 Found 845 hits with Last Name = 'menke' and Initial = 'j'
Found 845 hits with Last Name = 'menke' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50426601
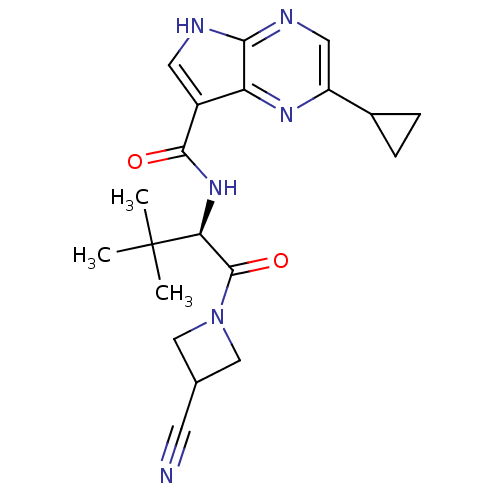
(CHEMBL2325895)Show SMILES CC(C)(C)[C@@H](NC(=O)c1c[nH]c2ncc(nc12)C1CC1)C(=O)N1CC(C1)C#N |r| Show InChI InChI=1S/C20H24N6O2/c1-20(2,3)16(19(28)26-9-11(6-21)10-26)25-18(27)13-7-22-17-15(13)24-14(8-23-17)12-4-5-12/h7-8,11-12,16H,4-5,9-10H2,1-3H3,(H,22,23)(H,25,27)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... |
J Med Chem 56: 345-56 (2013)
Article DOI: 10.1021/jm301646k
BindingDB Entry DOI: 10.7270/Q2Q241JX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50426601

(CHEMBL2325895)Show SMILES CC(C)(C)[C@@H](NC(=O)c1c[nH]c2ncc(nc12)C1CC1)C(=O)N1CC(C1)C#N |r| Show InChI InChI=1S/C20H24N6O2/c1-20(2,3)16(19(28)26-9-11(6-21)10-26)25-18(27)13-7-22-17-15(13)24-14(8-23-17)12-4-5-12/h7-8,11-12,16H,4-5,9-10H2,1-3H3,(H,22,23)(H,25,27)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) using [33gammaP]ATP and Biotin-KAIETDKEYYTVKD as substrate incubated for 10 mins prior to substrate addition meas... |
Bioorg Med Chem Lett 23: 2793-800 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.012
BindingDB Entry DOI: 10.7270/Q2QR4ZG3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50433243

(CHEMBL2376154)Show SMILES Cn1nc(-c2cnc3[nH]cc(C(=O)N[C@H](C4CC4)C(=O)N4CC(C4)C#N)c3n2)c2ccc(Cl)cc12 |r| Show InChI InChI=1S/C24H21ClN8O2/c1-32-18-6-14(25)4-5-15(18)20(31-32)17-9-28-22-21(29-17)16(8-27-22)23(34)30-19(13-2-3-13)24(35)33-10-12(7-26)11-33/h4-6,8-9,12-13,19H,2-3,10-11H2,1H3,(H,27,28)(H,30,34)/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) using [33gammaP]ATP and Biotin-KAIETDKEYYTVKD as substrate incubated for 10 mins prior to substrate addition meas... |
Bioorg Med Chem Lett 23: 2793-800 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.012
BindingDB Entry DOI: 10.7270/Q2QR4ZG3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50433240
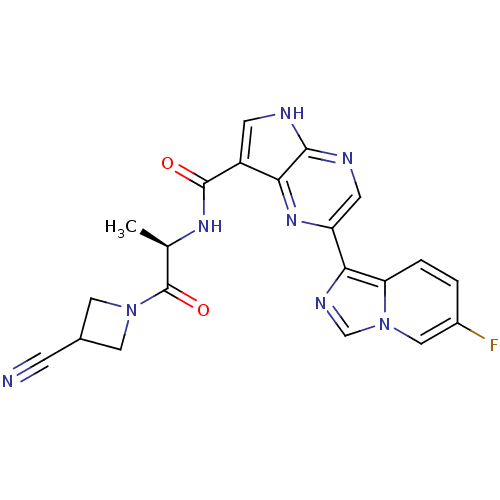
(CHEMBL2376157)Show SMILES C[C@@H](NC(=O)c1c[nH]c2ncc(nc12)-c1ncn2cc(F)ccc12)C(=O)N1CC(C1)C#N |r| Show InChI InChI=1S/C21H17FN8O2/c1-11(21(32)29-7-12(4-23)8-29)27-20(31)14-5-24-19-17(14)28-15(6-25-19)18-16-3-2-13(22)9-30(16)10-26-18/h2-3,5-6,9-12H,7-8H2,1H3,(H,24,25)(H,27,31)/t11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) using [33gammaP]ATP and Biotin-KAIETDKEYYTVKD as substrate incubated for 10 mins prior to substrate addition meas... |
Bioorg Med Chem Lett 23: 2793-800 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.012
BindingDB Entry DOI: 10.7270/Q2QR4ZG3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50433254
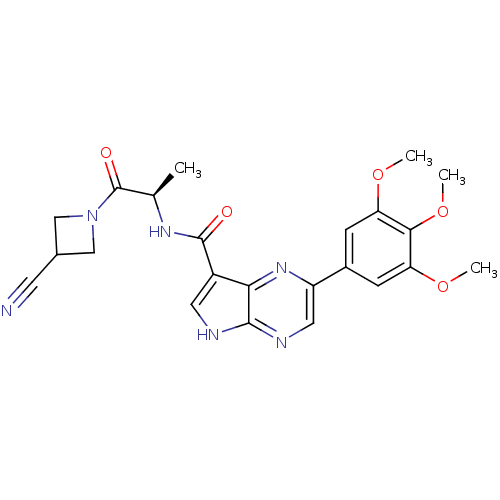
(CHEMBL2376040)Show SMILES COc1cc(cc(OC)c1OC)-c1cnc2[nH]cc(C(=O)N[C@H](C)C(=O)N3CC(C3)C#N)c2n1 |r| Show InChI InChI=1S/C23H24N6O5/c1-12(23(31)29-10-13(7-24)11-29)27-22(30)15-8-25-21-19(15)28-16(9-26-21)14-5-17(32-2)20(34-4)18(6-14)33-3/h5-6,8-9,12-13H,10-11H2,1-4H3,(H,25,26)(H,27,30)/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) using [33gammaP]ATP and Biotin-KAIETDKEYYTVKD as substrate incubated for 10 mins prior to substrate addition meas... |
Bioorg Med Chem Lett 23: 2793-800 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.012
BindingDB Entry DOI: 10.7270/Q2QR4ZG3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50426599
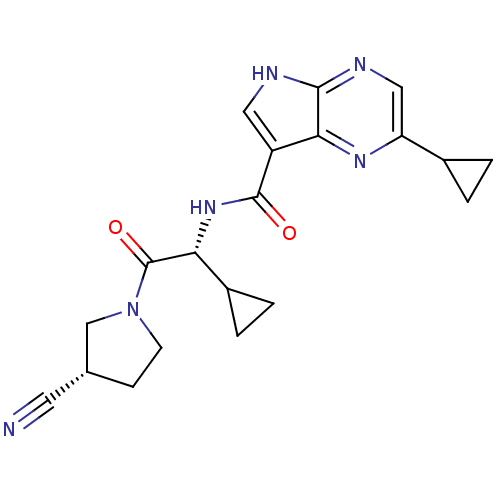
(CHEMBL2325898)Show SMILES O=C(N[C@H](C1CC1)C(=O)N1CC[C@@H](C1)C#N)c1c[nH]c2ncc(nc12)C1CC1 |r| Show InChI InChI=1S/C20H22N6O2/c21-7-11-5-6-26(10-11)20(28)16(13-3-4-13)25-19(27)14-8-22-18-17(14)24-15(9-23-18)12-1-2-12/h8-9,11-13,16H,1-6,10H2,(H,22,23)(H,25,27)/t11-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... |
J Med Chem 56: 345-56 (2013)
Article DOI: 10.1021/jm301646k
BindingDB Entry DOI: 10.7270/Q2Q241JX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50426607
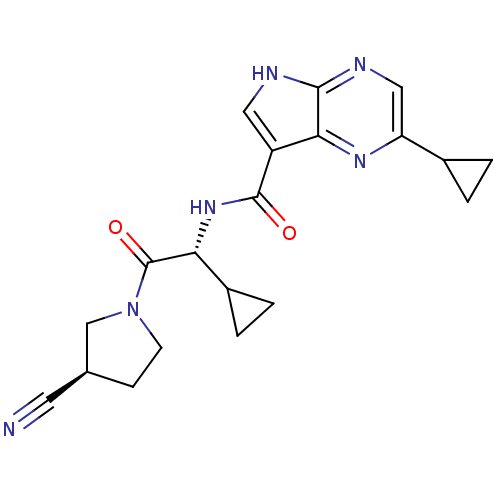
(CHEMBL2325897)Show SMILES O=C(N[C@H](C1CC1)C(=O)N1CC[C@H](C1)C#N)c1c[nH]c2ncc(nc12)C1CC1 |r| Show InChI InChI=1S/C20H22N6O2/c21-7-11-5-6-26(10-11)20(28)16(13-3-4-13)25-19(27)14-8-22-18-17(14)24-15(9-23-18)12-1-2-12/h8-9,11-13,16H,1-6,10H2,(H,22,23)(H,25,27)/t11-,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK1 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... |
J Med Chem 56: 345-56 (2013)
Article DOI: 10.1021/jm301646k
BindingDB Entry DOI: 10.7270/Q2Q241JX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50426599

