 Found 1167 hits with Last Name = 'mehta' and Initial = 's'
Found 1167 hits with Last Name = 'mehta' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Toll-like receptor 2
(Homo sapiens (Human)) | BDBM50406601

(CHEMBL5276854)Show SMILES CC(C)c1nc(c(C)c(-c2ccc(F)cc2)c1CCP(O)([O-])CC(=O)CC([O-])=O)-c1ccccc1 Show InChI InChI=1S/C27H30FNO5P/c1-17(2)26-23(13-14-35(33,34)16-22(30)15-24(31)32)25(19-9-11-21(28)12-10-19)18(3)27(29-26)20-7-5-4-6-8-20/h4-12,17,33,35H,13-16H2,1-3H3,(H,31,32)/q-1/p-1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Negative log concentration of antagonist was determined on 5-hydroxytryptamine 2B receptor of Rat stomach fundus |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Toll-like receptor 2
(Homo sapiens (Human)) | BDBM50406600
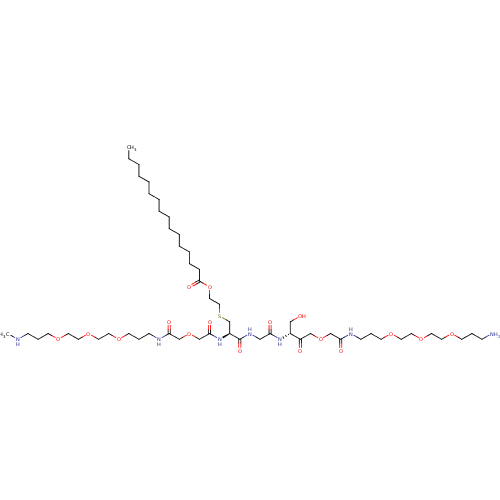
(CHEMBL5267159)Show SMILES CC(C)c1nc(c(C)c(-c2ccc(F)cc2)c1CCP(O)([O-])=CC(=O)CC([O-])=O)-c1ccccc1 Show InChI InChI=1S/C27H29FNO5P/c1-17(2)26-23(13-14-35(33,34)16-22(30)15-24(31)32)25(19-9-11-21(28)12-10-19)18(3)27(29-26)20-7-5-4-6-8-20/h4-12,16-17H,13-15H2,1-3H3,(H3,30,31,32,33,34)/p-2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Negative log concentration of antagonist was determined on 5-hydroxytryptamine 2B receptor of Rat stomach fundus |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Toll-like receptor 2
(Homo sapiens (Human)) | BDBM50406599

(CHEMBL5276410)Show SMILES Cc1c(nc(C2CC2)c(C=CP(O)([O-])=CC(=O)CC([O-])=O)c1-c1ccc(F)cc1)-c1ccccc1 |w:10.11| Show InChI InChI=1S/C27H25FNO5P/c1-17-25(18-9-11-21(28)12-10-18)23(13-14-35(33,34)16-22(30)15-24(31)32)27(20-7-8-20)29-26(17)19-5-3-2-4-6-19/h2-6,9-14,16,20H,7-8,15H2,1H3,(H3,30,31,32,33,34)/p-2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Negative log concentration of antagonistic compound was determined on 5-hydroxytryptamine 2B receptor of Rat stomach fundus |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Frizzled-7
(Homo sapiens) | BDBM50519340

(CHEMBL4435748)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(C)=O)[C@H](C)O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(C)=O)[C@H](C)O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |r| Show InChI InChI=1S/C144H212N34O38S4/c1-19-75(11)117(141(213)159-95(43-47-217-17)125(197)161-97(121(147)193)53-81-35-39-87(183)40-36-81)177-137(209)105(57-85-65-149-69-153-85)169-139(211)111(175-131(203)103(55-83-63-151-91-31-23-21-29-89(83)91)167-129(201)101(51-73(7)8)163-123(195)93(33-25-27-45-145)157-127(199)99(49-71(3)4)165-133(205)107(59-113(185)186)171-135(207)109(61-115(189)190)173-143(215)119(77(13)179)155-79(15)181)67-219-220-68-112(140(212)170-106(58-86-66-150-70-154-86)138(210)178-118(76(12)20-2)142(214)160-96(44-48-218-18)126(198)162-98(122(148)194)54-82-37-41-88(184)42-38-82)176-132(204)104(56-84-64-152-92-32-24-22-30-90(84)92)168-130(202)102(52-74(9)10)164-124(196)94(34-26-28-46-146)158-128(200)100(50-72(5)6)166-134(206)108(60-114(187)188)172-136(208)110(62-116(191)192)174-144(216)120(78(14)180)156-80(16)182/h21-24,29-32,35-42,63-66,69-78,93-112,117-120,151-152,179-180,183-184H,19-20,25-28,33-34,43-62,67-68,145-146H2,1-18H3,(H2,147,193)(H2,148,194)(H,149,153)(H,150,154)(H,155,181)(H,156,182)(H,157,199)(H,158,200)(H,159,213)(H,160,214)(H,161,197)(H,162,198)(H,163,195)(H,164,196)(H,165,205)(H,166,206)(H,167,201)(H,168,202)(H,169,211)(H,170,212)(H,171,207)(H,172,208)(H,173,215)(H,174,216)(H,175,203)(H,176,204)(H,177,209)(H,178,210)(H,185,186)(H,187,188)(H,189,190)(H,191,192)/t75-,76-,77-,78-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,109-,110-,111-,112-,117-,118-,119-,120-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Frizzled-7 in HEK293 cells expressing Wnt assessed as reduction in Wnt signaling by firefly luciferase reporter gene assay |
J Med Chem 62: 7739-7750 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00500
BindingDB Entry DOI: 10.7270/Q2MG7SVD |
More data for this
Ligand-Target Pair | |
Frizzled-7
(Homo sapiens) | BDBM50519331

