 Found 139 hits with Last Name = 'messere' and Initial = 'a'
Found 139 hits with Last Name = 'messere' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Smoothened homolog
(Homo sapiens (Human)) | BDBM24498
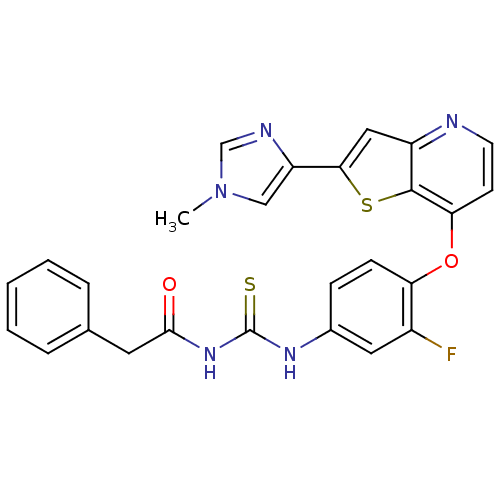
(3-(3-fluoro-4-{[2-(1-methyl-1H-imidazol-4-yl)thien...)Show SMILES Cn1cnc(c1)-c1cc2nccc(Oc3ccc(NC(=S)NC(=O)Cc4ccccc4)cc3F)c2s1 Show InChI InChI=1S/C26H20FN5O2S2/c1-32-14-20(29-15-32)23-13-19-25(36-23)22(9-10-28-19)34-21-8-7-17(12-18(21)27)30-26(35)31-24(33)11-16-5-3-2-4-6-16/h2-10,12-15H,11H2,1H3,(H2,30,31,33,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oncologia Medica, Dipartimento Medico-Chirurgico di Internistica Clinica e Sperimentale"F. Magrassi e A. Lanzara", UniversitÓ della Campania"Luigi Vanvitelli" , Via Pansini 6, 801
Curated by ChEMBL
| Assay Description
Displacement of [3H]-cyclopamine from human wild-type SMO receptor expressed in HEK293T cell membranes by liquid scintillation spectrometry |
J Med Chem 60: 7447-7458 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00794
BindingDB Entry DOI: 10.7270/Q22B91GS |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Homo sapiens (Human)) | BDBM50399540

(FORETINIB | US10464902, Foretinib | US10882853, Co...)Show SMILES COc1cc2c(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)ccnc2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C34H34F2N4O6/c1-43-30-20-25-27(21-31(30)45-16-2-13-40-14-17-44-18-15-40)37-12-9-28(25)46-29-8-7-24(19-26(29)36)39-33(42)34(10-11-34)32(41)38-23-5-3-22(35)4-6-23/h3-9,12,19-21H,2,10-11,13-18H2,1H3,(H,38,41)(H,39,42) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oncologia Medica, Dipartimento Medico-Chirurgico di Internistica Clinica e Sperimentale"F. Magrassi e A. Lanzara", UniversitÓ della Campania"Luigi Vanvitelli" , Via Pansini 6, 801
Curated by ChEMBL
| Assay Description
Displacement of [3H]-cyclopamine from human wild-type SMO receptor expressed in HEK293T cell membranes by liquid scintillation spectrometry |
J Med Chem 60: 7447-7458 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00794
BindingDB Entry DOI: 10.7270/Q22B91GS |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50250705
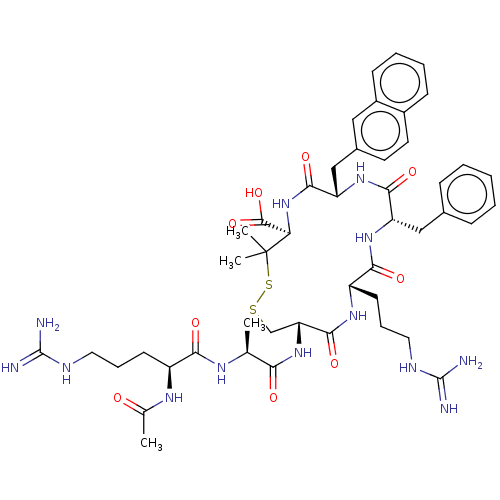
(CHEMBL4078698)Show SMILES C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(C)=O)C(=O)N[C@@H]1CSSC(C)(C)[C@H](NC(=O)[C@H](Cc2ccc3ccccc3c2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(O)=O |r| Show InChI InChI=1S/C47H65N13O9S2/c1-26(54-39(63)32(55-27(2)61)16-10-20-52-45(48)49)38(62)59-36-25-70-71-47(3,4)37(44(68)69)60-42(66)35(24-29-18-19-30-14-8-9-15-31(30)22-29)58-41(65)34(23-28-12-6-5-7-13-28)57-40(64)33(56-43(36)67)17-11-21-53-46(50)51/h5-9,12-15,18-19,22,26,32-37H,10-11,16-17,20-21,23-25H2,1-4H3,(H,54,63)(H,55,61)(H,56,67)(H,57,64)(H,58,65)(H,59,62)(H,60,66)(H,68,69)(H4,48,49,52)(H4,50,51,53)/t26-,32-,33-,34-,35-,36+,37+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL12 from CXCR4 in human CCRF-CEM cells after 1 hr by gamma counting method |
J Med Chem 60: 9641-9652 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01062
BindingDB Entry DOI: 10.7270/Q28S4SBJ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50027084