(CHEMBL2325898)Show SMILES O=C(N[C@H](C1CC1)C(=O)N1CC[C@@H](C1)C#N)c1c[nH]c2ncc(nc12)C1CC1 |r| Show InChI InChI=1S/C20H22N6O2/c21-7-11-5-6-26(10-11)20(28)16(13-3-4-13)25-19(27)14-8-22-18-17(14)24-15(9-23-18)12-1-2-12/h8-9,11-13,16H,1-6,10H2,(H,22,23)(H,25,27)/t11-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... |
J Med Chem 56: 345-56 (2013)
Article DOI: 10.1021/jm301646k
BindingDB Entry DOI: 10.7270/Q2Q241JX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50426599

(CHEMBL2325898)Show SMILES O=C(N[C@H](C1CC1)C(=O)N1CC[C@@H](C1)C#N)c1c[nH]c2ncc(nc12)C1CC1 |r| Show InChI InChI=1S/C20H22N6O2/c21-7-11-5-6-26(10-11)20(28)16(13-3-4-13)25-19(27)14-8-22-18-17(14)24-15(9-23-18)12-1-2-12/h8-9,11-13,16H,1-6,10H2,(H,22,23)(H,25,27)/t11-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK1 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... |
J Med Chem 56: 345-56 (2013)
Article DOI: 10.1021/jm301646k
BindingDB Entry DOI: 10.7270/Q2Q241JX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50433249
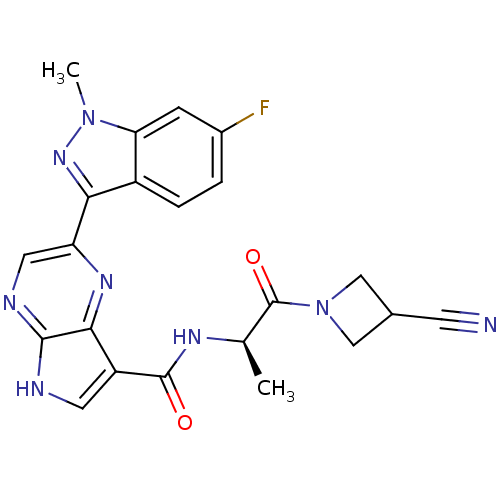
(CHEMBL2376147)Show SMILES C[C@@H](NC(=O)c1c[nH]c2ncc(nc12)-c1nn(C)c2cc(F)ccc12)C(=O)N1CC(C1)C#N |r| Show InChI InChI=1S/C22H19FN8O2/c1-11(22(33)31-9-12(6-24)10-31)27-21(32)15-7-25-20-19(15)28-16(8-26-20)18-14-4-3-13(23)5-17(14)30(2)29-18/h3-5,7-8,11-12H,9-10H2,1-2H3,(H,25,26)(H,27,32)/t11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) using [33gammaP]ATP and Biotin-KAIETDKEYYTVKD as substrate incubated for 10 mins prior to substrate addition meas... |
Bioorg Med Chem Lett 23: 2793-800 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.012
BindingDB Entry DOI: 10.7270/Q2QR4ZG3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50433253

(CHEMBL2376143)Show SMILES C[C@@H](NC(=O)c1c[nH]c2ncc(nc12)-c1cn(C)c2cc(Cl)ccc12)C(=O)N1CC(C1)C#N |r| Show InChI InChI=1S/C23H20ClN7O2/c1-12(23(33)31-9-13(6-25)10-31)28-22(32)16-7-26-21-20(16)29-18(8-27-21)17-11-30(2)19-5-14(24)3-4-15(17)19/h3-5,7-8,11-13H,9-10H2,1-2H3,(H,26,27)(H,28,32)/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) using [33gammaP]ATP and Biotin-KAIETDKEYYTVKD as substrate incubated for 10 mins prior to substrate addition meas... |
Bioorg Med Chem Lett 23: 2793-800 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.012
BindingDB Entry DOI: 10.7270/Q2QR4ZG3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50433254