(CHEMBL4438919)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |r| Show InChI InChI=1S/C140H198N32O42S4/c1-17-71(11)115(139(213)153-89(39-41-215-15)121(195)155-91(117(141)191)47-75-27-31-81(177)32-28-75)171-133(207)99(51-79-59-143-65-147-79)163-137(211)107(169-127(201)97(49-77-57-145-85-25-21-19-23-83(77)85)161-125(199)95(45-69(7)8)157-119(193)87(35-37-109(179)180)151-123(197)93(43-67(3)4)159-129(203)101(53-111(183)184)165-131(205)103(55-113(187)188)167-135(209)105(61-173)149-73(13)175)63-217-218-64-108(138(212)164-100(52-80-60-144-66-148-80)134(208)172-116(72(12)18-2)140(214)154-90(40-42-216-16)122(196)156-92(118(142)192)48-76-29-33-82(178)34-30-76)170-128(202)98(50-78-58-146-86-26-22-20-24-84(78)86)162-126(200)96(46-70(9)10)158-120(194)88(36-38-110(181)182)152-124(198)94(44-68(5)6)160-130(204)102(54-112(185)186)166-132(206)104(56-114(189)190)168-136(210)106(62-174)150-74(14)176/h19-34,57-60,65-72,87-108,115-116,145-146,173-174,177-178H,17-18,35-56,61-64H2,1-16H3,(H2,141,191)(H2,142,192)(H,143,147)(H,144,148)(H,149,175)(H,150,176)(H,151,197)(H,152,198)(H,153,213)(H,154,214)(H,155,195)(H,156,196)(H,157,193)(H,158,194)(H,159,203)(H,160,204)(H,161,199)(H,162,200)(H,163,211)(H,164,212)(H,165,205)(H,166,206)(H,167,209)(H,168,210)(H,169,201)(H,170,202)(H,171,207)(H,172,208)(H,179,180)(H,181,182)(H,183,184)(H,185,186)(H,187,188)(H,189,190)/t71-,72-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,115-,116-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Frizzled-7 in HEK293 cells expressing Wnt assessed as reduction in Wnt signaling by firefly luciferase reporter gene assay |
J Med Chem 62: 7739-7750 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00500
BindingDB Entry DOI: 10.7270/Q2MG7SVD |
More data for this
Ligand-Target Pair | |
Frizzled-7
(Homo sapiens) | BDBM50519332
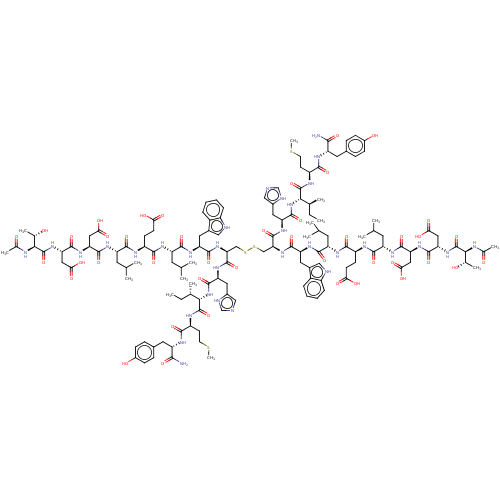
(CHEMBL4450933)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(C)=O)[C@H](C)O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(C)=O)[C@H](C)O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |r| Show InChI InChI=1S/C142H202N32O42S4/c1-19-71(11)115(139(213)155-91(41-43-217-17)123(197)157-93(119(143)193)49-77-29-33-83(179)34-30-77)173-135(209)101(53-81-61-145-65-149-81)165-137(211)107(171-129(203)99(51-79-59-147-87-27-23-21-25-85(79)87)163-127(201)97(47-69(7)8)159-121(195)89(37-39-109(181)182)153-125(199)95(45-67(3)4)161-131(205)103(55-111(185)186)167-133(207)105(57-113(189)190)169-141(215)117(73(13)175)151-75(15)177)63-219-220-64-108(138(212)166-102(54-82-62-146-66-150-82)136(210)174-116(72(12)20-2)140(214)156-92(42-44-218-18)124(198)158-94(120(144)194)50-78-31-35-84(180)36-32-78)172-130(204)100(52-80-60-148-88-28-24-22-26-86(80)88)164-128(202)98(48-70(9)10)160-122(196)90(38-40-110(183)184)154-126(200)96(46-68(5)6)162-132(206)104(56-112(187)188)168-134(208)106(58-114(191)192)170-142(216)118(74(14)176)152-76(16)178/h21-36,59-62,65-74,89-108,115-118,147-148,175-176,179-180H,19-20,37-58,63-64H2,1-18H3,(H2,143,193)(H2,144,194)(H,145,149)(H,146,150)(H,151,177)(H,152,178)(H,153,199)(H,154,200)(H,155,213)(H,156,214)(H,157,197)(H,158,198)(H,159,195)(H,160,196)(H,161,205)(H,162,206)(H,163,201)(H,164,202)(H,165,211)(H,166,212)(H,167,207)(H,168,208)(H,169,215)(H,170,216)(H,171,203)(H,172,204)(H,173,209)(H,174,210)(H,181,182)(H,183,184)(H,185,186)(H,187,188)(H,189,190)(H,191,192)/t71-,72-,73-,74-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,115-,116-,117-,118-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Frizzled-7 in HEK293 cells expressing Wnt assessed as reduction in Wnt signaling by firefly luciferase reporter gene assay |
J Med Chem 62: 7739-7750 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00500
BindingDB Entry DOI: 10.7270/Q2MG7SVD |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50078672
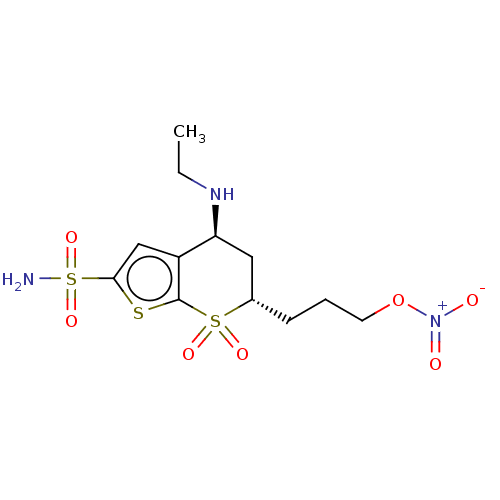
(CHEMBL3415379)Show SMILES CCN[C@H]1C[C@H](CCCO[N+]([O-])=O)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C12H19N3O7S3/c1-2-14-10-6-8(4-3-5-22-15(16)17)24(18,19)12-9(10)7-11(23-12)25(13,20)21/h7-8,10,14H,2-6H2,1H3,(H2,13,20,21)/t8-,10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 assessed as reduction in enzyme activity incubated for 30 mins by fluorescence polarization assay |
J Med Chem 58: 2821-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00043
BindingDB Entry DOI: 10.7270/Q2QF8VM2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50078643
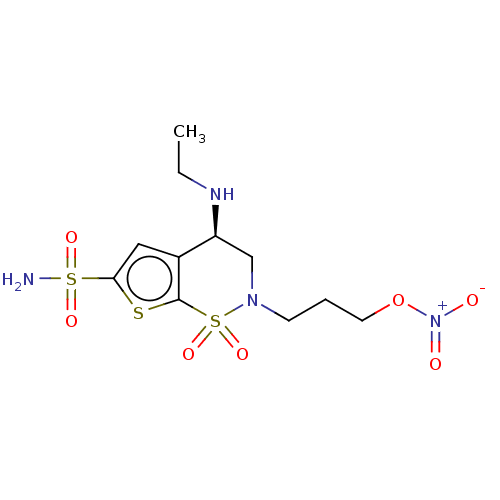
(CHEMBL3415383)Show SMILES CCN[C@H]1CN(CCCO[N+]([O-])=O)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C11H18N4O7S3/c1-2-13-9-7-14(4-3-5-22-15(16)17)25(20,21)11-8(9)6-10(23-11)24(12,18)19/h6,9,13H,2-5,7H2,1H3,(H2,12,18,19)/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 assessed as reduction in enzyme activity incubated for 30 mins by fluorescence polarization assay |
J Med Chem 58: 2821-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00043
BindingDB Entry DOI: 10.7270/Q2QF8VM2 |
More data for this
Ligand-Target Pair | |
Frizzled-7
(Homo sapiens) | BDBM50519308
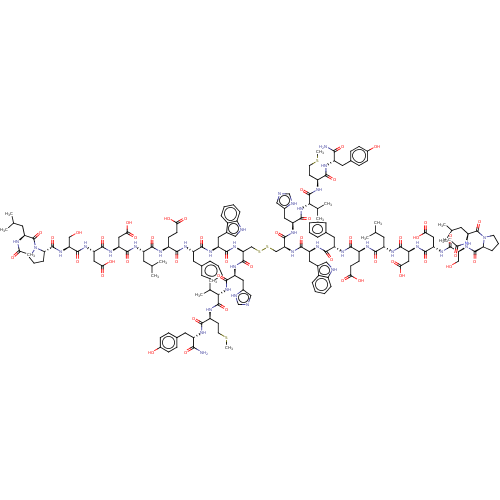
(CHEMBL4554351)Show SMILES CSCC[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(C)C)NC(C)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(C)C)NC(C)=O)C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |r| Show InChI InChI=1S/C166H226N36O46S4/c1-83(2)57-111(183-151(233)119(69-133(213)214)191-153(235)121(71-135(217)218)193-157(239)125(77-203)195-161(243)129-37-27-53-201(129)165(247)123(59-85(5)6)175-89(13)205)145(227)177-105(47-49-131(209)210)141(223)185-113(63-91-29-19-17-20-30-91)147(229)187-115(65-95-73-171-103-35-25-23-33-101(95)103)149(231)197-127(159(241)189-117(67-97-75-169-81-173-97)155(237)199-137(87(9)10)163(245)179-107(51-55-249-15)143(225)181-109(139(167)221)61-93-39-43-99(207)44-40-93)79-251-252-80-128(160(242)190-118(68-98-76-170-82-174-98)156(238)200-138(88(11)12)164(246)180-108(52-56-250-16)144(226)182-110(140(168)222)62-94-41-45-100(208)46-42-94)198-150(232)116(66-96-74-172-104-36-26-24-34-102(96)104)188-148(230)114(64-92-31-21-18-22-32-92)186-142(224)106(48-50-132(211)212)178-146(228)112(58-84(3)4)184-152(234)120(70-134(215)216)192-154(236)122(72-136(219)220)194-158(240)126(78-204)196-162(244)130-38-28-54-202(130)166(248)124(60-86(7)8)176-90(14)206/h17-26,29-36,39-46,73-76,81-88,105-130,137-138,171-172,203-204,207-208H,27-28,37-38,47-72,77-80H2,1-16H3,(H2,167,221)(H2,168,222)(H,169,173)(H,170,174)(H,175,205)(H,176,206)(H,177,227)(H,178,228)(H,179,245)(H,180,246)(H,181,225)(H,182,226)(H,183,233)(H,184,234)(H,185,223)(H,186,224)(H,187,229)(H,188,230)(H,189,241)(H,190,242)(H,191,235)(H,192,236)(H,193,239)(H,194,240)(H,195,243)(H,196,244)(H,197,231)(H,198,232)(H,199,237)(H,200,238)(H,209,210)(H,211,212)(H,213,214)(H,215,216)(H,217,218)(H,219,220)/t105-,106-,107-,108-,109-,110-,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,137-,138-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Frizzled-7 in HEK293 cells expressing Wnt assessed as reduction in Wnt signaling by firefly luciferase reporter gene assay |
J Med Chem 62: 7739-7750 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00500
BindingDB Entry DOI: 10.7270/Q2MG7SVD |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10884