(Melatonan)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01848
BindingDB Entry DOI: 10.7270/Q2J67N1M |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50027084

(Melatonan)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01848
BindingDB Entry DOI: 10.7270/Q2J67N1M |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50603799

(CHEMBL5180152)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CSCc2ccc(CSC[C@H](NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc3ccccc3)NC(=O)[C@H](Cc3c[nH]cn3)NC1=O)C(N)=O)cc2 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01848
BindingDB Entry DOI: 10.7270/Q2J67N1M |
More data for this
Ligand-Target Pair | |
Protein Mdm4
(Homo sapiens (Human)) | BDBM50241617

(CHEMBL4068667)Show SMILES CC(CC(=O)N[C@@H](Cc1ccc(OCCCNc2ccccn2)cc1)C(O)=O)c1ccccc1 |r| Show InChI InChI=1S/C27H31N3O4/c1-20(22-8-3-2-4-9-22)18-26(31)30-24(27(32)33)19-21-11-13-23(14-12-21)34-17-7-16-29-25-10-5-6-15-28-25/h2-6,8-15,20,24H,7,16-19H2,1H3,(H,28,29)(H,30,31)(H,32,33)/t20?,24-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Napoli Federico II
Curated by ChEMBL
| Assay Description
Inhibition of MDM4 in human SH-SY5Y cells assessed as reduction in MDM4 interaction with p53 after 10 mins by quantitative sandwich immune-enzymatic ... |
J Med Chem 60: 8115-8130 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00912
BindingDB Entry DOI: 10.7270/Q2348NJ0 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50603801
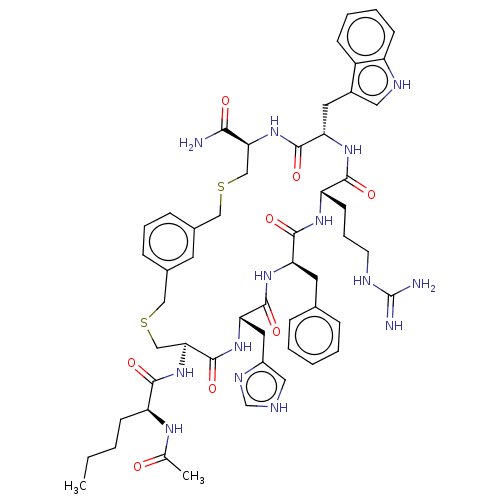
(CHEMBL5203580)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CSCc2cccc(CSC[C@H](NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc3ccccc3)NC(=O)[C@H](Cc3c[nH]cn3)NC1=O)C(N)=O)c2 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01848
BindingDB Entry DOI: 10.7270/Q2J67N1M |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50603801

(CHEMBL5203580)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CSCc2cccc(CSC[C@H](NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc3ccccc3)NC(=O)[C@H](Cc3c[nH]cn3)NC1=O)C(N)=O)c2 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01848
BindingDB Entry DOI: 10.7270/Q2J67N1M |
More data for this
Ligand-Target Pair | |
Protein Mdm4
(Homo sapiens (Human)) | BDBM50241630
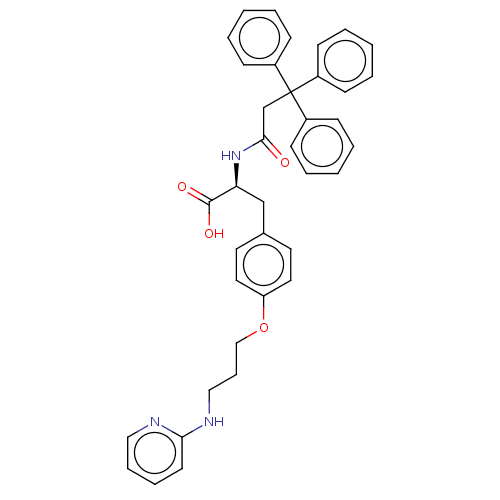
(CHEMBL4065471)Show SMILES OC(=O)[C@H](Cc1ccc(OCCCNc2ccccn2)cc1)NC(=O)CC(c1ccccc1)(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C38H37N3O4/c42-36(28-38(30-13-4-1-5-14-30,31-15-6-2-7-16-31)32-17-8-3-9-18-32)41-34(37(43)44)27-29-20-22-33(23-21-29)45-26-12-25-40-35-19-10-11-24-39-35/h1-11,13-24,34H,12,25-28H2,(H,39,40)(H,41,42)(H,43,44)/t34-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Napoli Federico II
Curated by ChEMBL
| Assay Description
Inhibition of MDM4 in human SH-SY5Y cells assessed as reduction in MDM4 interaction with p53 after 10 mins by quantitative sandwich immune-enzymatic ... |
J Med Chem 60: 8115-8130 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00912
BindingDB Entry DOI: 10.7270/Q2348NJ0 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50250733

