 Found 20 hits with Last Name = 'metcalf' and Initial = 'md'
Found 20 hits with Last Name = 'metcalf' and Initial = 'md' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50221416
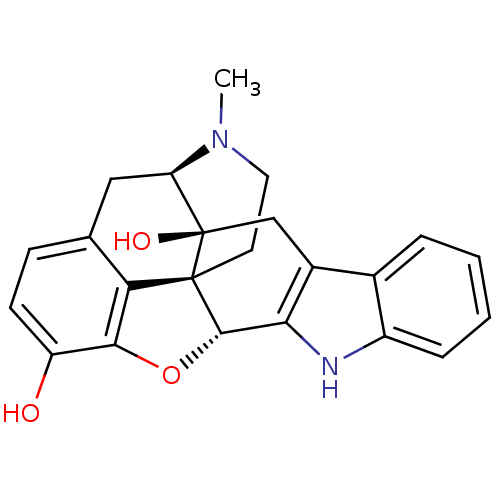
(22-methyl-(2S,13R)-14-oxa-11,22-diazaheptacyclo[13...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@]3(O)Cc1c4[nH]c2ccccc12)ccc5O |r| Show InChI InChI=1S/C23H22N2O3/c1-25-9-8-22-18-12-6-7-16(26)20(18)28-21(22)19-14(11-23(22,27)17(25)10-12)13-4-2-3-5-15(13)24-19/h2-7,17,21,24,26-27H,8-11H2,1H3/t17-,21+,22+,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Displacement of [3H]p-Cl-DPDPE from delta opioid receptor |
Bioorg Med Chem Lett 17: 5916-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.098
BindingDB Entry DOI: 10.7270/Q2RV0NFR |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50221413

((1S,2S,13R,21R)-7-fluoro-22-methyl-14-oxa-11,22-di...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@]3(O)Cc1c4[nH]c2ccc(F)cc12)ccc5O Show InChI InChI=1S/C23H21FN2O3/c1-26-7-6-22-18-11-2-5-16(27)20(18)29-21(22)19-14(10-23(22,28)17(26)8-11)13-9-12(24)3-4-15(13)25-19/h2-5,9,17,21,25,27-28H,6-8,10H2,1H3/t17-,21+,22+,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Displacement of [3H]p-Cl-DPDPE from delta opioid receptor |
Bioorg Med Chem Lett 17: 5916-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.098
BindingDB Entry DOI: 10.7270/Q2RV0NFR |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50221417
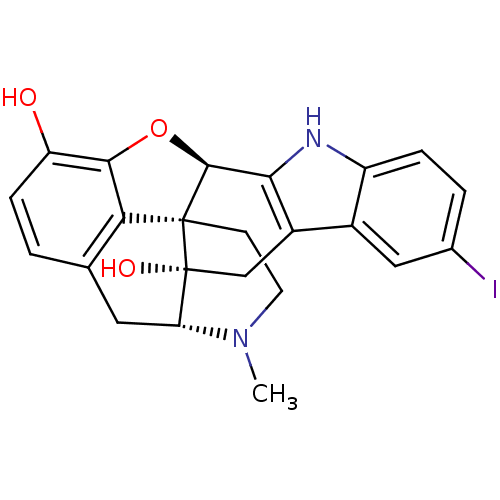
((1S,2S,13R,21R)-7-iodo-22-methyl-14-oxa-11,22-diaz...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@]3(O)Cc1c4[nH]c2ccc(I)cc12)ccc5O Show InChI InChI=1S/C23H21IN2O3/c1-26-7-6-22-18-11-2-5-16(27)20(18)29-21(22)19-14(10-23(22,28)17(26)8-11)13-9-12(24)3-4-15(13)25-19/h2-5,9,17,21,25,27-28H,6-8,10H2,1H3/t17-,21+,22+,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Displacement of [3H]p-Cl-DPDPE from delta opioid receptor |
Bioorg Med Chem Lett 17: 5916-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.098
BindingDB Entry DOI: 10.7270/Q2RV0NFR |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50221414