((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...)Show SMILES CCN[C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C10H16N2O4S3/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16)/t6-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 assessed as reduction in enzyme activity incubated for 30 mins by fluorescence polarization assay |
J Med Chem 58: 2821-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00043
BindingDB Entry DOI: 10.7270/Q2QF8VM2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10885

((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...)Show SMILES CCN[C@H]1CN(CCCOC)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C12H21N3O5S3/c1-3-14-10-8-15(5-4-6-20-2)23(18,19)12-9(10)7-11(21-12)22(13,16)17/h7,10,14H,3-6,8H2,1-2H3,(H2,13,16,17)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 assessed as reduction in enzyme activity incubated for 30 mins by fluorescence polarization assay |
J Med Chem 58: 2821-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00043
BindingDB Entry DOI: 10.7270/Q2QF8VM2 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50078671
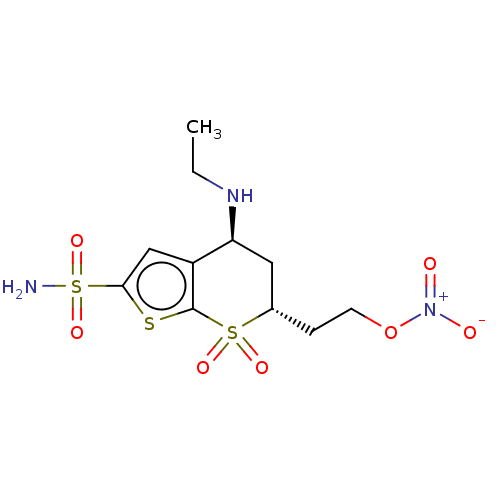
(CHEMBL3415380)Show SMILES CCN[C@H]1C[C@H](CCO[N+]([O-])=O)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C11H17N3O7S3/c1-2-13-9-5-7(3-4-21-14(15)16)23(17,18)11-8(9)6-10(22-11)24(12,19)20/h6-7,9,13H,2-5H2,1H3,(H2,12,19,20)/t7-,9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 assessed as reduction in enzyme activity incubated for 30 mins by fluorescence polarization assay |
J Med Chem 58: 2821-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00043
BindingDB Entry DOI: 10.7270/Q2QF8VM2 |
More data for this
Ligand-Target Pair | |
Frizzled-7
(Homo sapiens) | BDBM50519338