(CHEMBL4064373)Show SMILES C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(C)=O)C(=O)N[C@@H]1CSSC(C)(C)[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc3ccccc3c2)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(O)=O |r| Show InChI InChI=1S/C47H65N13O9S2/c1-26(54-39(63)32(55-27(2)61)16-10-20-52-45(48)49)38(62)59-36-25-70-71-47(3,4)37(44(68)69)60-42(66)34(23-28-12-6-5-7-13-28)58-41(65)35(24-29-18-19-30-14-8-9-15-31(30)22-29)57-40(64)33(56-43(36)67)17-11-21-53-46(50)51/h5-9,12-15,18-19,22,26,32-37H,10-11,16-17,20-21,23-25H2,1-4H3,(H,54,63)(H,55,61)(H,56,67)(H,57,64)(H,58,65)(H,59,62)(H,60,66)(H,68,69)(H4,48,49,52)(H4,50,51,53)/t26-,32-,33-,34-,35-,36+,37+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL12 from CXCR4 in human CCRF-CEM cells after 1 hr by gamma counting method |
J Med Chem 60: 9641-9652 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01062
BindingDB Entry DOI: 10.7270/Q28S4SBJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50250704

(CHEMBL4089168)Show SMILES C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(C)=O)C(=O)N[C@@H]1CSSC(C)(C)[C@H](NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](Cc2ccc3ccccc3c2)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(O)=O |r| Show InChI InChI=1S/C44H63N15O9S2/c1-23(53-36(62)29(54-24(2)60)11-7-15-50-42(45)46)35(61)58-33-21-69-70-44(3,4)34(41(67)68)59-39(65)32(19-28-20-49-22-52-28)57-38(64)31(18-25-13-14-26-9-5-6-10-27(26)17-25)56-37(63)30(55-40(33)66)12-8-16-51-43(47)48/h5-6,9-10,13-14,17,20,22-23,29-34H,7-8,11-12,15-16,18-19,21H2,1-4H3,(H,49,52)(H,53,62)(H,54,60)(H,55,66)(H,56,63)(H,57,64)(H,58,61)(H,59,65)(H,67,68)(H4,45,46,50)(H4,47,48,51)/t23-,29-,30-,31-,32-,33+,34+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL12 from CXCR4 in human CCRF-CEM cells after 1 hr by gamma counting method |
J Med Chem 60: 9641-9652 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01062
BindingDB Entry DOI: 10.7270/Q28S4SBJ |
More data for this
Ligand-Target Pair | |
Protein Mdm4
(Homo sapiens (Human)) | BDBM50241631
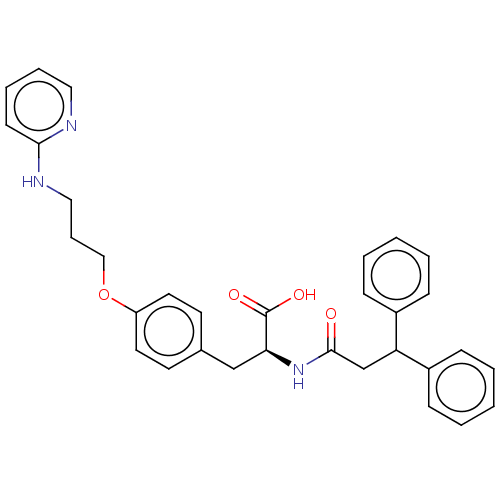
(CHEMBL4095983)Show SMILES OC(=O)[C@H](Cc1ccc(OCCCNc2ccccn2)cc1)NC(=O)CC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C32H33N3O4/c36-31(23-28(25-10-3-1-4-11-25)26-12-5-2-6-13-26)35-29(32(37)38)22-24-15-17-27(18-16-24)39-21-9-20-34-30-14-7-8-19-33-30/h1-8,10-19,28-29H,9,20-23H2,(H,33,34)(H,35,36)(H,37,38)/t29-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Napoli Federico II
Curated by ChEMBL
| Assay Description
Inhibition of MDM4 in human SH-SY5Y cells assessed as reduction in MDM4 interaction with p53 after 10 mins by quantitative sandwich immune-enzymatic ... |
J Med Chem 60: 8115-8130 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00912
BindingDB Entry DOI: 10.7270/Q2348NJ0 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50603799

(CHEMBL5180152)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CSCc2ccc(CSC[C@H](NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc3ccccc3)NC(=O)[C@H](Cc3c[nH]cn3)NC1=O)C(N)=O)cc2 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01848
BindingDB Entry DOI: 10.7270/Q2J67N1M |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50027084

(Melatonan)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01848
BindingDB Entry DOI: 10.7270/Q2J67N1M |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50241631

(CHEMBL4095983)Show SMILES OC(=O)[C@H](Cc1ccc(OCCCNc2ccccn2)cc1)NC(=O)CC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C32H33N3O4/c36-31(23-28(25-10-3-1-4-11-25)26-12-5-2-6-13-26)35-29(32(37)38)22-24-15-17-27(18-16-24)39-21-9-20-34-30-14-7-8-19-33-30/h1-8,10-19,28-29H,9,20-23H2,(H,33,34)(H,35,36)(H,37,38)/t29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Napoli Federico II
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 in human U87MG cells assessed as reduction in MDM2 interaction with p53 after 10 mins by quantitative sandwich immune-enzymatic as... |
J Med Chem 60: 8115-8130 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00912
BindingDB Entry DOI: 10.7270/Q2348NJ0 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50399540