((1S,2S,13R,21R)-7-chloro-22-methyl-14-oxa-11,22-di...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@]3(O)Cc1c4[nH]c2ccc(Cl)cc12)ccc5O Show InChI InChI=1S/C23H21ClN2O3/c1-26-7-6-22-18-11-2-5-16(27)20(18)29-21(22)19-14(10-23(22,28)17(26)8-11)13-9-12(24)3-4-15(13)25-19/h2-5,9,17,21,25,27-28H,6-8,10H2,1H3/t17-,21+,22+,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Displacement of [3H]p-Cl-DPDPE from delta opioid receptor |
Bioorg Med Chem Lett 17: 5916-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.098
BindingDB Entry DOI: 10.7270/Q2RV0NFR |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50221415
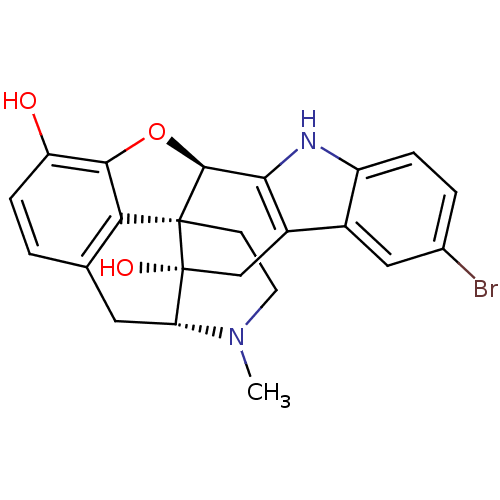
((1S,2S,13R,21R)-7-bromo-22-methyl-14-oxa-11,22-dia...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@]3(O)Cc1c4[nH]c2ccc(Br)cc12)ccc5O Show InChI InChI=1S/C23H21BrN2O3/c1-26-7-6-22-18-11-2-5-16(27)20(18)29-21(22)19-14(10-23(22,28)17(26)8-11)13-9-12(24)3-4-15(13)25-19/h2-5,9,17,21,25,27-28H,6-8,10H2,1H3/t17-,21+,22+,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Displacement of [3H]p-Cl-DPDPE from delta opioid receptor |
Bioorg Med Chem Lett 17: 5916-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.098
BindingDB Entry DOI: 10.7270/Q2RV0NFR |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50221413

((1S,2S,13R,21R)-7-fluoro-22-methyl-14-oxa-11,22-di...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@]3(O)Cc1c4[nH]c2ccc(F)cc12)ccc5O Show InChI InChI=1S/C23H21FN2O3/c1-26-7-6-22-18-11-2-5-16(27)20(18)29-21(22)19-14(10-23(22,28)17(26)8-11)13-9-12(24)3-4-15(13)25-19/h2-5,9,17,21,25,27-28H,6-8,10H2,1H3/t17-,21+,22+,23-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Activity at mu opioid receptor assessed as stimulation of [35S]GTP-gamma-S binding |
Bioorg Med Chem Lett 17: 5916-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.098
BindingDB Entry DOI: 10.7270/Q2RV0NFR |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50221414

((1S,2S,13R,21R)-7-chloro-22-methyl-14-oxa-11,22-di...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@]3(O)Cc1c4[nH]c2ccc(Cl)cc12)ccc5O Show InChI InChI=1S/C23H21ClN2O3/c1-26-7-6-22-18-11-2-5-16(27)20(18)29-21(22)19-14(10-23(22,28)17(26)8-11)13-9-12(24)3-4-15(13)25-19/h2-5,9,17,21,25,27-28H,6-8,10H2,1H3/t17-,21+,22+,23-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Activity at mu opioid receptor assessed as stimulation of [35S]GTP-gamma-S binding |
Bioorg Med Chem Lett 17: 5916-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.098
BindingDB Entry DOI: 10.7270/Q2RV0NFR |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50221417

((1S,2S,13R,21R)-7-iodo-22-methyl-14-oxa-11,22-diaz...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@]3(O)Cc1c4[nH]c2ccc(I)cc12)ccc5O Show InChI InChI=1S/C23H21IN2O3/c1-26-7-6-22-18-11-2-5-16(27)20(18)29-21(22)19-14(10-23(22,28)17(26)8-11)13-9-12(24)3-4-15(13)25-19/h2-5,9,17,21,25,27-28H,6-8,10H2,1H3/t17-,21+,22+,23-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Activity at mu opioid receptor assessed as stimulation of [35S]GTP-gamma-S binding |
Bioorg Med Chem Lett 17: 5916-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.098
BindingDB Entry DOI: 10.7270/Q2RV0NFR |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50221415

((1S,2S,13R,21R)-7-bromo-22-methyl-14-oxa-11,22-dia...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@]3(O)Cc1c4[nH]c2ccc(Br)cc12)ccc5O Show InChI InChI=1S/C23H21BrN2O3/c1-26-7-6-22-18-11-2-5-16(27)20(18)29-21(22)19-14(10-23(22,28)17(26)8-11)13-9-12(24)3-4-15(13)25-19/h2-5,9,17,21,25,27-28H,6-8,10H2,1H3/t17-,21+,22+,23-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Activity at mu opioid receptor assessed as stimulation of [35S]GTP-gamma-S binding |
Bioorg Med Chem Lett 17: 5916-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.098
BindingDB Entry DOI: 10.7270/Q2RV0NFR |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50221416