(CHEMBL4557583)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cccc(Cl)c1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(C)=O)[C@H](C)O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1cccc(Cl)c1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(C)=O)[C@H](C)O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |r| Show InChI InChI=1S/C148H196Cl2N32O42S4/c1-15-73(7)121(145(221)163-97(43-45-225-13)129(205)165-99(125(151)201)51-79-31-35-89(187)36-32-79)181-141(217)107(57-87-65-153-69-157-87)173-143(219)113(179-135(211)105(55-83-63-155-93-29-19-17-27-91(83)93)171-132(208)102(48-72(5)6)167-127(203)95(39-41-115(189)190)162-133(209)103(53-81-23-21-25-85(149)49-81)170-138(214)110(60-118(195)196)176-140(216)112(62-120(199)200)178-148(224)124(76(10)184)160-78(12)186)67-227-228-68-114(144(220)174-108(58-88-66-154-70-158-88)142(218)182-122(74(8)16-2)146(222)164-98(44-46-226-14)130(206)166-100(126(152)202)52-80-33-37-90(188)38-34-80)180-136(212)106(56-84-64-156-94-30-20-18-28-92(84)94)172-134(210)104(54-82-24-22-26-86(150)50-82)169-128(204)96(40-42-116(191)192)161-131(207)101(47-71(3)4)168-137(213)109(59-117(193)194)175-139(215)111(61-119(197)198)177-147(223)123(75(9)183)159-77(11)185/h17-38,49-50,63-66,69-76,95-114,121-124,155-156,183-184,187-188H,15-16,39-48,51-62,67-68H2,1-14H3,(H2,151,201)(H2,152,202)(H,153,157)(H,154,158)(H,159,185)(H,160,186)(H,161,207)(H,162,209)(H,163,221)(H,164,222)(H,165,205)(H,166,206)(H,167,203)(H,168,213)(H,169,204)(H,170,214)(H,171,208)(H,172,210)(H,173,219)(H,174,220)(H,175,215)(H,176,216)(H,177,223)(H,178,224)(H,179,211)(H,180,212)(H,181,217)(H,182,218)(H,189,190)(H,191,192)(H,193,194)(H,195,196)(H,197,198)(H,199,200)/t73-,74-,75-,76-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,109-,110-,111-,112-,113-,114-,121-,122-,123-,124-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Frizzled-7 in HEK293 cells expressing Wnt assessed as reduction in Wnt signaling by firefly luciferase reporter gene assay |
J Med Chem 62: 7739-7750 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00500
BindingDB Entry DOI: 10.7270/Q2MG7SVD |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50078644
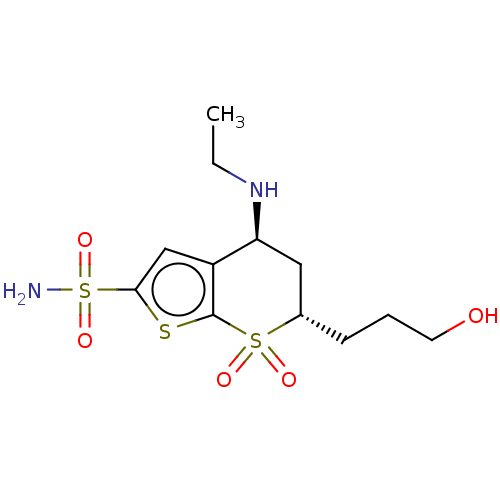
(CHEMBL3415382)Show SMILES CCN[C@H]1C[C@H](CCCO)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C12H20N2O5S3/c1-2-14-10-6-8(4-3-5-15)21(16,17)12-9(10)7-11(20-12)22(13,18)19/h7-8,10,14-15H,2-6H2,1H3,(H2,13,18,19)/t8-,10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 assessed as reduction in enzyme activity incubated for 30 mins by fluorescence polarization assay |
J Med Chem 58: 2821-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00043
BindingDB Entry DOI: 10.7270/Q2QF8VM2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50078670
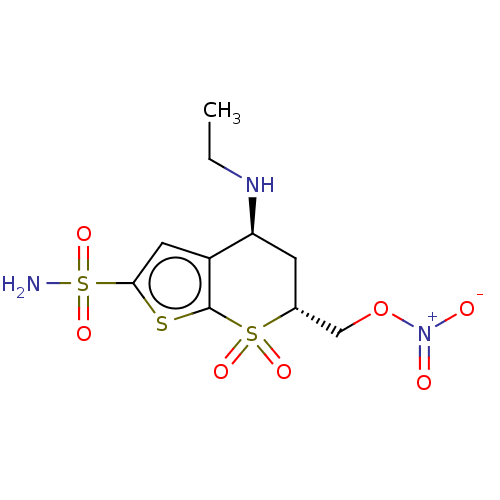
(CHEMBL3415381)Show SMILES CCN[C@H]1C[C@H](CO[N+]([O-])=O)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C10H15N3O7S3/c1-2-12-8-3-6(5-20-13(14)15)22(16,17)10-7(8)4-9(21-10)23(11,18)19/h4,6,8,12H,2-3,5H2,1H3,(H2,11,18,19)/t6-,8+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 156 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 assessed as reduction in enzyme activity incubated for 30 mins by fluorescence polarization assay |
J Med Chem 58: 2821-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00043
BindingDB Entry DOI: 10.7270/Q2QF8VM2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50078642
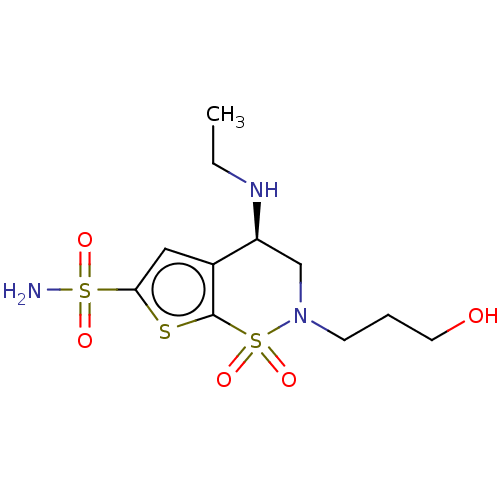
(CHEMBL3415384)Show SMILES CCN[C@H]1CN(CCCO)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C11H19N3O5S3/c1-2-13-9-7-14(4-3-5-15)22(18,19)11-8(9)6-10(20-11)21(12,16)17/h6,9,13,15H,2-5,7H2,1H3,(H2,12,16,17)/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 165 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 assessed as reduction in enzyme activity incubated for 30 mins by fluorescence polarization assay |
J Med Chem 58: 2821-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00043
BindingDB Entry DOI: 10.7270/Q2QF8VM2 |
More data for this
Ligand-Target Pair | |
Toll-like receptor 2
(Homo sapiens (Human)) | BDBM50406601

(CHEMBL5276854)Show SMILES CC(C)c1nc(c(C)c(-c2ccc(F)cc2)c1CCP(O)([O-])CC(=O)CC([O-])=O)-c1ccccc1 Show InChI InChI=1S/C27H30FNO5P/c1-17(2)26-23(13-14-35(33,34)16-22(30)15-24(31)32)25(19-9-11-21(28)12-10-19)18(3)27(29-26)20-7-5-4-6-8-20/h4-12,17,33,35H,13-16H2,1-3H3,(H,31,32)/q-1/p-1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 361 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against Bradykinin receptor B1 of rat ileum longitudinal smooth muscle. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50427852
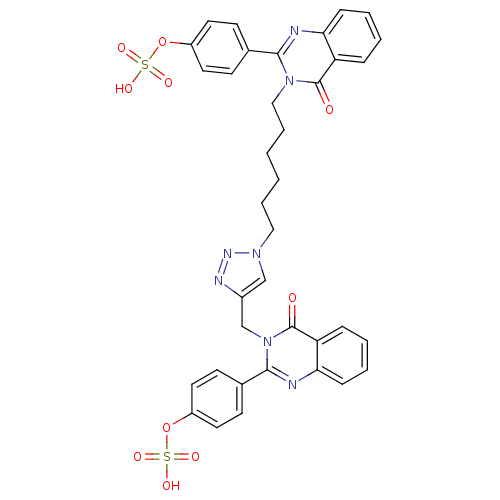
(CHEMBL2326346)Show SMILES OS(=O)(=O)Oc1ccc(cc1)-c1nc2ccccc2c(=O)n1CCCCCCn1cc(Cn2c(nc3ccccc3c2=O)-c2ccc(OS(O)(=O)=O)cc2)nn1 Show InChI InChI=1S/C37H33N7O10S2/c45-36-30-9-3-5-11-32(30)38-34(25-13-17-28(18-14-25)53-55(47,48)49)43(36)22-8-2-1-7-21-42-23-27(40-41-42)24-44-35(39-33-12-6-4-10-31(33)37(44)46)26-15-19-29(20-16-26)54-56(50,51)52/h3-6,9-20,23H,1-2,7-8,21-22,24H2,(H,47,48,49)(H,50,51,52) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a using S2366 as substrate after 10 mins by spectrophotometry |
J Med Chem 56: 2415-28 (2013)
Article DOI: 10.1021/jm301757v
BindingDB Entry DOI: 10.7270/Q27M0989 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50427853
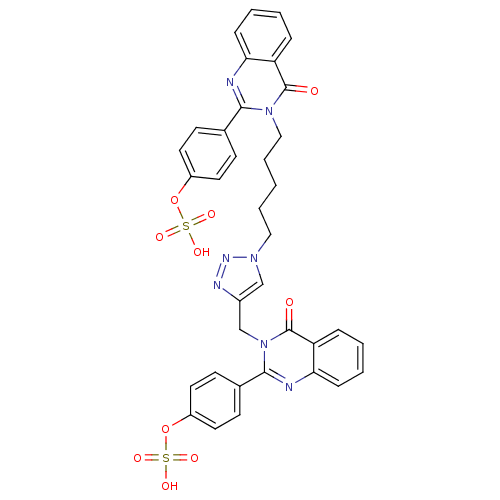
(CHEMBL2326345)Show SMILES OS(=O)(=O)Oc1ccc(cc1)-c1nc2ccccc2c(=O)n1CCCCCn1cc(Cn2c(nc3ccccc3c2=O)-c2ccc(OS(O)(=O)=O)cc2)nn1 Show InChI InChI=1S/C36H31N7O10S2/c44-35-29-8-2-4-10-31(29)37-33(24-12-16-27(17-13-24)52-54(46,47)48)42(35)21-7-1-6-20-41-22-26(39-40-41)23-43-34(38-32-11-5-3-9-30(32)36(43)45)25-14-18-28(19-15-25)53-55(49,50)51/h2-5,8-19,22H,1,6-7,20-21,23H2,(H,46,47,48)(H,49,50,51) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a using S2366 as substrate after 10 mins by spectrophotometry |
J Med Chem 56: 2415-28 (2013)
Article DOI: 10.1021/jm301757v
BindingDB Entry DOI: 10.7270/Q27M0989 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50427855
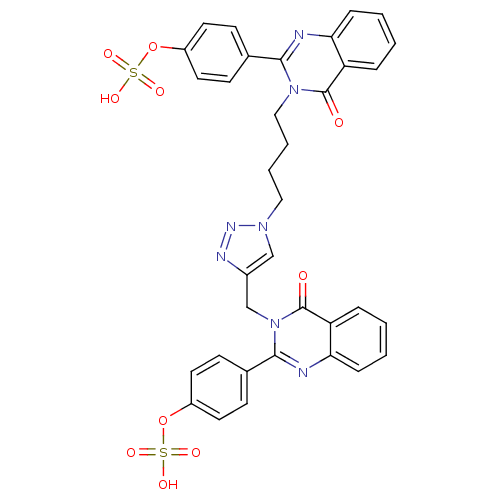
(CHEMBL2326343)Show SMILES OS(=O)(=O)Oc1ccc(cc1)-c1nc2ccccc2c(=O)n1CCCCn1cc(Cn2c(nc3ccccc3c2=O)-c2ccc(OS(O)(=O)=O)cc2)nn1 Show InChI InChI=1S/C35H29N7O10S2/c43-34-28-7-1-3-9-30(28)36-32(23-11-15-26(16-12-23)51-53(45,46)47)41(34)20-6-5-19-40-21-25(38-39-40)22-42-33(37-31-10-4-2-8-29(31)35(42)44)24-13-17-27(18-14-24)52-54(48,49)50/h1-4,7-18,21H,5-6,19-20,22H2,(H,45,46,47)(H,48,49,50) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a using S2366 as substrate after 10 mins by spectrophotometry |
J Med Chem 56: 2415-28 (2013)
Article DOI: 10.1021/jm301757v
BindingDB Entry DOI: 10.7270/Q27M0989 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50427843