(FORETINIB | US10464902, Foretinib | US10882853, Co...)Show SMILES COc1cc2c(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)ccnc2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C34H34F2N4O6/c1-43-30-20-25-27(21-31(30)45-16-2-13-40-14-17-44-18-15-40)37-12-9-28(25)46-29-8-7-24(19-26(29)36)39-33(42)34(10-11-34)32(41)38-23-5-3-22(35)4-6-23/h3-9,12,19-21H,2,10-11,13-18H2,1H3,(H,38,41)(H,39,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Oncologia Medica, Dipartimento Medico-Chirurgico di Internistica Clinica e Sperimentale"F. Magrassi e A. Lanzara", UniversitÓ della Campania"Luigi Vanvitelli" , Via Pansini 6, 801
Curated by ChEMBL
| Assay Description
Inhibition of MET (unknown origin) |
J Med Chem 60: 7447-7458 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00794
BindingDB Entry DOI: 10.7270/Q22B91GS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50603802
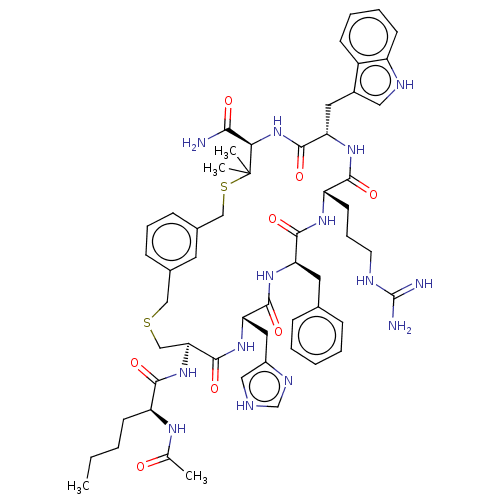
(CHEMBL5187368)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CSCc2cccc(CSC(C)(C)[C@H](NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc3ccccc3)NC(=O)[C@H](Cc3c[nH]cn3)NC1=O)C(N)=O)c2 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01848
BindingDB Entry DOI: 10.7270/Q2J67N1M |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50603804

(CHEMBL5206336)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CSCc2ccccc2CSC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]cn2)NC1=O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01848
BindingDB Entry DOI: 10.7270/Q2J67N1M |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50603801

(CHEMBL5203580)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CSCc2cccc(CSC[C@H](NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc3ccccc3)NC(=O)[C@H](Cc3c[nH]cn3)NC1=O)C(N)=O)c2 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01848
BindingDB Entry DOI: 10.7270/Q2J67N1M |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50241618

(CHEMBL4064911)Show SMILES OC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C31H29NO4/c33-30(21-28(25-12-6-2-7-13-25)26-14-8-3-9-15-26)32-29(31(34)35)20-23-16-18-27(19-17-23)36-22-24-10-4-1-5-11-24/h1-19,28-29H,20-22H2,(H,32,33)(H,34,35)/t29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Napoli Federico II
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 in human U87MG cells assessed as reduction in MDM2 interaction with p53 after 10 mins by quantitative sandwich immune-enzymatic as... |
J Med Chem 60: 8115-8130 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00912
BindingDB Entry DOI: 10.7270/Q2348NJ0 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50241623
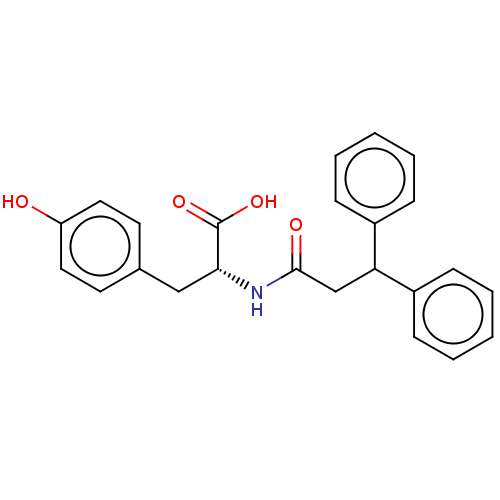
(CHEMBL4102803)Show SMILES OC(=O)[C@@H](Cc1ccc(O)cc1)NC(=O)CC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C24H23NO4/c26-20-13-11-17(12-14-20)15-22(24(28)29)25-23(27)16-21(18-7-3-1-4-8-18)19-9-5-2-6-10-19/h1-14,21-22,26H,15-16H2,(H,25,27)(H,28,29)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Napoli Federico II
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 in human U87MG cells assessed as reduction in MDM2 interaction with p53 after 10 mins by quantitative sandwich immune-enzymatic as... |
J Med Chem 60: 8115-8130 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00912
BindingDB Entry DOI: 10.7270/Q2348NJ0 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50027084

(Melatonan)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01848
BindingDB Entry DOI: 10.7270/Q2J67N1M |
More data for this
Ligand-Target Pair | |
Protein Mdm4
(Homo sapiens (Human)) | BDBM50241627