(22-methyl-(2S,13R)-14-oxa-11,22-diazaheptacyclo[13...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@]3(O)Cc1c4[nH]c2ccccc12)ccc5O |r| Show InChI InChI=1S/C23H22N2O3/c1-25-9-8-22-18-12-6-7-16(26)20(18)28-21(22)19-14(11-23(22,27)17(25)10-12)13-4-2-3-5-15(13)24-19/h2-7,17,21,24,26-27H,8-11H2,1H3/t17-,21+,22+,23-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Activity at mu opioid receptor assessed as stimulation of [35S]GTP-gamma-S binding |
Bioorg Med Chem Lett 17: 5916-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.098
BindingDB Entry DOI: 10.7270/Q2RV0NFR |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50221417

((1S,2S,13R,21R)-7-iodo-22-methyl-14-oxa-11,22-diaz...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@]3(O)Cc1c4[nH]c2ccc(I)cc12)ccc5O Show InChI InChI=1S/C23H21IN2O3/c1-26-7-6-22-18-11-2-5-16(27)20(18)29-21(22)19-14(10-23(22,28)17(26)8-11)13-9-12(24)3-4-15(13)25-19/h2-5,9,17,21,25,27-28H,6-8,10H2,1H3/t17-,21+,22+,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from cloned kappa opioid receptor |
Bioorg Med Chem Lett 17: 5916-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.098
BindingDB Entry DOI: 10.7270/Q2RV0NFR |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50221415

((1S,2S,13R,21R)-7-bromo-22-methyl-14-oxa-11,22-dia...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@]3(O)Cc1c4[nH]c2ccc(Br)cc12)ccc5O Show InChI InChI=1S/C23H21BrN2O3/c1-26-7-6-22-18-11-2-5-16(27)20(18)29-21(22)19-14(10-23(22,28)17(26)8-11)13-9-12(24)3-4-15(13)25-19/h2-5,9,17,21,25,27-28H,6-8,10H2,1H3/t17-,21+,22+,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from cloned kappa opioid receptor |
Bioorg Med Chem Lett 17: 5916-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.098
BindingDB Entry DOI: 10.7270/Q2RV0NFR |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50221413

((1S,2S,13R,21R)-7-fluoro-22-methyl-14-oxa-11,22-di...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@]3(O)Cc1c4[nH]c2ccc(F)cc12)ccc5O Show InChI InChI=1S/C23H21FN2O3/c1-26-7-6-22-18-11-2-5-16(27)20(18)29-21(22)19-14(10-23(22,28)17(26)8-11)13-9-12(24)3-4-15(13)25-19/h2-5,9,17,21,25,27-28H,6-8,10H2,1H3/t17-,21+,22+,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from cloned kappa opioid receptor |
Bioorg Med Chem Lett 17: 5916-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.098
BindingDB Entry DOI: 10.7270/Q2RV0NFR |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50221414

((1S,2S,13R,21R)-7-chloro-22-methyl-14-oxa-11,22-di...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@]3(O)Cc1c4[nH]c2ccc(Cl)cc12)ccc5O Show InChI InChI=1S/C23H21ClN2O3/c1-26-7-6-22-18-11-2-5-16(27)20(18)29-21(22)19-14(10-23(22,28)17(26)8-11)13-9-12(24)3-4-15(13)25-19/h2-5,9,17,21,25,27-28H,6-8,10H2,1H3/t17-,21+,22+,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from cloned kappa opioid receptor |
Bioorg Med Chem Lett 17: 5916-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.098
BindingDB Entry DOI: 10.7270/Q2RV0NFR |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50221416

(22-methyl-(2S,13R)-14-oxa-11,22-diazaheptacyclo[13...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@]3(O)Cc1c4[nH]c2ccccc12)ccc5O |r| Show InChI InChI=1S/C23H22N2O3/c1-25-9-8-22-18-12-6-7-16(26)20(18)28-21(22)19-14(11-23(22,27)17(25)10-12)13-4-2-3-5-15(13)24-19/h2-7,17,21,24,26-27H,8-11H2,1H3/t17-,21+,22+,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 515 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from cloned kappa opioid receptor |
Bioorg Med Chem Lett 17: 5916-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.098
BindingDB Entry DOI: 10.7270/Q2RV0NFR |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50221413

((1S,2S,13R,21R)-7-fluoro-22-methyl-14-oxa-11,22-di...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@]3(O)Cc1c4[nH]c2ccc(F)cc12)ccc5O Show InChI InChI=1S/C23H21FN2O3/c1-26-7-6-22-18-11-2-5-16(27)20(18)29-21(22)19-14(10-23(22,28)17(26)8-11)13-9-12(24)3-4-15(13)25-19/h2-5,9,17,21,25,27-28H,6-8,10H2,1H3/t17-,21+,22+,23-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Activity at mu opioid receptor assessed as stimulation of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 17: 5916-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.098
BindingDB Entry DOI: 10.7270/Q2RV0NFR |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50221413