(CHEMBL2326355)Show SMILES OS(=O)(=O)Oc1ccc(cc1)-c1nc2ccccc2c(=O)n1CCCCCn1nncc1Cn1c(nc2ccccc2c1=O)-c1ccc(OS(O)(=O)=O)cc1 Show InChI InChI=1S/C36H31N7O10S2/c44-35-29-8-2-4-10-31(29)38-33(24-12-16-27(17-13-24)52-54(46,47)48)41(35)20-6-1-7-21-43-26(22-37-40-43)23-42-34(39-32-11-5-3-9-30(32)36(42)45)25-14-18-28(19-15-25)53-55(49,50)51/h2-5,8-19,22H,1,6-7,20-21,23H2,(H,46,47,48)(H,49,50,51) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a using S2366 as substrate after 10 mins by spectrophotometry |
J Med Chem 56: 2415-28 (2013)
Article DOI: 10.1021/jm301757v
BindingDB Entry DOI: 10.7270/Q27M0989 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50427854

(CHEMBL2326344)Show SMILES OS(=O)(=O)Oc1ccc(cc1)-c1nc2ccccc2c(=O)n1Cc1cn(CCCCn2c(nc3ccccc3c2=O)-c2cccc(OS(O)(=O)=O)c2)nn1 Show InChI InChI=1S/C35H29N7O10S2/c43-34-28-10-1-3-12-30(28)37-33(24-8-7-9-27(20-24)52-54(48,49)50)41(34)19-6-5-18-40-21-25(38-39-40)22-42-32(36-31-13-4-2-11-29(31)35(42)44)23-14-16-26(17-15-23)51-53(45,46)47/h1-4,7-17,20-21H,5-6,18-19,22H2,(H,45,46,47)(H,48,49,50) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a using S2366 as substrate after 10 mins by spectrophotometry |
J Med Chem 56: 2415-28 (2013)
Article DOI: 10.1021/jm301757v
BindingDB Entry DOI: 10.7270/Q27M0989 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50427860
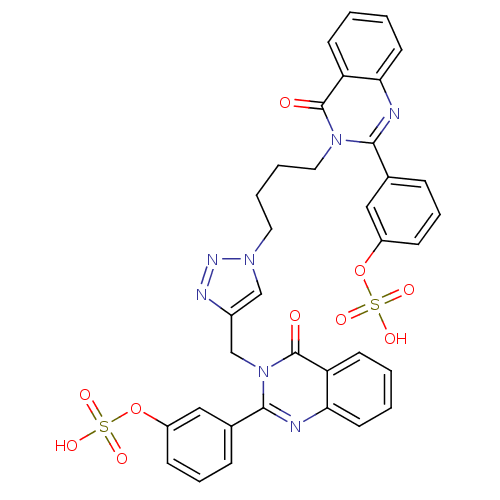
(CHEMBL2326078)Show SMILES OS(=O)(=O)Oc1cccc(c1)-c1nc2ccccc2c(=O)n1CCCCn1cc(Cn2c(nc3ccccc3c2=O)-c2cccc(OS(O)(=O)=O)c2)nn1 Show InChI InChI=1S/C35H29N7O10S2/c43-34-28-13-1-3-15-30(28)36-32(23-9-7-11-26(19-23)51-53(45,46)47)41(34)18-6-5-17-40-21-25(38-39-40)22-42-33(37-31-16-4-2-14-29(31)35(42)44)24-10-8-12-27(20-24)52-54(48,49)50/h1-4,7-16,19-21H,5-6,17-18,22H2,(H,45,46,47)(H,48,49,50) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a using S2366 as substrate after 10 mins by spectrophotometry |
J Med Chem 56: 2415-28 (2013)
Article DOI: 10.1021/jm301757v
BindingDB Entry DOI: 10.7270/Q27M0989 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50427858