(CHEMBL4105199)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CC(c1ccccc1)(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C31H29NO4/c1-36-30(35)28(21-23-17-19-27(33)20-18-23)32-29(34)22-31(24-11-5-2-6-12-24,25-13-7-3-8-14-25)26-15-9-4-10-16-26/h2-20,28,33H,21-22H2,1H3,(H,32,34)/t28-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Napoli Federico II
Curated by ChEMBL
| Assay Description
Inhibition of MDM4 in human SH-SY5Y cells assessed as reduction in MDM4 interaction with p53 after 10 mins by quantitative sandwich immune-enzymatic ... |
J Med Chem 60: 8115-8130 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00912
BindingDB Entry DOI: 10.7270/Q2348NJ0 |
More data for this
Ligand-Target Pair | |
Protein Mdm4
(Homo sapiens (Human)) | BDBM50241621
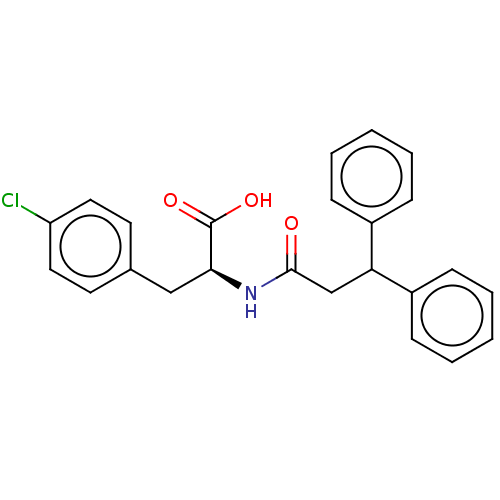
(CHEMBL4095042)Show SMILES OC(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)CC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C24H22ClNO3/c25-20-13-11-17(12-14-20)15-22(24(28)29)26-23(27)16-21(18-7-3-1-4-8-18)19-9-5-2-6-10-19/h1-14,21-22H,15-16H2,(H,26,27)(H,28,29)/t22-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Napoli Federico II
Curated by ChEMBL
| Assay Description
Inhibition of MDM4 in human SH-SY5Y cells assessed as reduction in MDM4 interaction with p53 after 10 mins by quantitative sandwich immune-enzymatic ... |
J Med Chem 60: 8115-8130 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00912
BindingDB Entry DOI: 10.7270/Q2348NJ0 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50241629
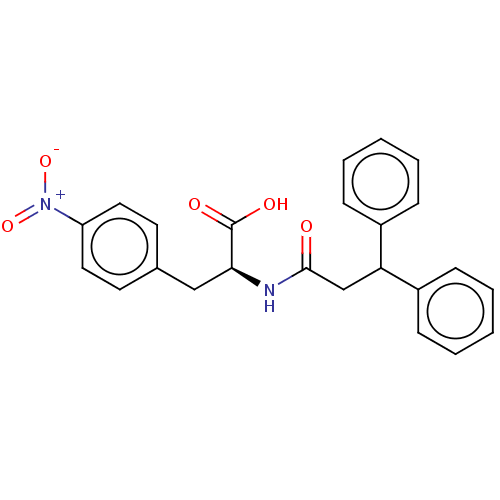
(CHEMBL4100348)Show SMILES OC(=O)[C@H](Cc1ccc(cc1)[N+]([O-])=O)NC(=O)CC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C24H22N2O5/c27-23(16-21(18-7-3-1-4-8-18)19-9-5-2-6-10-19)25-22(24(28)29)15-17-11-13-20(14-12-17)26(30)31/h1-14,21-22H,15-16H2,(H,25,27)(H,28,29)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Napoli Federico II
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 in human U87MG cells assessed as reduction in MDM2 interaction with p53 after 10 mins by quantitative sandwich immune-enzymatic as... |
J Med Chem 60: 8115-8130 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00912
BindingDB Entry DOI: 10.7270/Q2348NJ0 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50603799

(CHEMBL5180152)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CSCc2ccc(CSC[C@H](NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc3ccccc3)NC(=O)[C@H](Cc3c[nH]cn3)NC1=O)C(N)=O)cc2 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01848
BindingDB Entry DOI: 10.7270/Q2J67N1M |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50603800

(CHEMBL5205283)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CSCc2ccc(CSC(C)(C)[C@H](NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc3ccccc3)NC(=O)[C@H](Cc3c[nH]cn3)NC1=O)C(N)=O)cc2 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01848
BindingDB Entry DOI: 10.7270/Q2J67N1M |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50241626
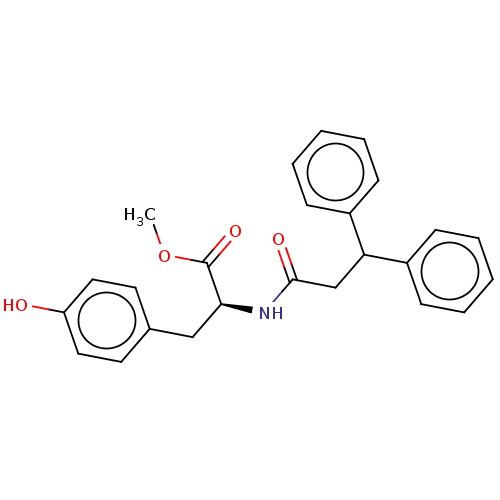
(CHEMBL4080951)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C25H25NO4/c1-30-25(29)23(16-18-12-14-21(27)15-13-18)26-24(28)17-22(19-8-4-2-5-9-19)20-10-6-3-7-11-20/h2-15,22-23,27H,16-17H2,1H3,(H,26,28)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Napoli Federico II
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 in human U87MG cells assessed as reduction in MDM2 interaction with p53 after 10 mins by quantitative sandwich immune-enzymatic as... |
J Med Chem 60: 8115-8130 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00912
BindingDB Entry DOI: 10.7270/Q2348NJ0 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50250704