(CHEMBL2376040)Show SMILES COc1cc(cc(OC)c1OC)-c1cnc2[nH]cc(C(=O)N[C@H](C)C(=O)N3CC(C3)C#N)c2n1 |r| Show InChI InChI=1S/C23H24N6O5/c1-12(23(31)29-10-13(7-24)11-29)27-22(30)15-8-25-21-19(15)28-16(9-26-21)14-5-17(32-2)20(34-4)18(6-14)33-3/h5-6,8-9,12-13H,10-11H2,1-4H3,(H,25,26)(H,27,30)/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) using [33gammaP]ATP and Biotin-KAIETDKEYYTVKD as substrate incubated for 10 mins prior to substrate addition meas... |
Bioorg Med Chem Lett 23: 2793-800 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.012
BindingDB Entry DOI: 10.7270/Q2QR4ZG3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50433242
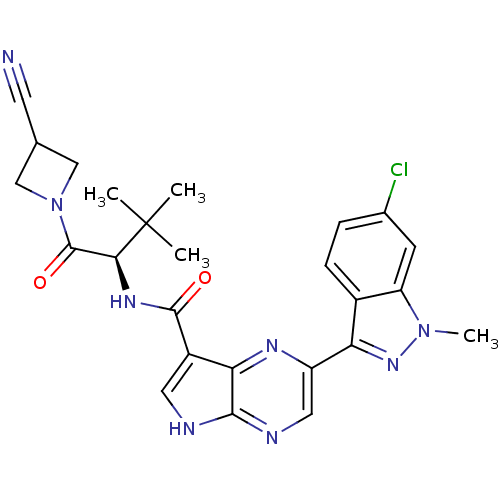
(CHEMBL2376155)Show SMILES Cn1nc(-c2cnc3[nH]cc(C(=O)N[C@@H](C(=O)N4CC(C4)C#N)C(C)(C)C)c3n2)c2ccc(Cl)cc12 |r| Show InChI InChI=1S/C25H25ClN8O2/c1-25(2,3)21(24(36)34-11-13(8-27)12-34)31-23(35)16-9-28-22-20(16)30-17(10-29-22)19-15-6-5-14(26)7-18(15)33(4)32-19/h5-7,9-10,13,21H,11-12H2,1-4H3,(H,28,29)(H,31,35)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) using [33gammaP]ATP and Biotin-KAIETDKEYYTVKD as substrate incubated for 10 mins prior to substrate addition meas... |
Bioorg Med Chem Lett 23: 2793-800 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.012
BindingDB Entry DOI: 10.7270/Q2QR4ZG3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50433243

(CHEMBL2376154)Show SMILES Cn1nc(-c2cnc3[nH]cc(C(=O)N[C@H](C4CC4)C(=O)N4CC(C4)C#N)c3n2)c2ccc(Cl)cc12 |r| Show InChI InChI=1S/C24H21ClN8O2/c1-32-18-6-14(25)4-5-15(18)20(31-32)17-9-28-22-21(29-17)16(8-27-22)23(34)30-19(13-2-3-13)24(35)33-10-12(7-26)11-33/h4-6,8-9,12-13,19H,2-3,10-11H2,1H3,(H,27,28)(H,30,34)/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK1 (unknown origin) using [33gammaP]ATP and Biotin-KAIETDKEYYTVKD as substrate incubated for 10 mins prior to substrate addition meas... |
Bioorg Med Chem Lett 23: 2793-800 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.012
BindingDB Entry DOI: 10.7270/Q2QR4ZG3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK1 (unknown origin) using [33gammaP]ATP and Biotin-KAIETDKEYYTVKD as substrate incubated for 10 mins prior to substrate addition meas... |
Bioorg Med Chem Lett 23: 2793-800 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.012
BindingDB Entry DOI: 10.7270/Q2QR4ZG3 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50383916

(CHEMBL2031657)Show SMILES O=c1[nH]nc(o1)-c1ccc(OCc2ccc3ccccc3n2)cc1[C@]1(C[C@H]2CC[C@H]1C2)c1ccccc1 |r,TLB:31:24:27.28:30,THB:23:24:27.28:30| Show InChI InChI=1S/C31H27N3O3/c35-30-34-33-29(37-30)26-15-14-25(36-19-24-13-11-21-6-4-5-9-28(21)32-24)17-27(26)31(22-7-2-1-3-8-22)18-20-10-12-23(31)16-20/h1-9,11,13-15,17,20,23H,10,12,16,18-19H2,(H,34,35)/t20-,23-,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]L-691,831 from 5-lipoxygenase-activating protein in human polymorphonuclear cells |
Bioorg Med Chem Lett 22: 4133-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.064
BindingDB Entry DOI: 10.7270/Q2348MCB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50426601

(CHEMBL2325895)Show SMILES CC(C)(C)[C@@H](NC(=O)c1c[nH]c2ncc(nc12)C1CC1)C(=O)N1CC(C1)C#N |r| Show InChI InChI=1S/C20H24N6O2/c1-20(2,3)16(19(28)26-9-11(6-21)10-26)25-18(27)13-7-22-17-15(13)24-14(8-23-17)12-4-5-12/h7-8,11-12,16H,4-5,9-10H2,1-3H3,(H,22,23)(H,25,27)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... |
J Med Chem 56: 345-56 (2013)
Article DOI: 10.1021/jm301646k
BindingDB Entry DOI: 10.7270/Q2Q241JX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50426601

(CHEMBL2325895)Show SMILES CC(C)(C)[C@@H](NC(=O)c1c[nH]c2ncc(nc12)C1CC1)C(=O)N1CC(C1)C#N |r| Show InChI InChI=1S/C20H24N6O2/c1-20(2,3)16(19(28)26-9-11(6-21)10-26)25-18(27)13-7-22-17-15(13)24-14(8-23-17)12-4-5-12/h7-8,11-12,16H,4-5,9-10H2,1-3H3,(H,22,23)(H,25,27)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) using [33gammaP]ATP and Biotin-KAIETDKEYYTVKD as substrate incubated for 10 mins prior to substrate addition meas... |
Bioorg Med Chem Lett 23: 2793-800 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.012
BindingDB Entry DOI: 10.7270/Q2QR4ZG3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... |
Bioorg Med Chem Lett 24: 4969-75 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.031
BindingDB Entry DOI: 10.7270/Q25X2BPB |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50167697