((1S,2S,13R,21R)-7-fluoro-22-methyl-14-oxa-11,22-di...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@]3(O)Cc1c4[nH]c2ccc(F)cc12)ccc5O Show InChI InChI=1S/C23H21FN2O3/c1-26-7-6-22-18-11-2-5-16(27)20(18)29-21(22)19-14(10-23(22,28)17(26)8-11)13-9-12(24)3-4-15(13)25-19/h2-5,9,17,21,25,27-28H,6-8,10H2,1H3/t17-,21+,22+,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Activity at delta opioid receptor assessed as stimulation of [35S]GTPgammaS binding |
Bioorg Med Chem Lett 17: 5916-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.098
BindingDB Entry DOI: 10.7270/Q2RV0NFR |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50373764
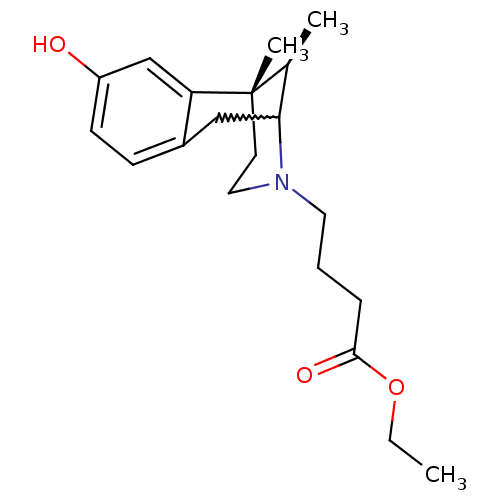
(CHEMBL401590)Show SMILES CCOC(=O)CCCN1CC[C@@]2(C)[C@@H](C)C1Cc1ccc(O)cc21 |w:15.16,TLB:7:8:13:17.23.16,THB:22:23:13:10.8.9| Show InChI InChI=1S/C20H29NO3/c1-4-24-19(23)6-5-10-21-11-9-20(3)14(2)18(21)12-15-7-8-16(22)13-17(15)20/h7-8,13-14,18,22H,4-6,9-12H2,1-3H3/t14-,18?,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.79E+4 | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Agonist activity at mu opioid receptor in Swiss mouse vas deferens assessed as inhibition of electrically-stimulated twitch |
Bioorg Med Chem 16: 869-73 (2008)
Article DOI: 10.1016/j.bmc.2007.10.030
BindingDB Entry DOI: 10.7270/Q2XW4KN1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50373765
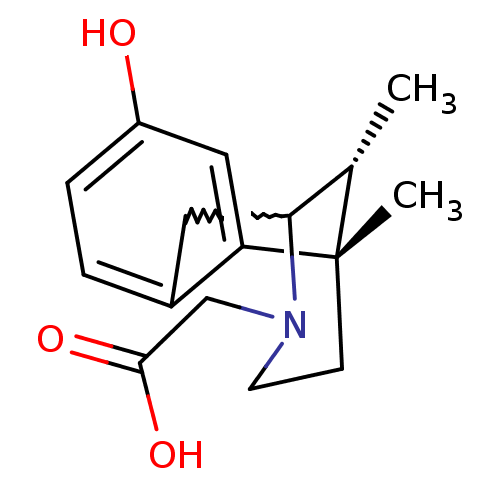
(CHEMBL271026)Show SMILES C[C@H]1C2Cc3ccc(O)cc3[C@@]1(C)CCN2CC(O)=O |w:2.2,TLB:9:10:1:13.15.14,16:15:1:4.10.3| Show InChI InChI=1S/C16H21NO3/c1-10-14-7-11-3-4-12(18)8-13(11)16(10,2)5-6-17(14)9-15(19)20/h3-4,8,10,14,18H,5-7,9H2,1-2H3,(H,19,20)/t10-,14?,16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.79E+3 | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Agonist activity at mu opioid receptor in Swiss mouse vas deferens assessed as inhibition of electrically-stimulated twitch |
Bioorg Med Chem 16: 869-73 (2008)
Article DOI: 10.1016/j.bmc.2007.10.030
BindingDB Entry DOI: 10.7270/Q2XW4KN1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 395 | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Agonist activity at mu opioid receptor in Swiss mouse vas deferens assessed as inhibition of electrically-stimulated twitch |
Bioorg Med Chem 16: 869-73 (2008)
Article DOI: 10.1016/j.bmc.2007.10.030
BindingDB Entry DOI: 10.7270/Q2XW4KN1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data