(CHEMBL4089168)Show SMILES C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(C)=O)C(=O)N[C@@H]1CSSC(C)(C)[C@H](NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](Cc2ccc3ccccc3c2)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(O)=O |r| Show InChI InChI=1S/C44H63N15O9S2/c1-23(53-36(62)29(54-24(2)60)11-7-15-50-42(45)46)35(61)58-33-21-69-70-44(3,4)34(41(67)68)59-39(65)32(19-28-20-49-22-52-28)57-38(64)31(18-25-13-14-26-9-5-6-10-27(26)17-25)56-37(63)30(55-40(33)66)12-8-16-51-43(47)48/h5-6,9-10,13-14,17,20,22-23,29-34H,7-8,11-12,15-16,18-19,21H2,1-4H3,(H,49,52)(H,53,62)(H,54,60)(H,55,66)(H,56,63)(H,57,64)(H,58,61)(H,59,65)(H,67,68)(H4,45,46,50)(H4,47,48,51)/t23-,29-,30-,31-,32-,33+,34+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL12 from CXCR4 in human CCRF-CEM cells after 1 hr by gamma counting method |
J Med Chem 60: 9641-9652 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01062
BindingDB Entry DOI: 10.7270/Q28S4SBJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50250710

(CHEMBL4061034)Show SMILES C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(C)=O)C(=O)N[C@@H]1CSSC(C)(C)[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2cccc3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(O)=O |r| Show InChI InChI=1S/C47H65N13O9S2/c1-26(54-39(63)32(55-27(2)61)19-11-21-52-45(48)49)38(62)59-36-25-70-71-47(3,4)37(44(68)69)60-42(66)34(23-28-13-6-5-7-14-28)57-41(65)35(24-30-17-10-16-29-15-8-9-18-31(29)30)58-40(64)33(56-43(36)67)20-12-22-53-46(50)51/h5-10,13-18,26,32-37H,11-12,19-25H2,1-4H3,(H,54,63)(H,55,61)(H,56,67)(H,57,65)(H,58,64)(H,59,62)(H,60,66)(H,68,69)(H4,48,49,52)(H4,50,51,53)/t26-,32-,33-,34-,35-,36+,37+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL12 from CXCR4 in human CCRF-CEM cells after 1 hr by gamma counting method |
J Med Chem 60: 9641-9652 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01062
BindingDB Entry DOI: 10.7270/Q28S4SBJ |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50241628
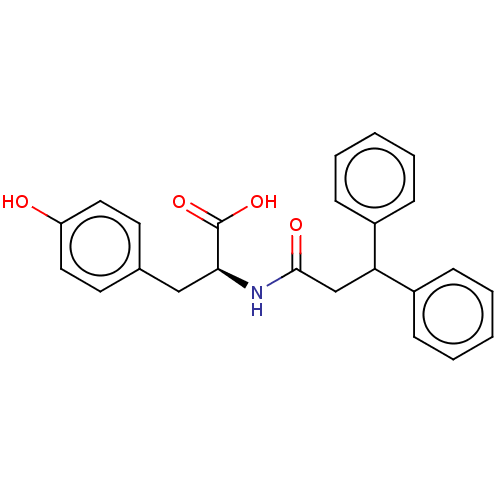
(CHEMBL4084848)Show SMILES OC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C24H23NO4/c26-20-13-11-17(12-14-20)15-22(24(28)29)25-23(27)16-21(18-7-3-1-4-8-18)19-9-5-2-6-10-19/h1-14,21-22,26H,15-16H2,(H,25,27)(H,28,29)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Napoli Federico II
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 in human U87MG cells assessed as reduction in MDM2 interaction with p53 after 10 mins by quantitative sandwich immune-enzymatic as... |
J Med Chem 60: 8115-8130 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00912
BindingDB Entry DOI: 10.7270/Q2348NJ0 |
More data for this
Ligand-Target Pair | |
Protein Mdm4
(Homo sapiens (Human)) | BDBM50241626

(CHEMBL4080951)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C25H25NO4/c1-30-25(29)23(16-18-12-14-21(27)15-13-18)26-24(28)17-22(19-8-4-2-5-9-19)20-10-6-3-7-11-20/h2-15,22-23,27H,16-17H2,1H3,(H,26,28)/t23-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Napoli Federico II
Curated by ChEMBL
| Assay Description
Inhibition of MDM4 in human SH-SY5Y cells assessed as reduction in MDM4 interaction with p53 after 10 mins by quantitative sandwich immune-enzymatic ... |
J Med Chem 60: 8115-8130 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00912
BindingDB Entry DOI: 10.7270/Q2348NJ0 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50603799

(CHEMBL5180152)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CSCc2ccc(CSC[C@H](NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc3ccccc3)NC(=O)[C@H](Cc3c[nH]cn3)NC1=O)C(N)=O)cc2 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01848
BindingDB Entry DOI: 10.7270/Q2J67N1M |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50603802

(CHEMBL5187368)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CSCc2cccc(CSC(C)(C)[C@H](NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc3ccccc3)NC(=O)[C@H](Cc3c[nH]cn3)NC1=O)C(N)=O)c2 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01848
BindingDB Entry DOI: 10.7270/Q2J67N1M |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50603801

(CHEMBL5203580)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CSCc2cccc(CSC[C@H](NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc3ccccc3)NC(=O)[C@H](Cc3c[nH]cn3)NC1=O)C(N)=O)c2 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01848
BindingDB Entry DOI: 10.7270/Q2J67N1M |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50241627