(CHEMBL2326080)Show SMILES COc1cc(ccc1OS(O)(=O)=O)-c1nc2ccccc2c(=O)n1CCCCn1cc(Cn2c(nc3ccccc3c2=O)-c2ccc(OS(O)(=O)=O)c(OC)c2)nn1 Show InChI InChI=1S/C37H33N7O12S2/c1-53-32-19-23(13-15-30(32)55-57(47,48)49)34-38-28-11-5-3-9-26(28)36(45)43(34)18-8-7-17-42-21-25(40-41-42)22-44-35(39-29-12-6-4-10-27(29)37(44)46)24-14-16-31(33(20-24)54-2)56-58(50,51)52/h3-6,9-16,19-21H,7-8,17-18,22H2,1-2H3,(H,47,48,49)(H,50,51,52) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.39E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a using S2366 as substrate after 10 mins by spectrophotometry |
J Med Chem 56: 2415-28 (2013)
Article DOI: 10.1021/jm301757v
BindingDB Entry DOI: 10.7270/Q27M0989 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50427859
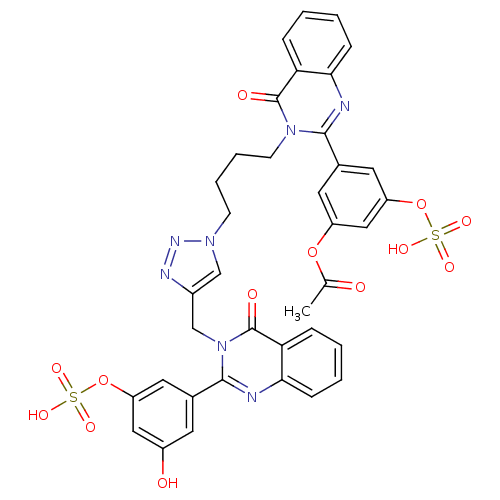
(CHEMBL2326079)Show SMILES CC(=O)Oc1cc(OS(O)(=O)=O)cc(c1)-c1nc2ccccc2c(=O)n1CCCCn1cc(Cn2c(nc3ccccc3c2=O)-c2cc(O)cc(OS(O)(=O)=O)c2)nn1 Show InChI InChI=1S/C37H31N7O13S2/c1-22(45)55-27-15-24(17-29(19-27)57-59(52,53)54)34-38-32-10-4-2-8-30(32)36(47)43(34)13-7-6-12-42-20-25(40-41-42)21-44-35(39-33-11-5-3-9-31(33)37(44)48)23-14-26(46)18-28(16-23)56-58(49,50)51/h2-5,8-11,14-20,46H,6-7,12-13,21H2,1H3,(H,49,50,51)(H,52,53,54) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.53E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a using S2366 as substrate after 10 mins by spectrophotometry |
J Med Chem 56: 2415-28 (2013)
Article DOI: 10.1021/jm301757v
BindingDB Entry DOI: 10.7270/Q27M0989 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50427856

(CHEMBL2326342)Show SMILES OS(=O)(=O)Oc1ccc(cc1)-c1nc2ccccc2c(=O)n1CCCCn1cc(Cn2c(nc3ccccc3c2=O)-c2cccc(OS(O)(=O)=O)c2)nn1 Show InChI InChI=1S/C35H29N7O10S2/c43-34-28-10-1-3-12-30(28)36-32(23-14-16-26(17-15-23)51-53(45,46)47)41(34)19-6-5-18-40-21-25(38-39-40)22-42-33(37-31-13-4-2-11-29(31)35(42)44)24-8-7-9-27(20-24)52-54(48,49)50/h1-4,7-17,20-21H,5-6,18-19,22H2,(H,45,46,47)(H,48,49,50) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.59E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a using S2366 as substrate after 10 mins by spectrophotometry |
J Med Chem 56: 2415-28 (2013)
Article DOI: 10.1021/jm301757v
BindingDB Entry DOI: 10.7270/Q27M0989 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50427851

(CHEMBL2326347)Show SMILES COc1cc(ccc1OS(O)(=O)=O)-c1nc2ccccc2c(=O)n1CCCCn1cc(Cn2c(nc3ccccc3c2=O)-c2cccc(OS(O)(=O)=O)c2)nn1 Show InChI InChI=1S/C36H31N7O11S2/c1-52-32-20-24(15-16-31(32)54-56(49,50)51)33-37-29-13-4-2-11-27(29)35(44)42(33)18-7-6-17-41-21-25(39-40-41)22-43-34(38-30-14-5-3-12-28(30)36(43)45)23-9-8-10-26(19-23)53-55(46,47)48/h2-5,8-16,19-21H,6-7,17-18,22H2,1H3,(H,46,47,48)(H,49,50,51) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.82E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a using S2366 as substrate after 10 mins by spectrophotometry |
J Med Chem 56: 2415-28 (2013)
Article DOI: 10.1021/jm301757v
BindingDB Entry DOI: 10.7270/Q27M0989 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50427850

(CHEMBL2326348)Show SMILES OS(=O)(=O)Oc1cccc(c1)-c1nc2ccccc2c(=O)n1CCCCn1cc(Cn2c(nc3ccccc3c2=O)-c2cc(OS(O)(=O)=O)cc(OS(O)(=O)=O)c2)nn1 Show InChI InChI=1S/C35H29N7O14S3/c43-34-28-10-1-3-12-30(28)36-32(22-8-7-9-25(16-22)54-57(45,46)47)41(34)15-6-5-14-40-20-24(38-39-40)21-42-33(37-31-13-4-2-11-29(31)35(42)44)23-17-26(55-58(48,49)50)19-27(18-23)56-59(51,52)53/h1-4,7-13,16-20H,5-6,14-15,21H2,(H,45,46,47)(H,48,49,50)(H,51,52,53) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.13E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a using S2366 as substrate after 10 mins by spectrophotometry |
J Med Chem 56: 2415-28 (2013)
Article DOI: 10.1021/jm301757v
BindingDB Entry DOI: 10.7270/Q27M0989 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50427846

(CHEMBL2326352)Show SMILES COc1cc(ccc1OS(O)(=O)=O)-c1nc2ccccc2c(=O)n1CCCCn1cc(COc2cc(ccc2OS(O)(=O)=O)-c2oc3cc(OS(O)(=O)=O)cc(OC)c3c(=O)c2OS(O)(=O)=O)nn1 Show InChI InChI=1S/C38H33N5O22S4/c1-58-29-16-22(10-12-27(29)63-67(49,50)51)37-39-26-8-4-3-7-25(26)38(45)43(37)14-6-5-13-42-19-23(40-41-42)20-60-30-15-21(9-11-28(30)64-68(52,53)54)35-36(65-69(55,56)57)34(44)33-31(59-2)17-24(18-32(33)61-35)62-66(46,47)48/h3-4,7-12,15-19H,5-6,13-14,20H2,1-2H3,(H,46,47,48)(H,49,50,51)(H,52,53,54)(H,55,56,57) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a using S2366 as substrate after 10 mins by spectrophotometry |
J Med Chem 56: 2415-28 (2013)
Article DOI: 10.1021/jm301757v
BindingDB Entry DOI: 10.7270/Q27M0989 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50427849

(CHEMBL2326349)Show SMILES CC(=O)Oc1cc(OS(O)(=O)=O)cc(c1)-c1nc2ccccc2c(=O)n1CCCCn1cc(Cn2c(nc3ccccc3c2=O)-c2cc(OS(O)(=O)=O)cc(OS(O)(=O)=O)c2)nn1 Show InChI InChI=1S/C37H31N7O16S3/c1-22(45)57-26-14-23(15-27(18-26)58-61(48,49)50)34-38-32-10-4-2-8-30(32)36(46)43(34)13-7-6-12-42-20-25(40-41-42)21-44-35(39-33-11-5-3-9-31(33)37(44)47)24-16-28(59-62(51,52)53)19-29(17-24)60-63(54,55)56/h2-5,8-11,14-20H,6-7,12-13,21H2,1H3,(H,48,49,50)(H,51,52,53)(H,54,55,56) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.73E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a using S2366 as substrate after 10 mins by spectrophotometry |
J Med Chem 56: 2415-28 (2013)
Article DOI: 10.1021/jm301757v
BindingDB Entry DOI: 10.7270/Q27M0989 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50427844