((4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,1...)Show SMILES CC1(CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21)C(=O)NC(=O)[C@@]1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21 Show InChI InChI=1S/C34H43NO4/c1-31-15-5-17-33(3,27(31)13-9-21-7-11-23(36)19-25(21)31)29(38)35-30(39)34(4)18-6-16-32(2)26-20-24(37)12-8-22(26)10-14-28(32)34/h7-8,11-12,19-20,27-28,36-37H,5-6,9-10,13-18H2,1-4H3,(H,35,38,39)/t27-,28-,31-,32-,33+,34?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-alpha in SPA assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50167697

((4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,1...)Show SMILES CC1(CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21)C(=O)NC(=O)[C@@]1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21 Show InChI InChI=1S/C34H43NO4/c1-31-15-5-17-33(3,27(31)13-9-21-7-11-23(36)19-25(21)31)29(38)35-30(39)34(4)18-6-16-32(2)26-20-24(37)12-8-22(26)10-14-28(32)34/h7-8,11-12,19-20,27-28,36-37H,5-6,9-10,13-18H2,1-4H3,(H,35,38,39)/t27-,28-,31-,32-,33+,34?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-beta in SPA assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50167698
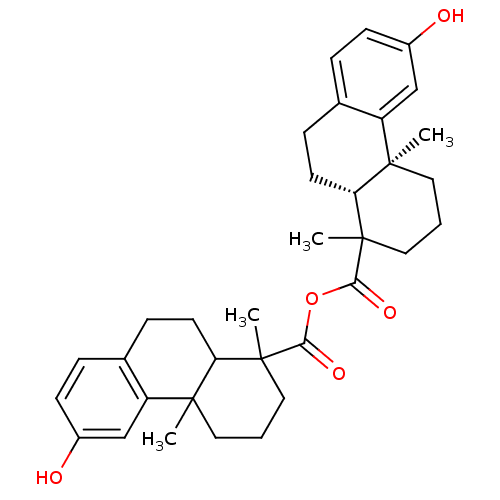
(13-hydroxy-2,6-dimethyl-(2S,7R)-tricyclo[8.4.0.02,...)Show SMILES CC1(CCCC2(C)C1CCc1ccc(O)cc21)C(=O)OC(=O)C1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21 Show InChI InChI=1S/C34H42O5/c1-31-15-5-17-33(3,27(31)13-9-21-7-11-23(35)19-25(21)31)29(37)39-30(38)34(4)18-6-16-32(2)26-20-24(36)12-8-22(26)10-14-28(32)34/h7-8,11-12,19-20,27-28,35-36H,5-6,9-10,13-18H2,1-4H3/t27-,28?,31-,32?,33?,34?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-beta in SPA assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50167697

((4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,1...)Show SMILES CC1(CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21)C(=O)NC(=O)[C@@]1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21 Show InChI InChI=1S/C34H43NO4/c1-31-15-5-17-33(3,27(31)13-9-21-7-11-23(36)19-25(21)31)29(38)35-30(39)34(4)18-6-16-32(2)26-20-24(37)12-8-22(26)10-14-28(32)34/h7-8,11-12,19-20,27-28,36-37H,5-6,9-10,13-18H2,1-4H3,(H,35,38,39)/t27-,28-,31-,32-,33+,34?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration in LXRSPA beta binding assay |
Bioorg Med Chem Lett 15: 4574-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.100
BindingDB Entry DOI: 10.7270/Q2154GKK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50400047

(BIIB-057 | CHEMBL2177736 | US9579320, Example 87)Show SMILES N[C@H]1CCCC[C@H]1Nc1ncc(C(N)=O)c(Nc2cccc(c2)-n2nccn2)n1 |r| Show InChI InChI=1S/C19H23N9O/c20-15-6-1-2-7-16(15)26-19-22-11-14(17(21)29)18(27-19)25-12-4-3-5-13(10-12)28-23-8-9-24-28/h3-5,8-11,15-16H,1-2,6-7,20H2,(H2,21,29)(H2,22,25,26,27)/t15-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant truncated SYK (360 to 365 amino acid residues) using N-terminally biotinylated EPEGDYEEVLE peptide as substrate asses... |
J Med Chem 56: 1677-92 (2013)
Article DOI: 10.1021/jm301720p
BindingDB Entry DOI: 10.7270/Q22N53M0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
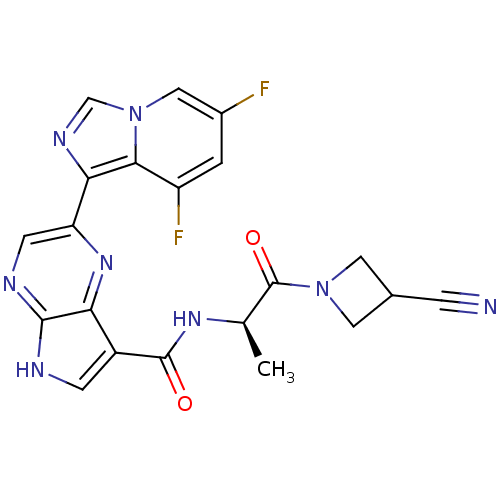
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50431385
(CHEMBL2346686)Show SMILES Cn1nc(-c2cnc3[nH]cc(C(=O)NC(C)(C)C)c3n2)c2cc(OC(F)F)ccc12 Show InChI InChI=1S/C20H20F2N6O2/c1-20(2,3)26-18(29)12-8-23-17-16(12)25-13(9-24-17)15-11-7-10(30-19(21)22)5-6-14(11)28(4)27-15/h5-9,19H,1-4H3,(H,23,24)(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant truncated SYK (360 to 365 amino acid residues) using N-terminally biotinylated EPEGDYEEVLE peptide as substrate asses... |
J Med Chem 56: 1677-92 (2013)
Article DOI: 10.1021/jm301720p
BindingDB Entry DOI: 10.7270/Q22N53M0 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50167697