(CHEMBL4105199)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CC(c1ccccc1)(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C31H29NO4/c1-36-30(35)28(21-23-17-19-27(33)20-18-23)32-29(34)22-31(24-11-5-2-6-12-24,25-13-7-3-8-14-25)26-15-9-4-10-16-26/h2-20,28,33H,21-22H2,1H3,(H,32,34)/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Napoli Federico II
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 in human U87MG cells assessed as reduction in MDM2 interaction with p53 after 10 mins by quantitative sandwich immune-enzymatic as... |
J Med Chem 60: 8115-8130 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00912
BindingDB Entry DOI: 10.7270/Q2348NJ0 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50192468
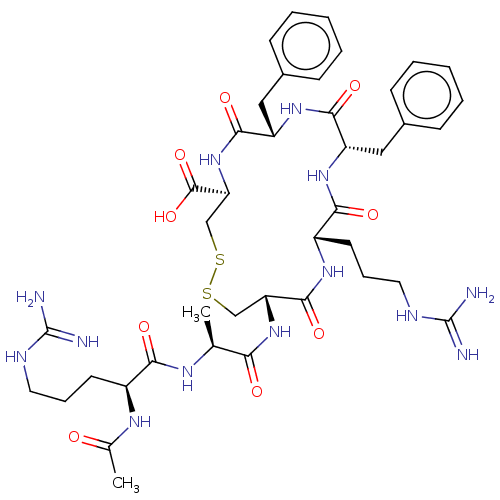
(CHEMBL3923282)Show SMILES C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(C)=O)C(=O)N[C@@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(O)=O |r| Show InChI InChI=1S/C41H59N13O9S2/c1-23(48-34(57)27(49-24(2)55)15-9-17-46-40(42)43)33(56)53-31-21-64-65-22-32(39(62)63)54-37(60)30(20-26-13-7-4-8-14-26)52-36(59)29(19-25-11-5-3-6-12-25)51-35(58)28(50-38(31)61)16-10-18-47-41(44)45/h3-8,11-14,23,27-32H,9-10,15-22H2,1-2H3,(H,48,57)(H,49,55)(H,50,61)(H,51,58)(H,52,59)(H,53,56)(H,54,60)(H,62,63)(H4,42,43,46)(H4,44,45,47)/t23-,27-,28-,29-,30-,31+,32-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL12 from CXCR4 in human CCRF-CEM cells after 1 hr by gamma counting method |
J Med Chem 60: 9641-9652 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01062
BindingDB Entry DOI: 10.7270/Q28S4SBJ |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50241622

(CHEMBL4074577)Show SMILES OC(=O)[C@H](Cc1ccccc1)NC(=O)CC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C24H23NO3/c26-23(25-22(24(27)28)16-18-10-4-1-5-11-18)17-21(19-12-6-2-7-13-19)20-14-8-3-9-15-20/h1-15,21-22H,16-17H2,(H,25,26)(H,27,28)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Napoli Federico II
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 in human U87MG cells assessed as reduction in MDM2 interaction with p53 after 10 mins by quantitative sandwich immune-enzymatic as... |
J Med Chem 60: 8115-8130 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00912
BindingDB Entry DOI: 10.7270/Q2348NJ0 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50603805
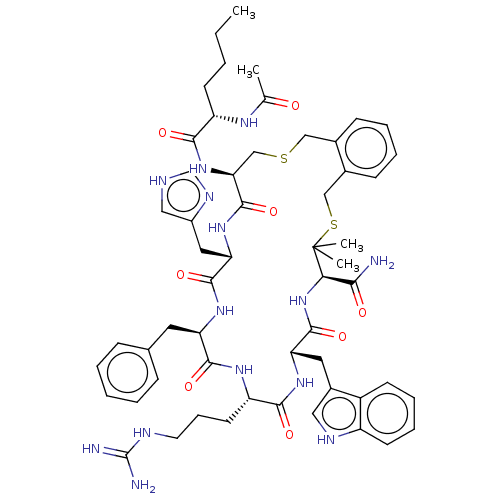
(CHEMBL5172938)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CSCc2ccccc2CSC(C)(C)[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]cn2)NC1=O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01848
BindingDB Entry DOI: 10.7270/Q2J67N1M |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50603805

(CHEMBL5172938)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CSCc2ccccc2CSC(C)(C)[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]cn2)NC1=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01848
BindingDB Entry DOI: 10.7270/Q2J67N1M |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50603805

(CHEMBL5172938)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CSCc2ccccc2CSC(C)(C)[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]cn2)NC1=O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01848
BindingDB Entry DOI: 10.7270/Q2J67N1M |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50241620