(CHEMBL2326354)Show SMILES OS(=O)(=O)Oc1ccc(cc1)-c1nc2ccccc2c(=O)n1Cc1cn(CCn2cc(Cn3c(nc4ccccc4c3=O)-c3ccc(OS(O)(=O)=O)cc3)nn2)nn1 Show InChI InChI=1S/C36H28N10O10S2/c47-35-29-5-1-3-7-31(29)37-33(23-9-13-27(14-10-23)55-57(49,50)51)45(35)21-25-19-43(41-39-25)17-18-44-20-26(40-42-44)22-46-34(38-32-8-4-2-6-30(32)36(46)48)24-11-15-28(16-12-24)56-58(52,53)54/h1-16,19-20H,17-18,21-22H2,(H,49,50,51)(H,52,53,54) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a using S2366 as substrate after 10 mins by spectrophotometry |
J Med Chem 56: 2415-28 (2013)
Article DOI: 10.1021/jm301757v
BindingDB Entry DOI: 10.7270/Q27M0989 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50427857

(CHEMBL2326341)Show SMILES OS(=O)(=O)Oc1cccc(c1)-c1nc2ccccc2c(=O)n1CCCn1cc(Cn2c(nc3ccccc3c2=O)-c2cccc(OS(O)(=O)=O)c2)nn1 Show InChI InChI=1S/C34H27N7O10S2/c42-33-27-12-1-3-14-29(27)35-31(22-8-5-10-25(18-22)50-52(44,45)46)40(33)17-7-16-39-20-24(37-38-39)21-41-32(36-30-15-4-2-13-28(30)34(41)43)23-9-6-11-26(19-23)51-53(47,48)49/h1-6,8-15,18-20H,7,16-17,21H2,(H,44,45,46)(H,47,48,49) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a using S2366 as substrate after 10 mins by spectrophotometry |
J Med Chem 56: 2415-28 (2013)
Article DOI: 10.1021/jm301757v
BindingDB Entry DOI: 10.7270/Q27M0989 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50427845

(CHEMBL2326353)Show SMILES COc1cc(OS(O)(=O)=O)cc2oc(-c3ccc(OS(O)(=O)=O)c(OCc4cn(CCCCn5c(nc6ccccc6c5=O)-c5cc(OC(C)=O)cc(OS(O)(=O)=O)c5)nn4)c3)c(OS(O)(=O)=O)c(=O)c12 Show InChI InChI=1S/C39H33N5O23S4/c1-21(45)62-25-13-23(14-26(16-25)64-68(48,49)50)38-40-29-8-4-3-7-28(29)39(47)44(38)12-6-5-11-43-19-24(41-42-43)20-61-31-15-22(9-10-30(31)66-70(54,55)56)36-37(67-71(57,58)59)35(46)34-32(60-2)17-27(18-33(34)63-36)65-69(51,52)53/h3-4,7-10,13-19H,5-6,11-12,20H2,1-2H3,(H,48,49,50)(H,51,52,53)(H,54,55,56)(H,57,58,59) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.07E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a using S2366 as substrate after 10 mins by spectrophotometry |
J Med Chem 56: 2415-28 (2013)
Article DOI: 10.1021/jm301757v
BindingDB Entry DOI: 10.7270/Q27M0989 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50427847
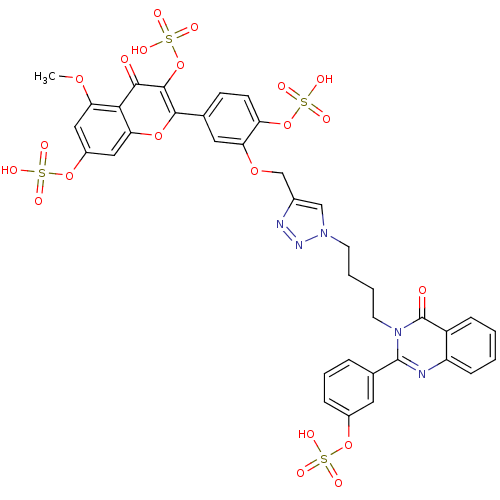
(CHEMBL2326351)Show SMILES COc1cc(OS(O)(=O)=O)cc2oc(-c3ccc(OS(O)(=O)=O)c(OCc4cn(CCCCn5c(nc6ccccc6c5=O)-c5cccc(OS(O)(=O)=O)c5)nn4)c3)c(OS(O)(=O)=O)c(=O)c12 Show InChI InChI=1S/C37H31N5O21S4/c1-57-30-17-25(61-65(48,49)50)18-31-32(30)33(43)35(63-67(54,55)56)34(59-31)21-11-12-28(62-66(51,52)53)29(16-21)58-20-23-19-41(40-39-23)13-4-5-14-42-36(38-27-10-3-2-9-26(27)37(42)44)22-7-6-8-24(15-22)60-64(45,46)47/h2-3,6-12,15-19H,4-5,13-14,20H2,1H3,(H,45,46,47)(H,48,49,50)(H,51,52,53)(H,54,55,56) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a using S2366 as substrate after 10 mins by spectrophotometry |
J Med Chem 56: 2415-28 (2013)
Article DOI: 10.1021/jm301757v
BindingDB Entry DOI: 10.7270/Q27M0989 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50427861
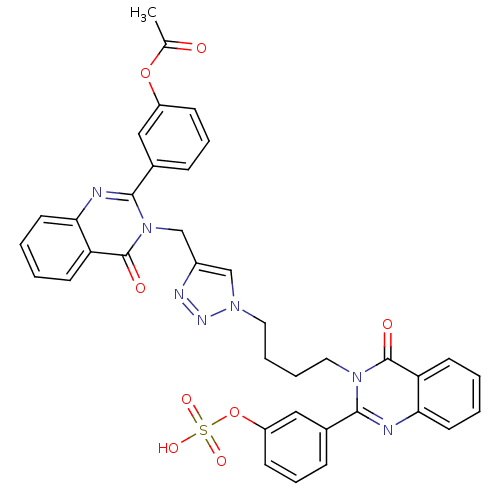
(CHEMBL2326077)Show SMILES CC(=O)Oc1cccc(c1)-c1nc2ccccc2c(=O)n1Cc1cn(CCCCn2c(nc3ccccc3c2=O)-c2cccc(OS(O)(=O)=O)c2)nn1 Show InChI InChI=1S/C37H31N7O8S/c1-24(45)51-28-12-8-10-25(20-28)35-39-33-17-5-3-15-31(33)37(47)44(35)23-27-22-42(41-40-27)18-6-7-19-43-34(38-32-16-4-2-14-30(32)36(43)46)26-11-9-13-29(21-26)52-53(48,49)50/h2-5,8-17,20-22H,6-7,18-19,23H2,1H3,(H,48,49,50) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a using S2366 as substrate after 10 mins by spectrophotometry |
J Med Chem 56: 2415-28 (2013)
Article DOI: 10.1021/jm301757v
BindingDB Entry DOI: 10.7270/Q27M0989 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50427848