((4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,1...)Show SMILES CC1(CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21)C(=O)NC(=O)[C@@]1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21 Show InChI InChI=1S/C34H43NO4/c1-31-15-5-17-33(3,27(31)13-9-21-7-11-23(36)19-25(21)31)29(38)35-30(39)34(4)18-6-16-32(2)26-20-24(37)12-8-22(26)10-14-28(32)34/h7-8,11-12,19-20,27-28,36-37H,5-6,9-10,13-18H2,1-4H3,(H,35,38,39)/t27-,28-,31-,32-,33+,34?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration in LXRSPA alpha binding assay |
Bioorg Med Chem Lett 15: 4574-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.100
BindingDB Entry DOI: 10.7270/Q2154GKK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM112546
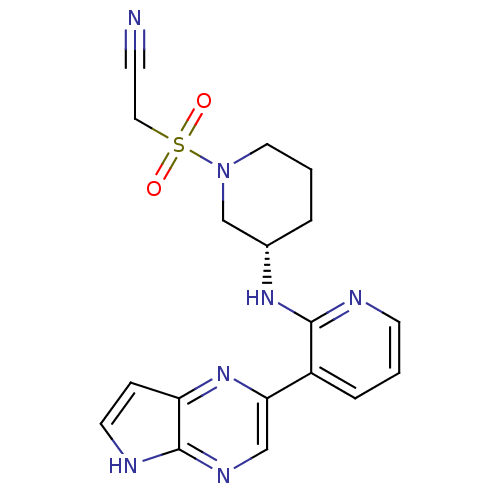
(US8618103, I-42)Show SMILES O=S(=O)(CC#N)N1CCC[C@@H](C1)Nc1ncccc1-c1cnc2[nH]ccc2n1 |r| Show InChI InChI=1S/C18H19N7O2S/c19-6-10-28(26,27)25-9-2-3-13(12-25)23-17-14(4-1-7-20-17)16-11-22-18-15(24-16)5-8-21-18/h1,4-5,7-8,11,13H,2-3,9-10,12H2,(H,20,23)(H,21,22)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... |
Bioorg Med Chem Lett 24: 4969-75 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.031
BindingDB Entry DOI: 10.7270/Q25X2BPB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50433253

(CHEMBL2376143)Show SMILES C[C@@H](NC(=O)c1c[nH]c2ncc(nc12)-c1cn(C)c2cc(Cl)ccc12)C(=O)N1CC(C1)C#N |r| Show InChI InChI=1S/C23H20ClN7O2/c1-12(23(33)31-9-13(6-25)10-31)28-22(32)16-7-26-21-20(16)29-18(8-27-21)17-11-30(2)19-5-14(24)3-4-15(17)19/h3-5,7-8,11-13H,9-10H2,1-2H3,(H,26,27)(H,28,32)/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK1 (unknown origin) using [33gammaP]ATP and Biotin-KAIETDKEYYTVKD as substrate incubated for 10 mins prior to substrate addition meas... |
Bioorg Med Chem Lett 23: 2793-800 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.012
BindingDB Entry DOI: 10.7270/Q2QR4ZG3 |
More data for this
Ligand-Target Pair | |
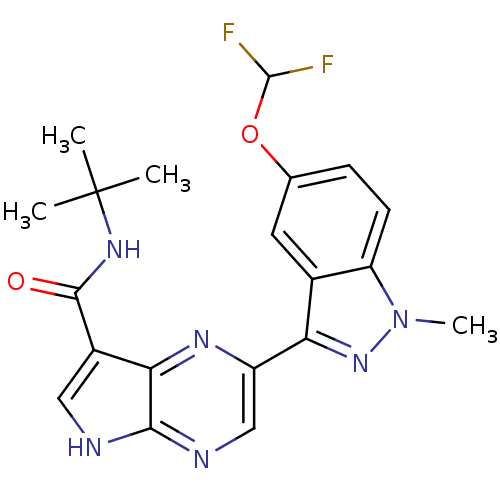
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50433239
(CHEMBL2376158)Show SMILES C[C@@H](NC(=O)c1c[nH]c2ncc(nc12)-c1ncn2cc(F)cc(F)c12)C(=O)N1CC(C1)C#N |r| Show InChI InChI=1S/C21H16F2N8O2/c1-10(21(33)30-6-11(3-24)7-30)28-20(32)13-4-25-19-16(13)29-15(5-26-19)17-18-14(23)2-12(22)8-31(18)9-27-17/h2,4-5,8-11H,6-7H2,1H3,(H,25,26)(H,28,32)/t10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) using [33gammaP]ATP and Biotin-KAIETDKEYYTVKD as substrate incubated for 10 mins prior to substrate addition meas... |
Bioorg Med Chem Lett 23: 2793-800 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.012
BindingDB Entry DOI: 10.7270/Q2QR4ZG3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50433252
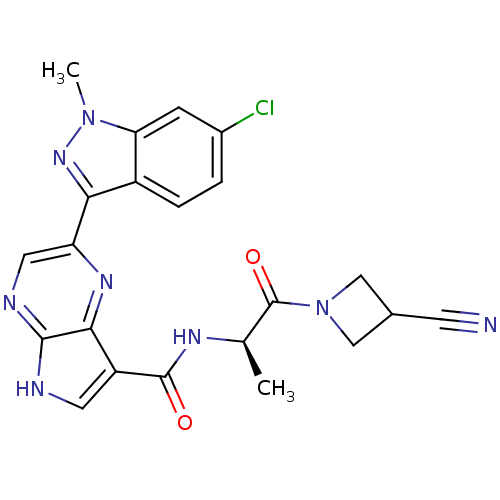
(CHEMBL2376144)Show SMILES C[C@@H](NC(=O)c1c[nH]c2ncc(nc12)-c1nn(C)c2cc(Cl)ccc12)C(=O)N1CC(C1)C#N |r| Show InChI InChI=1S/C22H19ClN8O2/c1-11(22(33)31-9-12(6-24)10-31)27-21(32)15-7-25-20-19(15)28-16(8-26-20)18-14-4-3-13(23)5-17(14)30(2)29-18/h3-5,7-8,11-12H,9-10H2,1-2H3,(H,25,26)(H,27,32)/t11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) using [33gammaP]ATP and Biotin-KAIETDKEYYTVKD as substrate incubated for 10 mins prior to substrate addition meas... |
Bioorg Med Chem Lett 23: 2793-800 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.012
BindingDB Entry DOI: 10.7270/Q2QR4ZG3 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50383906
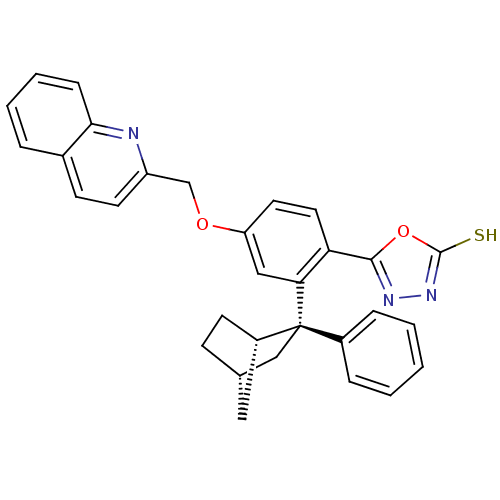
(CHEMBL2031647)Show SMILES Sc1nnc(o1)-c1ccc(OCc2ccc3ccccc3n2)cc1[C@@]1(C[C@H]2CC[C@H]1C2)c1ccccc1 |r,TLB:31:24:28.27:30,THB:23:24:28.27:30| Show InChI InChI=1S/C31H27N3O2S/c37-30-34-33-29(36-30)26-15-14-25(35-19-24-13-11-21-6-4-5-9-28(21)32-24)17-27(26)31(22-7-2-1-3-8-22)18-20-10-12-23(31)16-20/h1-9,11,13-15,17,20,23H,10,12,16,18-19H2,(H,34,37)/t20-,23-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]L-691,831 from 5-lipoxygenase-activating protein in human polymorphonuclear cells |
Bioorg Med Chem Lett 22: 4133-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.064
BindingDB Entry DOI: 10.7270/Q2348MCB |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50383917
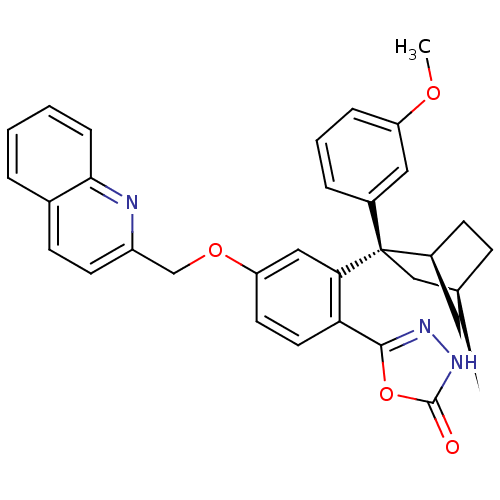
(CHEMBL2031658)Show SMILES COc1cccc(c1)[C@@]1(C[C@H]2CC[C@H]1C2)c1cc(OCc2ccc3ccccc3n2)ccc1-c1n[nH]c(=O)o1 |r,TLB:6:8:11.12:14,THB:15:8:11.12:14| Show InChI InChI=1S/C32H29N3O4/c1-37-25-7-4-6-22(16-25)32(18-20-9-11-23(32)15-20)28-17-26(13-14-27(28)30-34-35-31(36)39-30)38-19-24-12-10-21-5-2-3-8-29(21)33-24/h2-8,10,12-14,16-17,20,23H,9,11,15,18-19H2,1H3,(H,35,36)/t20-,23-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]L-691,831 from 5-lipoxygenase-activating protein in human polymorphonuclear cells |
Bioorg Med Chem Lett 22: 4133-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.064
BindingDB Entry DOI: 10.7270/Q2348MCB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50433243