(CHEMBL4073512)Show SMILES Nc1ccc(C[C@H](NC(=O)CC(c2ccccc2)c2ccccc2)C(O)=O)cc1 |r| Show InChI InChI=1S/C24H24N2O3/c25-20-13-11-17(12-14-20)15-22(24(28)29)26-23(27)16-21(18-7-3-1-4-8-18)19-9-5-2-6-10-19/h1-14,21-22H,15-16,25H2,(H,26,27)(H,28,29)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Napoli Federico II
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 in human U87MG cells assessed as reduction in MDM2 interaction with p53 after 10 mins by quantitative sandwich immune-enzymatic as... |
J Med Chem 60: 8115-8130 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00912
BindingDB Entry DOI: 10.7270/Q2348NJ0 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50192652
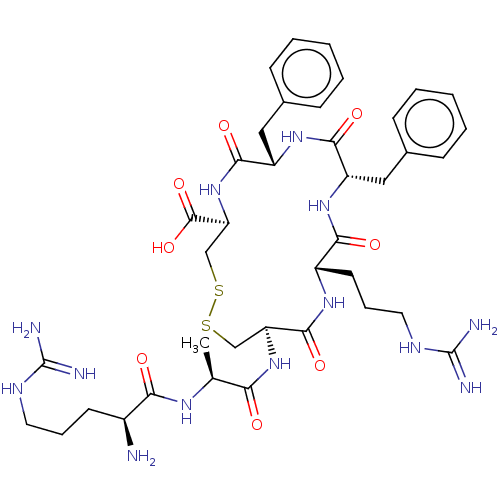
(CHEMBL3901189)Show SMILES C[C@H](NC(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(O)=O |r| Show InChI InChI=1S/C39H57N13O8S2/c1-22(47-32(54)25(40)14-8-16-45-38(41)42)31(53)51-29-20-61-62-21-30(37(59)60)52-35(57)28(19-24-12-6-3-7-13-24)50-34(56)27(18-23-10-4-2-5-11-23)49-33(55)26(48-36(29)58)15-9-17-46-39(43)44/h2-7,10-13,22,25-30H,8-9,14-21,40H2,1H3,(H,47,54)(H,48,58)(H,49,55)(H,50,56)(H,51,53)(H,52,57)(H,59,60)(H4,41,42,45)(H4,43,44,46)/t22-,25-,26-,27-,28-,29-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL12 from CXCR4 in human CCRF-CEM cells after 1 hr by gamma counting method |
J Med Chem 60: 9641-9652 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01062
BindingDB Entry DOI: 10.7270/Q28S4SBJ |
More data for this
Ligand-Target Pair | |
Protein Mdm4
(Homo sapiens (Human)) | BDBM50241618

(CHEMBL4064911)Show SMILES OC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C31H29NO4/c33-30(21-28(25-12-6-2-7-13-25)26-14-8-3-9-15-26)32-29(31(34)35)20-23-16-18-27(19-17-23)36-22-24-10-4-1-5-11-24/h1-19,28-29H,20-22H2,(H,32,33)(H,34,35)/t29-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Napoli Federico II
Curated by ChEMBL
| Assay Description
Inhibition of MDM4 in human SH-SY5Y cells assessed as reduction in MDM4 interaction with p53 after 10 mins by quantitative sandwich immune-enzymatic ... |
J Med Chem 60: 8115-8130 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00912
BindingDB Entry DOI: 10.7270/Q2348NJ0 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50603800

(CHEMBL5205283)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CSCc2ccc(CSC(C)(C)[C@H](NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc3ccccc3)NC(=O)[C@H](Cc3c[nH]cn3)NC1=O)C(N)=O)cc2 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01848
BindingDB Entry DOI: 10.7270/Q2J67N1M |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50603800

(CHEMBL5205283)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CSCc2ccc(CSC(C)(C)[C@H](NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc3ccccc3)NC(=O)[C@H](Cc3c[nH]cn3)NC1=O)C(N)=O)cc2 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01848
BindingDB Entry DOI: 10.7270/Q2J67N1M |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50241621

(CHEMBL4095042)Show SMILES OC(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)CC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C24H22ClNO3/c25-20-13-11-17(12-14-20)15-22(24(28)29)26-23(27)16-21(18-7-3-1-4-8-18)19-9-5-2-6-10-19/h1-14,21-22H,15-16H2,(H,26,27)(H,28,29)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Napoli Federico II
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 in human U87MG cells assessed as reduction in MDM2 interaction with p53 after 10 mins by quantitative sandwich immune-enzymatic as... |
J Med Chem 60: 8115-8130 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00912
BindingDB Entry DOI: 10.7270/Q2348NJ0 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50603800

(CHEMBL5205283)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CSCc2ccc(CSC(C)(C)[C@H](NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc3ccccc3)NC(=O)[C@H](Cc3c[nH]cn3)NC1=O)C(N)=O)cc2 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01848
BindingDB Entry DOI: 10.7270/Q2J67N1M |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50250721

(CHEMBL4093767)Show SMILES C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(C)=O)C(=O)N[C@@H]1CSSC(C)(C)[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(O)=O |r| Show InChI InChI=1S/C43H63N13O10S2/c1-23(50-35(60)28(51-24(2)57)12-8-18-48-41(44)45)34(59)55-32-22-67-68-43(3,4)33(40(65)66)56-38(63)31(20-25-10-6-5-7-11-25)54-37(62)30(21-26-14-16-27(58)17-15-26)53-36(61)29(52-39(32)64)13-9-19-49-42(46)47/h5-7,10-11,14-17,23,28-33,58H,8-9,12-13,18-22H2,1-4H3,(H,50,60)(H,51,57)(H,52,64)(H,53,61)(H,54,62)(H,55,59)(H,56,63)(H,65,66)(H4,44,45,48)(H4,46,47,49)/t23-,28-,29-,30-,31-,32+,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL12 from CXCR4 in human CCRF-CEM cells after 1 hr by gamma counting method |
J Med Chem 60: 9641-9652 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01062
BindingDB Entry DOI: 10.7270/Q28S4SBJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data