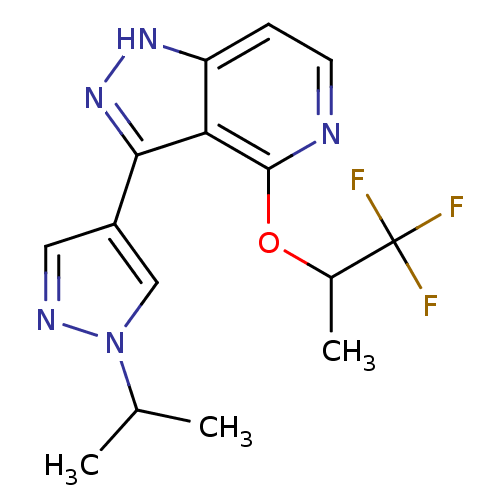 (US8569281, 78) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105118 (US8569281, 138) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105158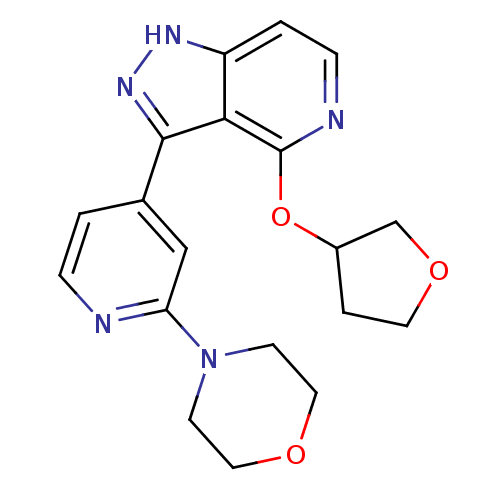 (US8569281, 190) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105156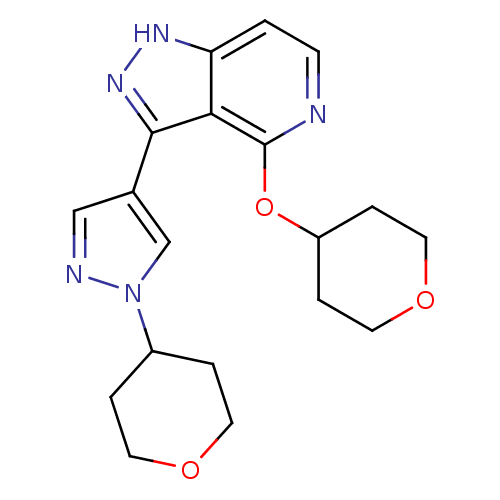 (US8569281, 186) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105120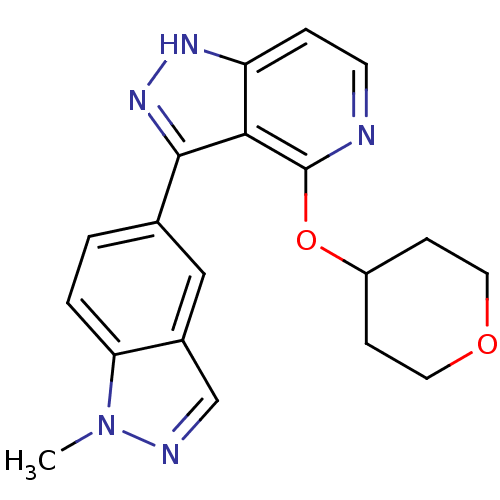 (US8569281, 140) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105119 (US8569281, 139) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105077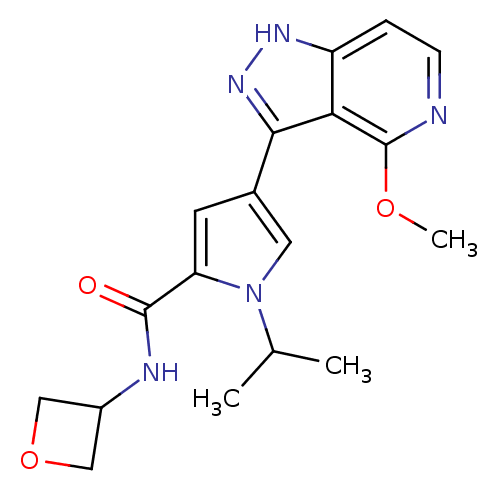 (US8569281, 64) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105062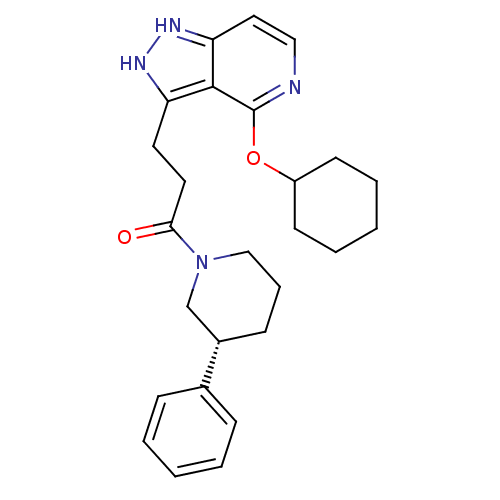 (US8569281, 6) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105173 (US8569281, 211) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105150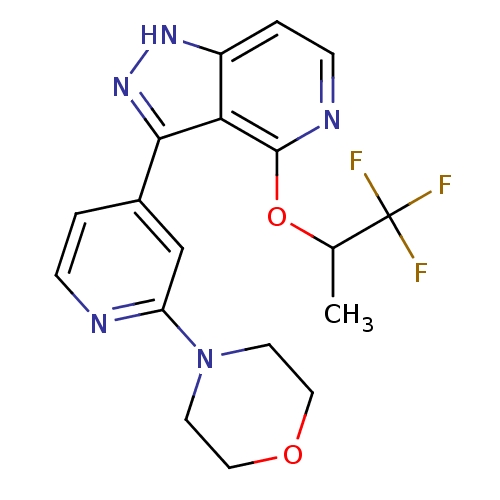 (US8569281, 177) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105063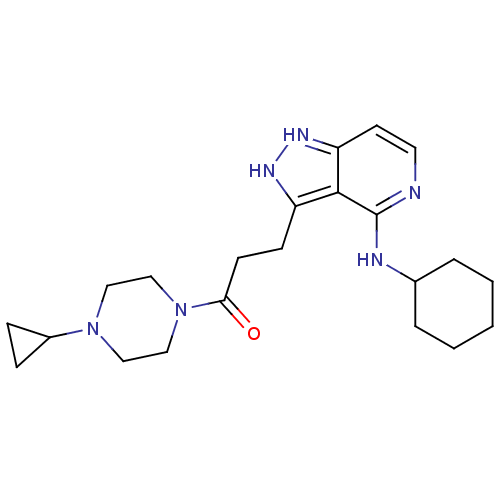 (US8569281, 8) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105129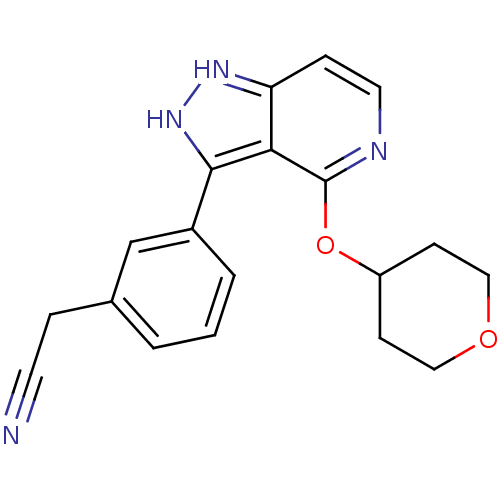 (US8569281, 151) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105073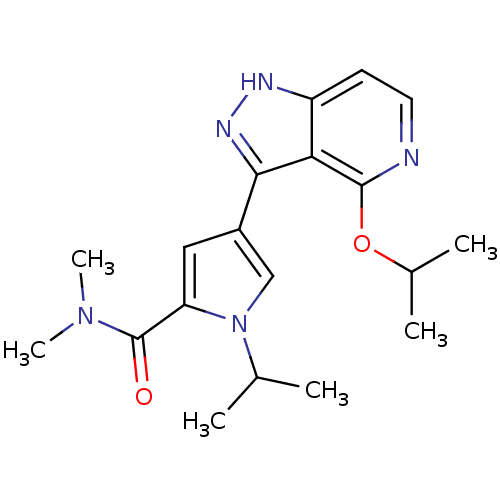 (US8569281, 54) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM105165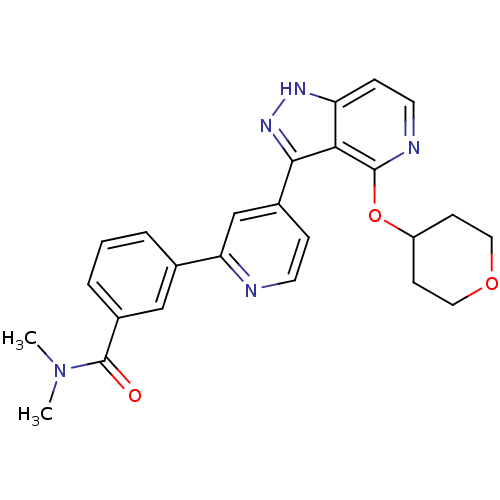 (US8569281, 203) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical Research Council Technology; Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining Kiapp, IC50 or percent inhibition values. | US Patent US8569281 (2013) BindingDB Entry DOI: 10.7270/Q2DN43PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 421 total ) | Next | Last >> |