(CHEMBL2376154)Show SMILES Cn1nc(-c2cnc3[nH]cc(C(=O)N[C@H](C4CC4)C(=O)N4CC(C4)C#N)c3n2)c2ccc(Cl)cc12 |r| Show InChI InChI=1S/C24H21ClN8O2/c1-32-18-6-14(25)4-5-15(18)20(31-32)17-9-28-22-21(29-17)16(8-27-22)23(34)30-19(13-2-3-13)24(35)33-10-12(7-26)11-33/h4-6,8-9,12-13,19H,2-3,10-11H2,1H3,(H,27,28)(H,30,34)/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) using [33gammaP]ATP and Biotin-KAIETDKEYYTVKD as substrate incubated for 10 mins prior to substrate addition meas... |
Bioorg Med Chem Lett 23: 2793-800 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.012
BindingDB Entry DOI: 10.7270/Q2QR4ZG3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50433242

(CHEMBL2376155)Show SMILES Cn1nc(-c2cnc3[nH]cc(C(=O)N[C@@H](C(=O)N4CC(C4)C#N)C(C)(C)C)c3n2)c2ccc(Cl)cc12 |r| Show InChI InChI=1S/C25H25ClN8O2/c1-25(2,3)21(24(36)34-11-13(8-27)12-34)31-23(35)16-9-28-22-20(16)30-17(10-29-22)19-15-6-5-14(26)7-18(15)33(4)32-19/h5-7,9-10,13,21H,11-12H2,1-4H3,(H,28,29)(H,31,35)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) using [33gammaP]ATP and Biotin-KAIETDKEYYTVKD as substrate incubated for 10 mins prior to substrate addition meas... |
Bioorg Med Chem Lett 23: 2793-800 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.012
BindingDB Entry DOI: 10.7270/Q2QR4ZG3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50426606
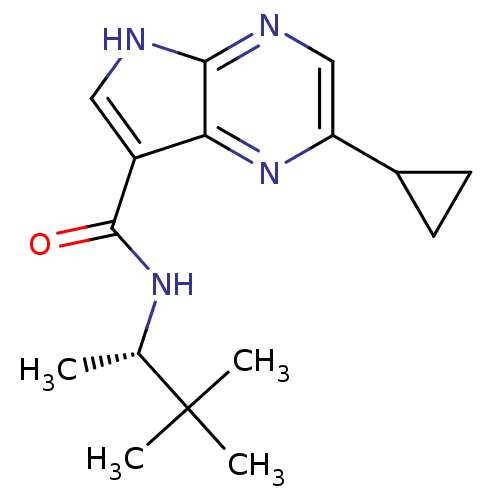
(CHEMBL2325903)Show SMILES C[C@H](NC(=O)c1c[nH]c2ncc(nc12)C1CC1)C(C)(C)C |r| Show InChI InChI=1S/C16H22N4O/c1-9(16(2,3)4)19-15(21)11-7-17-14-13(11)20-12(8-18-14)10-5-6-10/h7-10H,5-6H2,1-4H3,(H,17,18)(H,19,21)/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... |
J Med Chem 56: 345-56 (2013)
Article DOI: 10.1021/jm301646k
BindingDB Entry DOI: 10.7270/Q2Q241JX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK1 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... |
J Med Chem 56: 345-56 (2013)
Article DOI: 10.1021/jm301646k
BindingDB Entry DOI: 10.7270/Q2Q241JX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK1 (unknown origin) using [33gammaP]ATP and Biotin-KAIETDKEYYTVKD as substrate incubated for 10 mins prior to substrate addition meas... |
Bioorg Med Chem Lett 23: 2793-800 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.012
BindingDB Entry DOI: 10.7270/Q2QR4ZG3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50383910
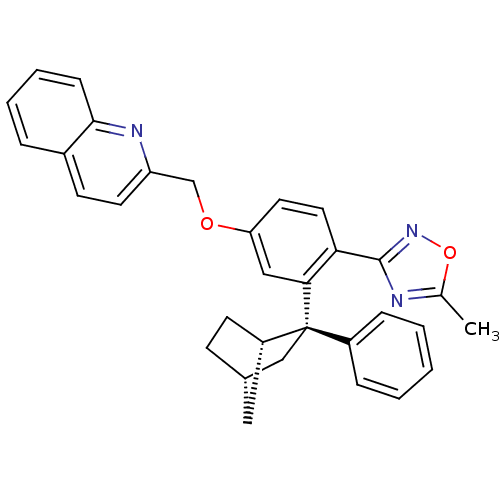
(CHEMBL2031651)Show SMILES Cc1nc(no1)-c1ccc(OCc2ccc3ccccc3n2)cc1[C@@]1(C[C@H]2CC[C@H]1C2)c1ccccc1 |r,TLB:31:24:28.27:30,THB:23:24:28.27:30| Show InChI InChI=1S/C32H29N3O2/c1-21-33-31(35-37-21)28-16-15-27(36-20-26-14-12-23-7-5-6-10-30(23)34-26)18-29(28)32(24-8-3-2-4-9-24)19-22-11-13-25(32)17-22/h2-10,12,14-16,18,22,25H,11,13,17,19-20H2,1H3/t22-,25-,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]L-691,831 from 5-lipoxygenase-activating protein in human polymorphonuclear cells |
Bioorg Med Chem Lett 22: 4133-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.064
BindingDB Entry DOI: 10.7270/Q2348MCB |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50383914