(CHEMBL2326350)Show SMILES OS(=O)(=O)Oc1cc(OS(O)(=O)=O)cc(c1)-c1nc2ccccc2c(=O)n1CCCCn1cc(Cn2c(nc3ccccc3c2=O)-c2cc(OS(O)(=O)=O)cc(OS(O)(=O)=O)c2)nn1 Show InChI InChI=1S/C35H29N7O18S4/c43-34-28-7-1-3-9-30(28)36-32(21-13-24(57-61(45,46)47)17-25(14-21)58-62(48,49)50)41(34)12-6-5-11-40-19-23(38-39-40)20-42-33(37-31-10-4-2-8-29(31)35(42)44)22-15-26(59-63(51,52)53)18-27(16-22)60-64(54,55)56/h1-4,7-10,13-19H,5-6,11-12,20H2,(H,45,46,47)(H,48,49,50)(H,51,52,53)(H,54,55,56) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a using S2366 as substrate after 10 mins by spectrophotometry |
J Med Chem 56: 2415-28 (2013)
Article DOI: 10.1021/jm301757v
BindingDB Entry DOI: 10.7270/Q27M0989 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1 [201-500]
(Homo sapiens (Human)) | BDBM13530

(4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | 2 | n/a | n/a | n/a | 7.4 | 25 |
Ambit, Inc.
| Assay Description
Competition binding assay was used to measure the interaction between the phage-tagged kinase, immobilized competitive ligand, and unlinked test comp... |
Proc Natl Acad Sci U S A 102: 11011-6 (2005)
Article DOI: 10.1073/pnas.0504952102
BindingDB Entry DOI: 10.7270/Q29C6VNB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL1 [201-500,Q252H]
(Homo sapiens (Human)) | BDBM13530

(4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 20 | n/a | n/a | n/a | 7.4 | 25 |
Ambit, Inc.
| Assay Description
Competition binding assay was used to measure the interaction between the phage-tagged kinase, immobilized competitive ligand, and unlinked test comp... |
Proc Natl Acad Sci U S A 102: 11011-6 (2005)
Article DOI: 10.1073/pnas.0504952102
BindingDB Entry DOI: 10.7270/Q29C6VNB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL1 [201-500,Y253F]
(Homo sapiens (Human)) | BDBM13530

(4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 40 | n/a | n/a | n/a | 7.4 | 25 |
Ambit, Inc.
| Assay Description
Competition binding assay was used to measure the interaction between the phage-tagged kinase, immobilized competitive ligand, and unlinked test comp... |
Proc Natl Acad Sci U S A 102: 11011-6 (2005)
Article DOI: 10.1073/pnas.0504952102
BindingDB Entry DOI: 10.7270/Q29C6VNB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL1 [201-500,E255K]
(Homo sapiens (Human)) | BDBM13530

(4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 100 | n/a | n/a | n/a | 7.4 | 25 |
Ambit, Inc.
| Assay Description
Competition binding assay was used to measure the interaction between the phage-tagged kinase, immobilized competitive ligand, and unlinked test comp... |
Proc Natl Acad Sci U S A 102: 11011-6 (2005)
Article DOI: 10.1073/pnas.0504952102
BindingDB Entry DOI: 10.7270/Q29C6VNB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL1 [201-500,M351T]
(Homo sapiens (Human)) | BDBM13530

(4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 10 | n/a | n/a | n/a | 7.4 | 25 |
Ambit, Inc.
| Assay Description
Competition binding assay was used to measure the interaction between the phage-tagged kinase, immobilized competitive ligand, and unlinked test comp... |
Proc Natl Acad Sci U S A 102: 11011-6 (2005)
Article DOI: 10.1073/pnas.0504952102
BindingDB Entry DOI: 10.7270/Q29C6VNB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL1 [201-500]
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | 7.4 | 25 |
Ambit, Inc.
| Assay Description
Competition binding assay was used to measure the interaction between the phage-tagged kinase, immobilized competitive ligand, and unlinked test comp... |
Proc Natl Acad Sci U S A 102: 11011-6 (2005)
Article DOI: 10.1073/pnas.0504952102
BindingDB Entry DOI: 10.7270/Q29C6VNB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL1 [201-500,Q252H]
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 1 | n/a | n/a | n/a | 7.4 | 25 |
Ambit, Inc.
| Assay Description
Competition binding assay was used to measure the interaction between the phage-tagged kinase, immobilized competitive ligand, and unlinked test comp... |
Proc Natl Acad Sci U S A 102: 11011-6 (2005)
Article DOI: 10.1073/pnas.0504952102
BindingDB Entry DOI: 10.7270/Q29C6VNB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL1 [201-500,Y253F]
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 1 | n/a | n/a | n/a | 7.4 | 25 |
Ambit, Inc.
| Assay Description
Competition binding assay was used to measure the interaction between the phage-tagged kinase, immobilized competitive ligand, and unlinked test comp... |
Proc Natl Acad Sci U S A 102: 11011-6 (2005)
Article DOI: 10.1073/pnas.0504952102
BindingDB Entry DOI: 10.7270/Q29C6VNB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL1 [201-500,E255K]
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 2 | n/a | n/a | n/a | 7.4 | 25 |
Ambit, Inc.
| Assay Description
Competition binding assay was used to measure the interaction between the phage-tagged kinase, immobilized competitive ligand, and unlinked test comp... |
Proc Natl Acad Sci U S A 102: 11011-6 (2005)
Article DOI: 10.1073/pnas.0504952102
BindingDB Entry DOI: 10.7270/Q29C6VNB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL1 [201-500,M351T]
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 0.700 | n/a | n/a | n/a | 7.4 | 25 |
Ambit, Inc.
| Assay Description
Competition binding assay was used to measure the interaction between the phage-tagged kinase, immobilized competitive ligand, and unlinked test comp... |
Proc Natl Acad Sci U S A 102: 11011-6 (2005)
Article DOI: 10.1073/pnas.0504952102
BindingDB Entry DOI: 10.7270/Q29C6VNB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL1 [201-500,F359V]
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | 7.4 | 25 |
Ambit, Inc.
| Assay Description
Competition binding assay was used to measure the interaction between the phage-tagged kinase, immobilized competitive ligand, and unlinked test comp... |
Proc Natl Acad Sci U S A 102: 11011-6 (2005)
Article DOI: 10.1073/pnas.0504952102
BindingDB Entry DOI: 10.7270/Q29C6VNB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL1 [201-500,H396P]
(Homo sapiens (Human)) | BDBM13530

(4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a |
Ambit, Inc.
| Assay Description
Competition binding assay was used to measure the interaction between the phage-tagged kinase, immobilized competitive ligand, and unlinked test comp... |
Proc Natl Acad Sci U S A 102: 11011-6 (2005)
Article DOI: 10.1073/pnas.0504952102
BindingDB Entry DOI: 10.7270/Q29C6VNB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL1 [201-500,T315I]
(Homo sapiens (Human)) | BDBM13530

(4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit, Inc.
| Assay Description
Competition binding assay was used to measure the interaction between the phage-tagged kinase, immobilized competitive ligand, and unlinked test comp... |
Proc Natl Acad Sci U S A 102: 11011-6 (2005)
Article DOI: 10.1073/pnas.0504952102
BindingDB Entry DOI: 10.7270/Q29C6VNB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mast/stem cell growth factor receptor Kit [N822K]
(Homo sapiens (Human)) | BDBM13530

(4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a |
Ambit, Inc.
| Assay Description
Competition binding assay was used to measure the interaction between the phage-tagged kinase, immobilized competitive ligand, and unlinked test comp... |
Proc Natl Acad Sci U S A 102: 11011-6 (2005)
Article DOI: 10.1073/pnas.0504952102
BindingDB Entry DOI: 10.7270/Q29C6VNB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data