(CHEMBL2031655)Show SMILES CC(C)n1nnc(n1)-c1ccc(OCc2ccc3ccccc3n2)cc1[C@@]1(C[C@H]2CC[C@H]1C2)c1ccccc1 |r,TLB:33:26:30.29:32,THB:25:26:30.29:32| Show InChI InChI=1S/C33H33N5O/c1-22(2)38-36-32(35-37-38)29-17-16-28(39-21-27-15-13-24-8-6-7-11-31(24)34-27)19-30(29)33(25-9-4-3-5-10-25)20-23-12-14-26(33)18-23/h3-11,13,15-17,19,22-23,26H,12,14,18,20-21H2,1-2H3/t23-,26-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]L-691,831 from 5-lipoxygenase-activating protein in human polymorphonuclear cells |
Bioorg Med Chem Lett 22: 4133-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.064
BindingDB Entry DOI: 10.7270/Q2348MCB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50433247

(CHEMBL2376149)Show SMILES COc1ccc2c(nn(C)c2c1)-c1cnc2[nH]cc(C(=O)N[C@H](C)C(=O)N3CC(C3)C#N)c2n1 |r| Show InChI InChI=1S/C23H22N8O3/c1-12(23(33)31-10-13(7-24)11-31)27-22(32)16-8-25-21-20(16)28-17(9-26-21)19-15-5-4-14(34-3)6-18(15)30(2)29-19/h4-6,8-9,12-13H,10-11H2,1-3H3,(H,25,26)(H,27,32)/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) using [33gammaP]ATP and Biotin-KAIETDKEYYTVKD as substrate incubated for 10 mins prior to substrate addition meas... |
Bioorg Med Chem Lett 23: 2793-800 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.012
BindingDB Entry DOI: 10.7270/Q2QR4ZG3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50433244
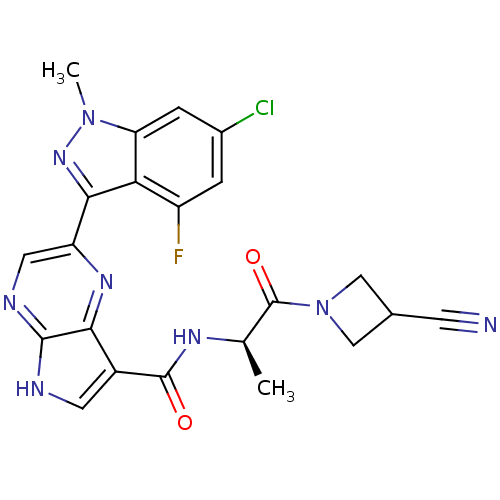
(CHEMBL2376152)Show SMILES C[C@@H](NC(=O)c1c[nH]c2ncc(nc12)-c1nn(C)c2cc(Cl)cc(F)c12)C(=O)N1CC(C1)C#N |r| Show InChI InChI=1S/C22H18ClFN8O2/c1-10(22(34)32-8-11(5-25)9-32)28-21(33)13-6-26-20-18(13)29-15(7-27-20)19-17-14(24)3-12(23)4-16(17)31(2)30-19/h3-4,6-7,10-11H,8-9H2,1-2H3,(H,26,27)(H,28,33)/t10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) using [33gammaP]ATP and Biotin-KAIETDKEYYTVKD as substrate incubated for 10 mins prior to substrate addition meas... |
Bioorg Med Chem Lett 23: 2793-800 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.012
BindingDB Entry DOI: 10.7270/Q2QR4ZG3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50426604

(CHEMBL2325906)Show SMILES C[C@H](NC(=O)c1c[nH]c2ncc(nc12)C1CC1)C(C)(C)C#N |r| Show InChI InChI=1S/C16H19N5O/c1-9(16(2,3)8-17)20-15(22)11-6-18-14-13(11)21-12(7-19-14)10-4-5-10/h6-7,9-10H,4-5H2,1-3H3,(H,18,19)(H,20,22)/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... |
J Med Chem 56: 345-56 (2013)
Article DOI: 10.1021/jm301646k
BindingDB Entry DOI: 10.7270/Q2Q241JX |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50383918
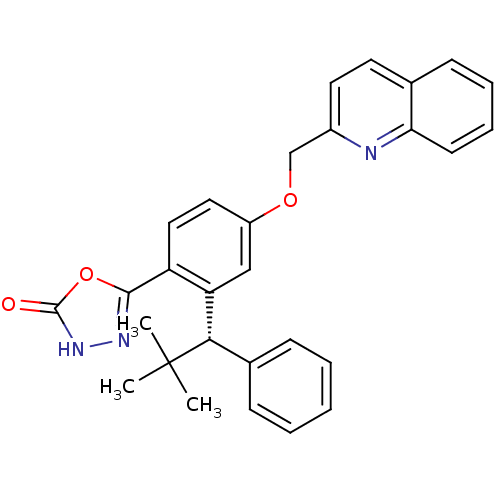
(CHEMBL2031659)Show SMILES CC(C)(C)[C@@H](c1ccccc1)c1cc(OCc2ccc3ccccc3n2)ccc1-c1n[nH]c(=O)o1 |r| Show InChI InChI=1S/C29H27N3O3/c1-29(2,3)26(20-10-5-4-6-11-20)24-17-22(15-16-23(24)27-31-32-28(33)35-27)34-18-21-14-13-19-9-7-8-12-25(19)30-21/h4-17,26H,18H2,1-3H3,(H,32,33)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]L-691,831 from 5-lipoxygenase-activating protein in human polymorphonuclear cells |
Bioorg Med Chem Lett 22: 4133-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.064
BindingDB Entry DOI: 10.7270/Q2348MCB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50431372
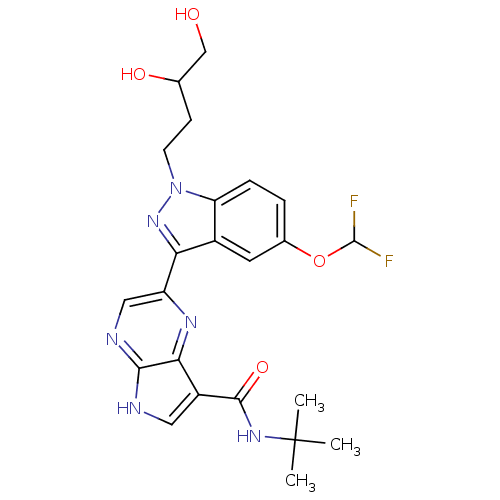
(CHEMBL2347988)Show SMILES CC(C)(C)NC(=O)c1c[nH]c2ncc(nc12)-c1nn(CCC(O)CO)c2ccc(OC(F)F)cc12 Show InChI InChI=1S/C23H26F2N6O4/c1-23(2,3)29-21(34)15-9-26-20-19(15)28-16(10-27-20)18-14-8-13(35-22(24)25)4-5-17(14)31(30-18)7-6-12(33)11-32/h4-5,8-10,12,22,32-33H,6-7,11H2,1-3H3,(H,26,27)(H,29,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant truncated SYK (360 to 365 amino acid residues) using N-terminally biotinylated EPEGDYEEVLE peptide as substrate asses... |
J Med Chem 56: 1677-92 (2013)
Article DOI: 10.1021/jm301720p
BindingDB Entry DOI: 10.7270/Q22N53M0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50431383
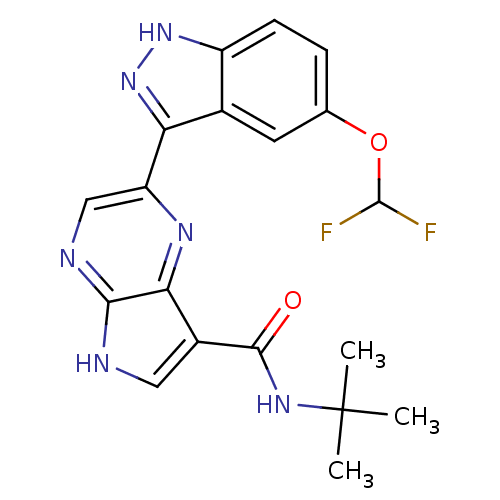
(CHEMBL2347405)Show SMILES CC(C)(C)NC(=O)c1c[nH]c2ncc(nc12)-c1n[nH]c2ccc(OC(F)F)cc12 Show InChI InChI=1S/C19H18F2N6O2/c1-19(2,3)25-17(28)11-7-22-16-15(11)24-13(8-23-16)14-10-6-9(29-18(20)21)4-5-12(10)26-27-14/h4-8,18H,1-3H3,(H,22,23)(H,25,28)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant truncated SYK (360 to 365 amino acid residues) using N-terminally biotinylated EPEGDYEEVLE peptide as substrate asses... |
J Med Chem 56: 1677-92 (2013)
Article DOI: 10.1021/jm301720p
BindingDB Entry DOI: 10.7270/Q22N53M0 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50167694

(2,6-dimethyl-13-methylcarbonyloxy-(2S)-tricyclo[8....)Show SMILES CC(=O)Oc1ccc2CC[C@H]3C(C)(CCC[C@]3(C)c2c1)C(=O)OC(=O)[C@@]1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(OC(C)=O)cc21 Show InChI InChI=1S/C38H46O7/c1-23(39)43-27-13-9-25-11-15-31-35(3,29(25)21-27)17-7-19-37(31,5)33(41)45-34(42)38(6)20-8-18-36(4)30-22-28(44-24(2)40)14-10-26(30)12-16-32(36)38/h9-10,13-14,21-22,31-32H,7-8,11-12,15-20H2,1-6H3/t31-,32-,35-,36-,37+,38?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-alpha in SPA assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50167698

(13-hydroxy-2,6-dimethyl-(2S,7R)-tricyclo[8.4.0.02,...)Show SMILES CC1(CCCC2(C)C1CCc1ccc(O)cc21)C(=O)OC(=O)C1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21 Show InChI InChI=1S/C34H42O5/c1-31-15-5-17-33(3,27(31)13-9-21-7-11-23(35)19-25(21)31)29(37)39-30(38)34(4)18-6-16-32(2)26-20-24(36)12-8-22(26)10-14-28(32)34/h7-8,11-12,19-20,27-28,35-36H,5-6,9-10,13-18H2,1-4H3/t27-,28?,31-,32?,33?,34?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-alpha in SPA assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50167694

(2,6-dimethyl-13-methylcarbonyloxy-(2S)-tricyclo[8....)Show SMILES CC(=O)Oc1ccc2CC[C@H]3C(C)(CCC[C@]3(C)c2c1)C(=O)OC(=O)[C@@]1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(OC(C)=O)cc21 Show InChI InChI=1S/C38H46O7/c1-23(39)43-27-13-9-25-11-15-31-35(3,29(25)21-27)17-7-19-37(31,5)33(41)45-34(42)38(6)20-8-18-36(4)30-22-28(44-24(2)40)14-10-26(30)12-16-32(36)38/h9-10,13-14,21-22,31-32H,7-8,11-12,15-20H2,1-6H3/t31-,32-,35-,36-,37+,38?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration in LXRSPA alpha binding assay |
Bioorg Med Chem Lett 15: 4574-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.100
BindingDB Entry DOI: 10.7270/Q2154GKK |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50167694

(2,6-dimethyl-13-methylcarbonyloxy-(2S)-tricyclo[8....)Show SMILES CC(=O)Oc1ccc2CC[C@H]3C(C)(CCC[C@]3(C)c2c1)C(=O)OC(=O)[C@@]1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(OC(C)=O)cc21 Show InChI InChI=1S/C38H46O7/c1-23(39)43-27-13-9-25-11-15-31-35(3,29(25)21-27)17-7-19-37(31,5)33(41)45-34(42)38(6)20-8-18-36(4)30-22-28(44-24(2)40)14-10-26(30)12-16-32(36)38/h9-10,13-14,21-22,31-32H,7-8,11-12,15-20H2,1-6H3/t31-,32-,35-,36-,37+,38?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration in LXRSPA beta binding assay |
Bioorg Med Chem Lett 15: 4574-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.100
BindingDB Entry DOI: 10.7270/Q2154GKK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK1 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... |
Bioorg Med Chem Lett 24: 4969-75 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.031
BindingDB Entry DOI: 10.7270/Q25X2BPB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data